Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Fabrication of Reinforced and Unreinforced Constructs
2.3. Dynamic Culture of Constructs
2.4. Post-Culture Analyses
2.4.1. Histological Analysis
2.4.2. Digestion of Hydrogels
2.4.3. Measurement of GAG Production
2.4.4. Measurement of Total Collagen Production
2.4.5. Evaluation and Quantitation of Immunofluorescence Staining
2.5. Terminology
2.6. Statistical Analysis
3. Results
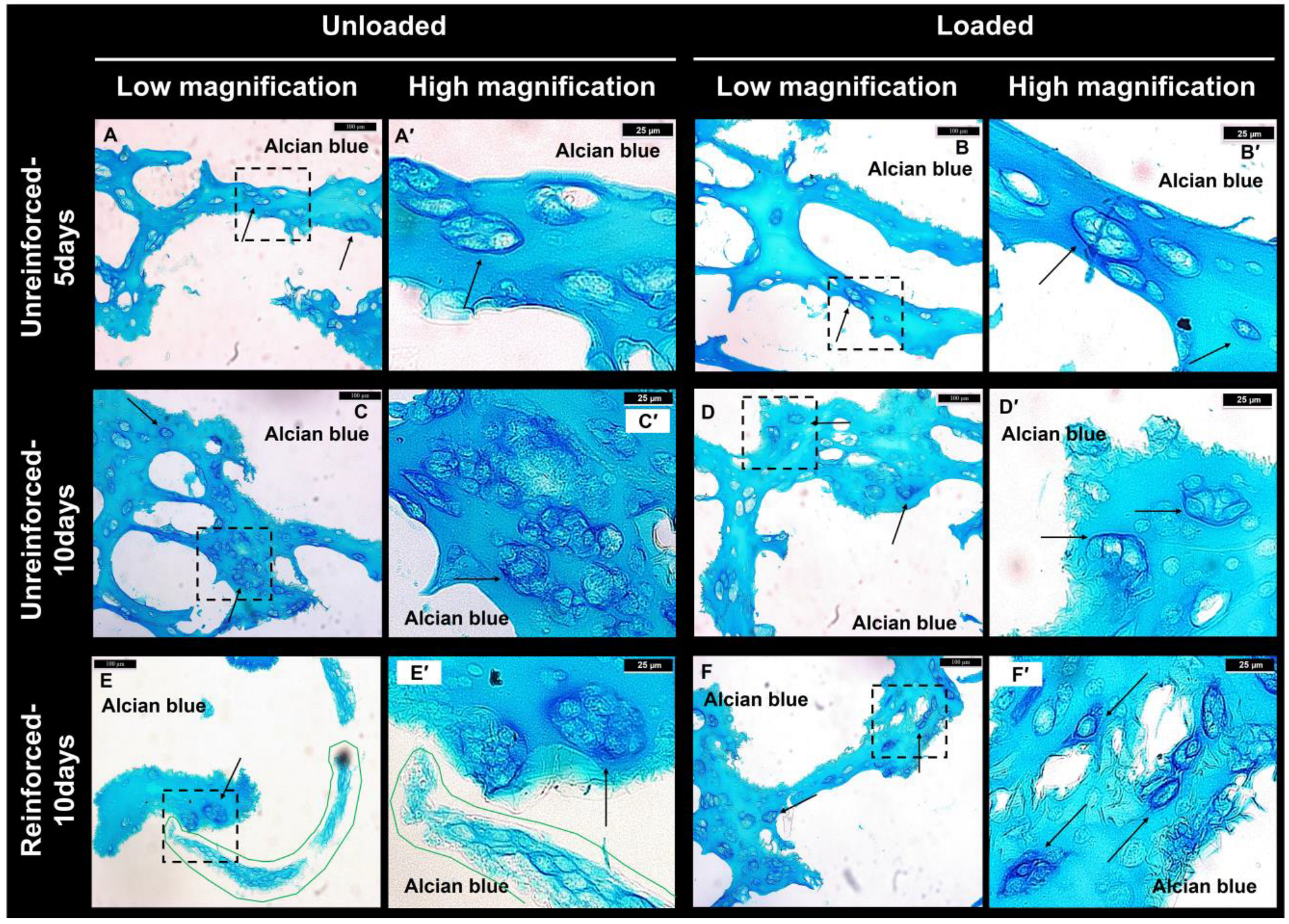
3.1. GAG Deposition Confirmed Chondrogenic Differentiation
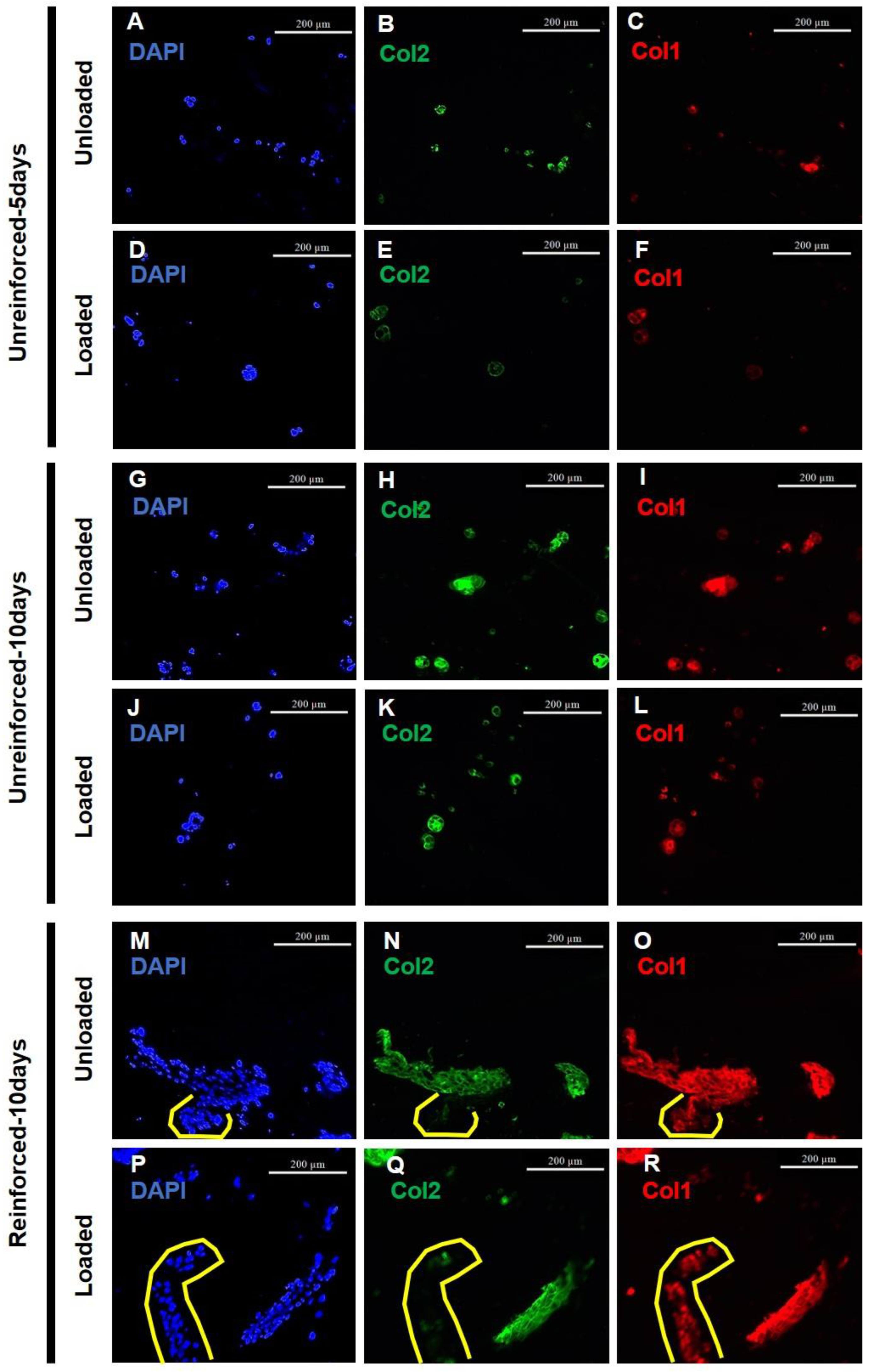
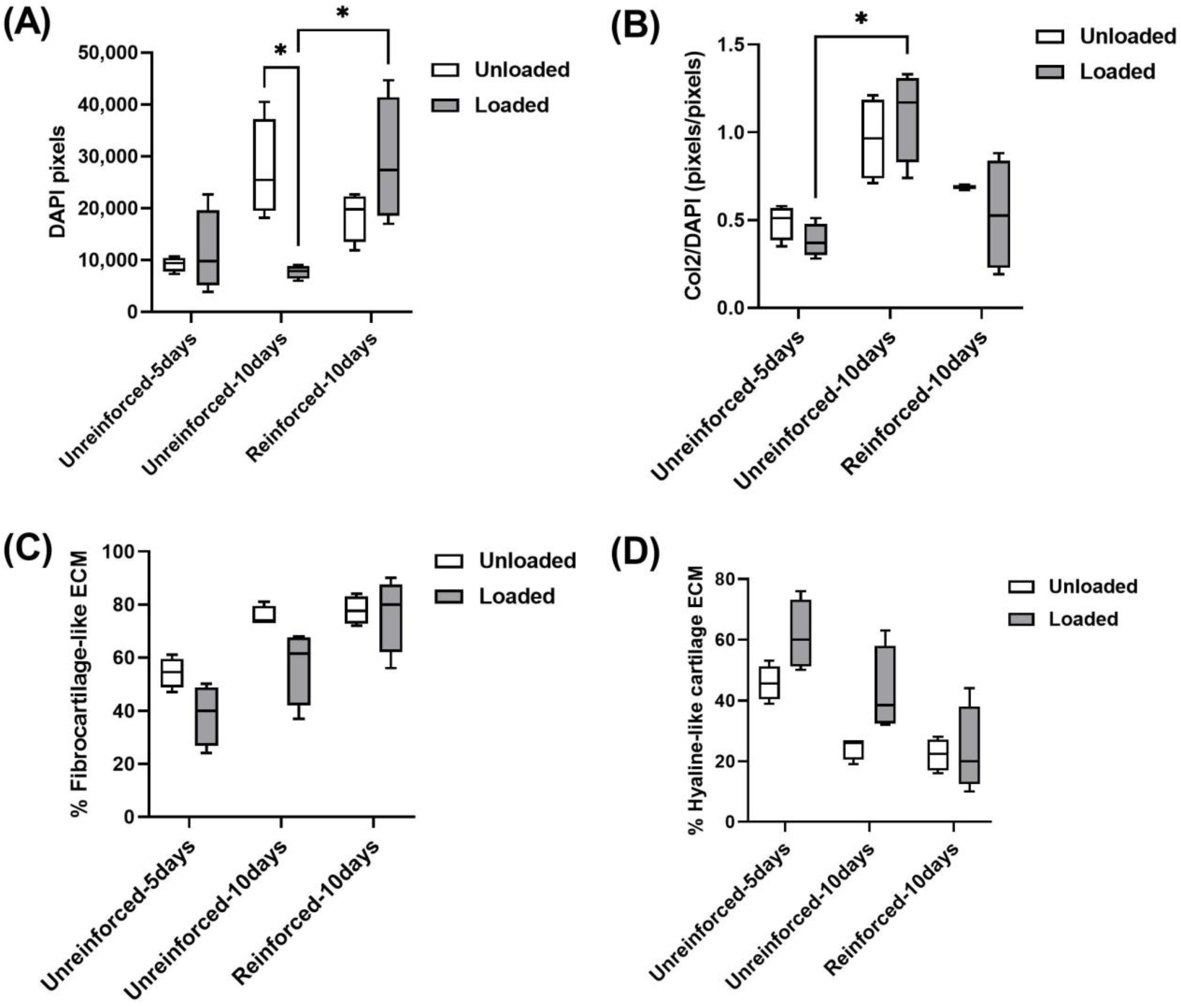
3.2. GAG and Collagen Productions Tended Higher in Reinforced Constructs
3.3. Reinforced Constructs Tending to Increase Cell Numbers and Fibrocartilage Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kopeček, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, D.; Yang, W.; Song, Y. Compressive mechanical properties and microstructure of PVA–HA hydrogels for cartilage repair. RSC Adv. 2016, 6, 20166–20172. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Naghieh, S.; Yazdanpanah, Z.; Sardroud, H.A.; Sharma, N.; Wilson, L.D.; Chen, X. Fabrication of chitosan/alginate/hydroxyapatite hybrid scaffolds using 3D printing and impregnating techniques for potential cartilage regeneration. Int. J. Biol. Macromol. 2022, 204, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Vega, S.L.; Kwon, M.Y.; Burdick, J.A. Recent advances in hydrogels for cartilage tissue engineering. Eur. Cells Mater. 2017, 33, 59. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Zhu, N.; Lei, M.; Eames, B.F.; Chen, X. 3D printing of porous cell-laden hydrogel constructs for potential applications in cartilage tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 1200–1210. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef]
- Farokhi, M.; Jonidi Shariatzadeh, F.; Solouk, A.; Mirzadeh, H. Alginate based scaffolds for cartilage tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 230–247. [Google Scholar] [CrossRef]
- Salati, M.A.; Khazai, J.; Tahmuri, A.M.; Samadi, A.; Taghizadeh, A.; Taghizadeh, M.; Zarrintaj, P.; Ramsey, J.D.; Habibzadeh, S.; Seidi, F. Agarose-based biomaterials: Opportunities and challenges in cartilage tissue engineering. Polymers 2020, 12, 1150. [Google Scholar] [CrossRef]
- Irawan, V.; Sung, T.-C.; Higuchi, A.; Ikoma, T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng. Regen. Med. 2018, 15, 673–697. [Google Scholar] [CrossRef]
- Aguero, L.; Alpdagtas, S.; Ilhan, E.; Zaldivar-Silva, D.; Gunduz, O. Functional role of crosslinking in alginate scaffold for drug delivery and tissue engineering: A review. Eur. Polym. J. 2021, 160, 110807. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hagg, D.; Gatenholm, P. 3D bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Zhang, E.C.; Mauck, R.L.; Burdick, J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng. Part A 2012, 18, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Chang, T.; Kulyk, W.; Chen, X.; Eames, B.F. Analyzing biological performance of 3D-printed, cell-impregnated hybrid constructs for cartilage tissue engineering. Tissue Eng. Part C Methods 2016, 22, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Becerra, J.; Andrades, J.A.; Guerado, E.; Zamora-Navas, P.; López-Puertas, J.M.; Reddi, A.H. Articular cartilage: Structure and regeneration. Tissue Eng. Part B: Rev. 2010, 16, 617–627. [Google Scholar] [CrossRef]
- Wu, D.-Q.; Wang, T.; Lu, B.; Xu, X.-D.; Cheng, S.-X.; Jiang, X.-J.; Zhang, X.-Z.; Zhuo, R.-X. Fabrication of supramolecular hydrogels for drug delivery and stem cell encapsulation. Langmuir 2008, 24, 10306–10312. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef]
- Skardal, A.; Atala, A. Biomaterials for integration with 3-D bioprinting. Ann. Biomed. Eng. 2015, 43, 730–746. [Google Scholar] [CrossRef]
- You, F.; Eames, B.F.; Chen, X. Application of extrusion-based hydrogel bioprinting for cartilage tissue engineering. Int. J. Mol. Sci. 2017, 18, 1597. [Google Scholar] [CrossRef]
- You, F.; Wu, X.; Chen, X. 3D printing of porous alginate/gelatin hydrogel scaffolds and their mechanical property characterization. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 299–306. [Google Scholar] [CrossRef]
- Yazdanpanah, Z.; Johnston, J.D.; Cooper, D.M.; Chen, X. 3D bioprinted scaffolds for bone tissue engineering: State-of-the-art and emerging technologies. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; Sharma, N.; Chen, X. 3D biofabrication of vascular networks for tissue regeneration: A report on recent advances. J. Pharm. Anal. 2018, 8, 277–296. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Chen, X. A brief review of extrusion-based tissue scaffold bio-printing. Biotechnol. J. 2017, 12, 1600671. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Chen, X. Printability—A key issue in extrusion-based bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Sadeghianmaryan, A.; Naghieh, S.; Sardroud, H.A.; Yazdanpanah, Z.; Soltani, Y.A.; Sernaglia, J.; Chen, X. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020, 164, 3179–3192. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Ren, Y.; Qiang, L.; Liu, Y.; Wang, J.; Dai, K. 3D printed hydrogel for articular cartilage regeneration. Compos. Part B Eng. 2022, 109863. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D bioprinting of hydrogels for cartilage tissue engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef]
- Koo, Y.; Choi, E.-J.; Lee, J.; Kim, H.-J.; Kim, G.; Do, S.H. 3D printed cell-laden collagen and hybrid scaffolds for in vivo articular cartilage tissue regeneration. J. Ind. Eng. Chem. 2018, 66, 343–355. [Google Scholar] [CrossRef]
- Alizadeh Sardroud, H.; Chen, X.; Eames, B.F. Applied Compressive Strain Governs Hyaline-like Cartilage versus Fibrocartilage-like ECM Produced within Hydrogel Constructs. Int. J. Mol. Sci. 2023, 24, 7410. [Google Scholar] [CrossRef]
- Xu, T.; Binder, K.W.; Albanna, M.Z.; Dice, D.; Zhao, W.; Yoo, J.J.; Atala, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2012, 5, 015001. [Google Scholar] [CrossRef]
- Olubamiji, A.D.; Izadifar, Z.; Si, J.L.; Cooper, D.M.; Eames, B.F.; Chen, D.X. Modulating mechanical behaviour of 3D-printed cartilage-mimetic PCL scaffolds: Influence of molecular weight and pore geometry. Biofabrication 2016, 8, 025020. [Google Scholar] [CrossRef]
- Bothe, F.; Deubel, A.-K.; Hesse, E.; Lotz, B.; Groll, J.; Werner, C.; Richter, W.; Hagmann, S. Treatment of focal cartilage defects in minipigs with zonal chondrocyte/mesenchymal progenitor cell constructs. Int. J. Mol. Sci. 2019, 20, 653. [Google Scholar] [CrossRef]
- Urtaza, U.; Guaresti, O.; Gorroñogoitia, I.; Zubiarrain-Laserna, A.; Muiños-López, E.; Granero-Moltó, F.; de Espinosa, J.L.; López-Martinez, T.; Mazo, M.; Prósper, F. 3D printed bioresorbable scaffolds for articular cartilage tissue engineering: A comparative study between neat polycaprolactone (PCL) and poly (lactide-b-ethylene glycol)(PLA-PEG) block copolymer. Biomed. Mater. 2022, 17, 045028. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Cheng, P.; Sang, S.; Xiang, C.; An, Y.; Wei, X.; Yan, Y.; Li, P. 3D printed PCL/GelMA biphasic scaffold boosts cartilage regeneration using co-culture of mesenchymal stem cells and chondrocytes: In vivo study. Mater. Des. 2021, 210, 110065. [Google Scholar] [CrossRef]
- Shim, J.-H.; Kim, J.Y.; Park, M.; Park, J.; Cho, D.-W. Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication 2011, 3, 034102. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Melchels, F.P.; Jeon, J.E.; Van Bussel, E.M.; Kimpton, L.S.; Byrne, H.M.; Dhert, W.J.; Dalton, P.D.; Hutmacher, D.W.; Malda, J. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat. Commun. 2015, 6, 6933. [Google Scholar] [CrossRef]
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143. [Google Scholar] [CrossRef]
- Pang, L.; Sun, P.; Dong, X.; Tang, T.; Chen, Y.; Liu, Q.; Qi, M. Shear viscoelasticity of electrospinning PCL nanofibers reinforced alginate hydrogels. Mater. Res. Express 2021, 8, 055402. [Google Scholar] [CrossRef]
- Lee, H.-p.; Gu, L.; Mooney, D.J.; Levenston, M.E.; Chaudhuri, O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017, 16, 1243–1251. [Google Scholar] [CrossRef]
- Castilho, M.; Mouser, V.; Chen, M.; Malda, J.; Ito, K. Bi-layered micro-fibre reinforced hydrogels for articular cartilage regeneration. Acta Biomater. 2019, 95, 297–306. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Z.; He, D.; Li, H. Modulating degradation of sodium alginate/bioglass hydrogel for improving tissue infiltration and promoting wound healing. Bioact. Mater. 2021, 6, 3692–3704. [Google Scholar] [CrossRef]
- Meinert, C.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. A novel bioreactor system for biaxial mechanical loading enhances the properties of tissue-engineered human cartilage. Sci. Rep. 2017, 7, 16997. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, K.; Williams, R.J., III; Warren, R.F.; Potter, H.G.; Spock, C.R.; Jones, E.C.; Wickiewicz, T.L.; Marx, R.G. The microfracture technique for the treatment of articular cartilage lesions in the knee: A prospective cohort study. JBJS 2005, 87, 1911–1920. [Google Scholar] [CrossRef]
- Mitchell, N.; Shepard, N. The resurfacing of adult rabbit articular cartilage by multiple perforations through the subchondral bone. JBJS 1976, 58, 230–233. [Google Scholar] [CrossRef]
- Pridie, K. A method of resurfacing osteoarthritic knee joint. J. Bone J. Surg. Br. 1959, 41, 618–619. [Google Scholar]
- Schagemann, J.C.; Erggelet, C.; Chung, H.-W.; Lahm, A.; Kurz, H.; Mrosek, E.H. Cell-laden and cell-free biopolymer hydrogel for the treatment of osteochondral defects in a sheep model. Tissue Eng. Part A 2009, 15, 75–82. [Google Scholar] [CrossRef]
- Lee, C.R.; Grad, S.; Gorna, K.; Gogolewski, S.; Goessl, A.; Alini, M. Fibrin–polyurethane composites for articular cartilage tissue engineering: A preliminary analysis. Tissue Eng. 2005, 11, 1562–1573. [Google Scholar] [CrossRef]
- Marquass, B.; Schulz, R.; Hepp, P.; Zscharnack, M.; Aigner, T.; Schmidt, S.; Stein, F.; Richter, R.; Osterhoff, G.; Aust, G. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: In vivo results of cartilage repair after 1 year. Am. J. Sport. Med. 2011, 39, 1401–1412. [Google Scholar] [CrossRef]
- Alizadeh Sardroud, H.; Wanlin, T.; Chen, X.; Eames, B.F. Cartilage tissue engineering approaches need to assess fibrocartilage when hydrogel constructs are mechanically loaded. Front. Bioeng. Biotechnol. 2022, 9, 1388. [Google Scholar] [CrossRef]
- Vaca-González, J.J.; Guevara, J.M.; Moncayo, M.A.; Castro-Abril, H.; Hata, Y.; Garzón-Alvarado, D.A. Biophysical stimuli: A review of electrical and mechanical stimulation in hyaline cartilage. Cartilage 2019, 10, 157–172. [Google Scholar] [CrossRef]
- Kelly, T.-A.; Ng, K.W.; Ateshian, G.A.; Hung, C.T. Analysis of radial variations in material properties and matrix composition of chondrocyte-seeded agarose hydrogel constructs. Osteoarthr. Cartil. 2009, 17, 73–82. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, Y. ATDC5: An excellent in vitro model cell line for skeletal development. J. Cell. Biochem. 2013, 114, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Olubamiji, A.D.; Izadifar, Z.; Zhu, N.; Chang, T.; Chen, X.; Eames, B.F. Using synchrotron radiation inline phase-contrast imaging computed tomography to visualize three-dimensional printed hybrid constructs for cartilage tissue engineering. J. Synchrotron Radiat. 2016, 23, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.G.; Widmyer, M.R.; Utturkar, G.M.; Spritzer, C.E.; Garrett, W.E., Jr.; DeFrate, L.E. In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am. J. Sport. Med. 2015, 43, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Little, C.J.; Kulyk, W.M.; Chen, X. The effect of chondroitin sulphate and hyaluronic acid on chondrocytes cultured within a fibrin-alginate hydrogel. J. Funct. Biomater. 2014, 5, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C 2018, 83, 195–201. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef]
- Park, K.H.; Na, K. Effect of growth factors on chondrogenic differentiation of rabbit mesenchymal cells embedded in injectable hydrogels. J. Biosci. Bioeng. 2008, 106, 74–79. [Google Scholar] [CrossRef]
- Bitto, A.; Minutoli, L.; Altavilla, D.; Polito, F.; Fiumara, T.; Marini, H.; Galeano, M.; Calò, M.; Cascio, P.L.; Bonaiuto, M. Simvastatin enhances VEGF production and ameliorates impaired wound healing in experimental diabetes. Pharmacol. Res. 2008, 57, 159–169. [Google Scholar] [CrossRef]
- Fan, C.; Liao, L.; Zhang, C.; Liu, L. A tough double network hydrogel for cartilage tissue engineering. J. Mater. Chem. B 2013, 1, 4251–4258. [Google Scholar] [CrossRef]
- Chuang, E.-Y.; Chiang, C.-W.; Wong, P.-C.; Chen, C.-H. Hydrogels for the application of articular cartilage tissue engineering: A review of hydrogels. Adv. Mater. Sci. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Kisiday, J.D.; Jin, M.; DiMicco, M.A.; Kurz, B.; Grodzinsky, A.J. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J. Biomech. 2004, 37, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Chow, H.H.; Lai, J.Y.; Liu, H.L.; Tsai, W.B. Dynamic compression modulates chondrocyte proliferation and matrix biosynthesis in chitosan/gelatin scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Grodzinsky, A.; Spector, M. Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2003, 64, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Noguchi, T.; Frean, S.P.; Lees, P.; Bader, D.L. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology 2000, 37, 149–161. [Google Scholar]
- Terraciano, V.; Hwang, N.; Moroni, L.; Park, H.B.; Zhang, Z.; Mizrahi, J.; Seliktar, D.; Elisseeff, J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 2007, 25, 2730–2738. [Google Scholar] [CrossRef]
- Anderson, D.E.; Johnstone, B. Dynamic mechanical compression of chondrocytes for tissue engineering: A critical review. Front. Bioeng. Biotechnol. 2017, 5, 76. [Google Scholar] [CrossRef]
- Jeon, J.E.; Schrobback, K.; Hutmacher, D.W.; Klein, T.J. Dynamic compression improves biosynthesis of human zonal chondrocytes from osteoarthritis patients. Osteoarthr. Cartil. 2012, 20, 906–915. [Google Scholar] [CrossRef]
- Nicodemus, G.; Bryant, S. Mechanical loading regimes affect the anabolic and catabolic activities by chondrocytes encapsulated in PEG hydrogels. Osteoarthr. Cartil. 2010, 18, 126–137. [Google Scholar] [CrossRef]
- Nebelung, S.; Gavenis, K.; Lüring, C.; Zhou, B.; Mueller-Rath, R.; Stoffel, M.; Tingart, M.; Rath, B. Simultaneous anabolic and catabolic responses of human chondrocytes seeded in collagen hydrogels to long-term continuous dynamic compression. Ann. Anat.-Anat. Anz. 2012, 194, 351–358. [Google Scholar] [CrossRef]
- Hunter, C.J.; Imler, S.M.; Malaviya, P.; Nerem, R.M.; Levenston, M.E. Mechanical compression alters gene expression and extracellular matrix synthesis by chondrocytes cultured in collagen I gels. Biomaterials 2002, 23, 1249–1259. [Google Scholar] [CrossRef]
- Nicodemus, G.; Bryant, S.J. The role of hydrogel structure and dynamic loading on chondrocyte gene expression and matrix formation. J. Biomech. 2008, 41, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Camarero-Espinosa, S.; Rothen-Rutishauser, B.; Foster, E.J.; Weder, C. Articular cartilage: From formation to tissue engineering. Biomater. Sci. 2016, 4, 734–767. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Lin, W.; Cai, H.; Chen, Y.; Man, Y.; Liang, J.; Wang, Q.; Sun, Y.; Fan, Y.; Zhang, X. Dynamic mechanical loading facilitated chondrogenic differentiation of rabbit BMSCs in collagen scaffolds. Regen. Biomater. 2019, 6, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Antunes, B.P.; Vainieri, M.L.; Alini, M.; Monsonego-Ornan, E.; Grad, S.; Yayon, A. Enhanced chondrogenic phenotype of primary bovine articular chondrocytes in Fibrin-Hyaluronan hydrogel by multi-axial mechanical loading and FGF18. Acta Biomater. 2020, 105, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Vickers, S.M.; Squitieri, L.S.; Spector, M. Effects of cross-linking type II collagen-GAG scaffolds on chondrogenesis in vitro: Dynamic pore reduction promotes cartilage formation. Tissue Eng. 2006, 12, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lim, T.C.; Kurisawa, M.; Spector, M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 2012, 33, 3835–3845. [Google Scholar] [CrossRef]
- Arora, A.; Kothari, A.; Katti, D.S. Pericellular plasma clot negates the influence of scaffold stiffness on chondrogenic differentiation. Acta Biomater. 2016, 46, 68–78. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh Sardroud, H.; Chen, X.; Eames, B.F. Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression. J. Funct. Biomater. 2023, 14, 313. https://doi.org/10.3390/jfb14060313
Alizadeh Sardroud H, Chen X, Eames BF. Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression. Journal of Functional Biomaterials. 2023; 14(6):313. https://doi.org/10.3390/jfb14060313
Chicago/Turabian StyleAlizadeh Sardroud, Hamed, Xiongbiao Chen, and B. Frank Eames. 2023. "Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression" Journal of Functional Biomaterials 14, no. 6: 313. https://doi.org/10.3390/jfb14060313
APA StyleAlizadeh Sardroud, H., Chen, X., & Eames, B. F. (2023). Reinforcement of Hydrogels with a 3D-Printed Polycaprolactone (PCL) Structure Enhances Cell Numbers and Cartilage ECM Production under Compression. Journal of Functional Biomaterials, 14(6), 313. https://doi.org/10.3390/jfb14060313








