Abstract
Bioactive nanomaterials are increasingly being applied in oral health research. Specifically, they have shown great potential for periodontal tissue regeneration and have substantially improved oral health in translational and clinical applications. However, their limitations and side effects still need to be explored and elucidated. This article aims to review the recent advancements in nanomaterials applied for periodontal tissue regeneration and to discuss future research directions in this field, especially focusing on research using nanomaterials to improve oral health. The biomimetic and physiochemical properties of nanomaterials such as metals and polymer composites are described in detail, including their effects on the regeneration of alveolar bone, periodontal ligament, cementum and gingiva. Finally, the biomedical safety issues of their application as regenerative materials are updated, with a discussion about their complications and future perspectives. Although the applications of bioactive nanomaterials in the oral cavity are still at an initial stage, and pose numerous challenges, recent research suggests that they are a promising alternative in periodontal tissue regeneration.
1. Introduction
The oral cavity plays a crucial role in essential body functions such as mastication, speech or deglutition, and its influence on facial aesthetics, especially of teeth, is undeniable. Periodontal tissue, including the alveolar bone, periodontal ligament (PDL), cementum and gingiva, is essential to maintain the integrity and stability of the teeth, absorb the chewing forces, and protect against bacterial invasion and infection. There are a variety of factors causing periodontal tissue defects and threatening the patients’ quality of life, such as caries, periodontitis, tumors, cysts and trauma [1]. Due to the complex functions of the oral tissue and the unique characteristics of the oral environment, it has always been a great challenge to reconstruct the periodontal tissue and restore its physical function.
For periodontal tissue reconstruction, traditional techniques based on allogenic grafts replacing the missing or damaged tissue from living donors or even cadavers are still used in dental and other medical fields [2]. For example, autologous and allogenic alveolar bone grafts are currently considered a gold standard to overcome bone atrophy, although in clinical settings the best results seem to be obtained with autologous bone [3]. However, these methods encounter limitations such as limited supply of graft tissue, donor site morbidity, graft failure, immunological rejection and lengthy hospitalization periods [4]. In addition, these techniques exhibit great difficulty in regenerating the cementum–ligament–bone complex. Furthermore, traditional dental materials such as composites and cement in macro and micro sizes are also widely applied in clinics. Despite their low cost, easy application and good biocompatibility, these materials also present several complications, such as degradation [5], cure shrinkage [6], stress fatigue [7], marginal microleakage [8] and high susceptibility to microbial adhesion. Thus, there is a critical need for alternative techniques and materials.
In this respect, nanotechnology can provide an innovative alternative. Nanomaterials, in the range of 1–100 nanometers, have gained significant attention in regeneration medicine due to their unique optical, mechanical, magnetic, electronic and catalytic properties [9], which explain their high biocompatibility, permeability, tunability and immune evasion capability. Hence, they exhibit tremendous potential in tissue engineering [10], as anti-bacterial agents [11], for drug delivery [12], tissue repair [13] and functional imaging (such as MRI and CT) [14]. Recently, various types of nanomaterials, such as nanoparticles, nanocapsules, nanocomposites, nanofibers, nanotubes and nanosheets, have achieved satisfying outcomes and could therefore be used to reconstruct structures and restore the functions of oral tissues [15].
However, nanotechnology and nanomaterials also face great challenges that prevent them from advancing from the bench to the clinic. Firstly, there is a shortage of established protocols that allow their construction with the desired composition, structure parameters and physiochemical properties. It is difficult to modify or improve the behavior or performance of nanosystems in vivo because of the limited development of their surface chemistry [16]. Second, the biosafety issues and adverse events of nanomaterials remain concerning. For instance, in vivo application of nanomaterials could induce immunologic reactions [17]. Moreover, nanomaterials have shown high permeability [18], and therefore their cytotoxicity can significantly increase as they can penetrate through physiological barriers and may accumulate in nontarget tissues [19]. Third, it is very difficult to control their biodegradation. Their biodistribution and pharmacokinetics largely depend on the size, shape and surface chemistry and homogeneous production of nanomaterials is still a challenge [20]. Moreover, when nanomaterials are applied for the local delivery of drugs, it is essential to control their biodegradation rate [21]. Although many papers emphasize their promising perspective in tissue regeneration, these challenges are rarely discussed.
This review focuses on the most recent publications regarding periodontal tissue regeneration with nanotechnology and nanomaterials (Figure 1). First, the current designs, structures and functions of nanomaterials are introduced and discussed. Secondly, the related factors that may interact with the behaviors and bioactivities of nanomaterials are summarized and elucidated. Lastly, the potential complications of nanomaterials are presented and some remarks for future research are proposed in order to overcome the current limitations.
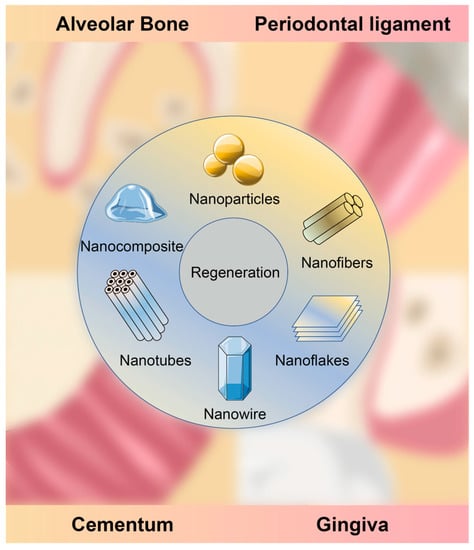
Figure 1.
Periodontal tissue regeneration with nanomaterials.
2. Materials and Methods
2.1. Literature Search Strategy
An electronic literature search was conducted on PubMed using the following combination of search terms and Boolean operators: “((alveolar bone regeneration) OR (cementum regeneration) OR (gingiva regeneration) OR (periodontal ligament regeneration)) AND (nanomaterials OR nanotechnology) NOT review.” to identify articles published in the last 5 years (between January 2017 and February 2023). No filters were activated and similar additional searches were performed on Embase and Cochrane Library.
2.2. Literature Screening and Selection Criteria
Literature screening and selection were carried out by one reviewer. The titles and abstracts of the publications identified by the different databases were screened and the reference lists of critical articles were additionally and hand search for relevant articles. The full text was examined at the second stage to determine whether it matched the selection criteria.
The inclusion criteria were as follows:
- (1)
- The reports on nanomaterials were not older than 5 years (published before 2017).
- (2)
- The nanomaterials should be proposed to promote periodontal tissue regeneration, including alveolar bone, PDL, cementum and gingiva.
- (3)
- The literature should involve research in not only physiochemistry but also biomedicine, for which the nanomaterials were tested in cells and/or animal models.
The exclusion criteria were as follows:
- (1)
- The materials used for periodontal tissue regeneration are not nanomaterials.
- (2)
- The nanomaterials were not designed for periodontal tissue regeneration but rather for osseointegration of dental implants.
- (3)
- Case reports with a sample size smaller than three subjects.
2.3. Data Management
The following data were extracted from the included articles in an Excel Table: application, nanomaterials, morphology, in vitro experiments, in vivo experiments, outcomes and references. Articles were sorted according to the type of tissue they intended to regenerate (alveolar bone, PDL, cementum, gingiva).
3. Results
Literature Search
A total of 276 literature studies were acquired from the three databases, from which 99 were selected and read as full text after the screening. Forty-one studies met the eligibility criteria and were included in this study, as shown in Figure 2.
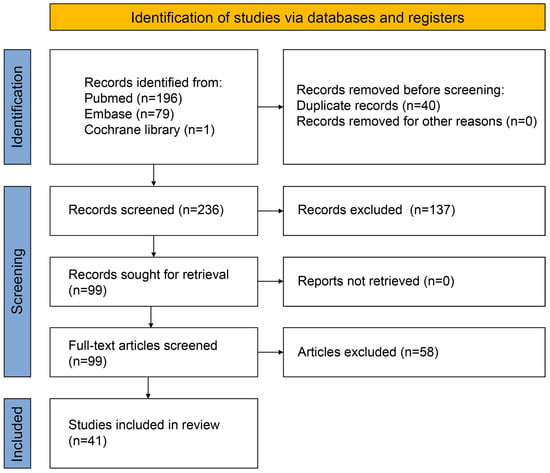
Figure 2.
Flowchart of the retrieved and selected literature.
4. Discussion
4.1. Alveolar Bone Regeneration
The alveolar bone is a specific type of bone tissue that forms the sockets where the teeth are supported and anchored to the jaws. The alveolar bone is constantly remodeling itself in response to various factors, such as chewing forces and hormonal changes. Under physiological conditions, this process involves the breakdown and replacement of old bone tissue by new bone tissue, which helps to maintain the integrity and strength of the bone. However, under pathological conditions, the breakdown of alveolar bone is spontaneously irreversible and can lead to tooth mobility and loss [22]. Most of the studies involving periodontal tissue regeneration focus on alveolar bone regeneration. Various techniques have been proposed to guide or control bone regeneration, such as bone grafting [23], guided tissue regeneration (GTR) [24] and platelet-rich plasma (PRP) therapy [25]. Although effective to a certain level, these techniques lack in repeatability and do not completely reconstruct the original periodontium [26,27]. Recently, the application of nanomaterials smaller than 100 nm on the above techniques has shown promising results, since they have multiple advantages over traditional materials, such as versatility, biocompatibility, enhanced cellular interactions, improved tissue integration, controlled drug release and mechanical strength [28,29,30].
Among the included studies, the nanomaterials applied in alveolar bone regeneration are listed in Table 1. Nano-hydroxyapatite (nHA) [31,32,33,34,35,36,37,38,39] and collagen [31,39,40,41,42,43] are the most commonly investigated biomaterials for alveolar bone regeneration. Hydroxyapatite is a naturally occurring mineral that is the main component of bone tissue, and nHA has a higher surface area-to-volume ratio compared to conventional hydroxyapatite, making it more suitable as a substitute for bone graft [44]. Collagen is a crucial component of the extracellular matrix in many tissues, including alveolar bone. It provides structural support, enables cell–biomaterial interaction and increases cell adhesion, which helps regulate cell behavior, making it an attractive biomaterial for alveolar bone regeneration [45]. Both nHA and collagen have excellent osteoconductive properties, which can support the formation of new bone tissue by serving as a scaffold for bone growth, with good biocompatibility and mechanical properties.
Poly(lactic-co-glycolic acid) (PLGA) [41,42,46,47,48,49] and polycaprolactone (PCL) [38,46,50,51,52] are biodegradable polymers that have been widely studied as materials for nanoparticles but also in other applications in tissue engineering and regenerative medicine. Both PLGA and PCL degrade over time into natural compounds that can be metabolized and eliminated through normal metabolic pathways. This facilitates their application in bone grafting, guided tissue regeneration and especially drug delivery. When engineered to release therapeutic agents, such as growth factors or antibiotics, they can provide sustained long-term therapeutic effects that can promote bone regeneration and prevent inflammation [53]. However, PLGA and PCL also present shortcomings. For example, the mechanical strength of PLGA alone is inadequate for bone tissue regeneration, so PLGA often needs to be incorporated with other ceramic nanoparticles such as nHA, PCL and flourhydroxyapatite [54]. Moreover, although PCL displays adequate cell adhesion and good mechanical strength, its slow biodegradation, which takes more than 2 years, greatly exceeds the time required for new tissue formation [51], which can negatively impact the bone tissue regeneration process [55].
Gold nanoparticles (AuNPs) have attracted much attention as multifunctional contrast agents for computerized tomography (CT) due to their chemical inertness, versatile surface functionalization and biocompatibility, high radiopacity and low cytotoxicity [56,57]. In addition, in vivo cell labeling and tracking using AuNPs with CT have become a cost-effective and time-efficient approach [58]. Among the included studies, AuNPs have also been shown to have high potential in alveolar bone regeneration [13,59,60]. Besides the advantages mentioned above, AuNPs can promote the proliferation and differentiation of bone-forming cells, such as osteoblasts and mesenchymal stem cells [61,62]. Furthermore, AuNCs exhibit antibacterial properties, which can be useful in preventing infection during the bone regeneration process [63]. Finally, their property of X-ray attenuation can be useful to distinguish the transplant from the host tissue, monitor the regeneration process and evaluate the efficacy of the treatment.
Bone morphogenic protein 2 (BMP-2) is one of the most popular drugs loaded in nanomaterials for alveolar bone regeneration [42,50,64,65]. BMP-2, a member of the transforming growth factor-β superfamily, has been shown to successfully promote alveolar bone regeneration by enhancing vertical bone height in animal studies [66,67]. However, it is important to note that BMP-2 is a potent growth factor and should be used with caution since it can lead to complications such as excessive bone growth, inflammation, fatty tissue formation and deteriorated bone quality [68].
The number of studies on alveolar bone regeneration greatly exceeds those focused on PDL, cementum or gingiva regeneration. However, it should be noted that periodontal tissue defects are usually accompanied by an inflammatory microenvironment, so alveolar bone regeneration has higher requirements regarding anti-inflammatory and immune regulation, which differs from regeneration of other bony structures [69]. This might be one of the most important limitations of studies applying an animal model of surgically inflicted bone defects since the surgical insult induces acute inflammation, in contrast to, i.e., a ligature-induced periodontitis model, which causes the chronic inflammation characteristic of human periodontitis [70]. The effect of nanomaterials in alveolar bone regeneration should be tested under particular conditions similar to clinical conditions to facilitate their translational application from bench to bedside.

Table 1.
Nanomaterials used for alveolar bone regeneration.
Table 1.
Nanomaterials used for alveolar bone regeneration.
| Application | Nanomaterials | Morphology | In Vitro Experiments | In Vivo Experiments | Outcomes | References |
|---|---|---|---|---|---|---|
| Alveolar bone regeneration | SP600125, bone morphogenic protein 2 (BMP-2) | nanofiber | Beagle PDLCs and bone marrow stem cells (BMSCs) | Beagle model of mandible class II furcation defect | suppress the expression of pro-inflammatory factors and recover bone defects covering the periodontitis site within 2 month | Liu et al., 2020 [64] |
| Electrospun fish collagen/bioactive glass/chitosan (Col/BG/CS) | nanofiber | hPDLCs | Beagle model of mandibular furcation defect | enhance the cell viability and osteogenic gene expression, increase the expression of RUNX-2 and OPN protein, and promote bone regeneration | Zhou et al., 2017 [40] | |
| Ferroelectric BaTiO3/poly(vinylidene fluoride-trifluoroethylene) | nanocomposite | N/A | Mini-pig model of bone defect | prevent the vertical and horizontal dimension resorption of the alveolar ridge, promote buccal alveolar bone regeneration and maturation | Li et al., 2023 [71] | |
| Nano β-tricalcium phosphate/chitosan /glycerophosphate/glyoxal hydrogel | nanoparticle | Wish normal cells, hepatocellular carcinoma and breast cancer cell lines | Mongrel dog model of mandible bone defects | promote new bone in infected teeth | Abdel-Fattah et al., 2017 [72] | |
| Poly(L-lactide-co-D,L-lactide) encapsulating platelet-derived growth factor or metronidazole | nanofiber | MSCs line | Murine model of dentoalveolar defect | show high biocompatibility, facilitate wound healing and enhance alveolar ridge regeneration | Ho et al., 2017 [73] | |
| Lysophosphatidic acid, zinc oxide, poly(lactic-co-glycolic acid) (PLGA)/PCL and deferoxamine | nanofiber | Murine calvarial osteoblast cell line (MC3T3-E1) and Human umbilical vein endothelial cells | Murine model of maxillary alveolar bone defect, rat model of mandibular fenestration | exhibit remarkable biocompatibility and osteogenesis, antibacterial activity, neovascularization and new bone formation | Xing et al., 2022 [46] | |
| PCL biomembranes, BMP-2 and ibuprofen | nanoreservoir | hBMSCs | Murine model of maxillary alveolar bone lesion | regenerate maxillary bone for periods between 90 and 150 days after implantation | Stutz et al., 2020 [50] | |
| Autologous BMMNCs loaded collagen sponge with nano-HA and autologous platelet-rich fibrin | nanoparticle | N/A | Patients with unilateral alveolar cleft defects | exhibit less complications and better tissue healing. 90% of the cases exhibit complete alveolar bone union | Al-Ahmady et al., 2018 [31] | |
| silica coated nanoHA-gelatin reinforced with electrospun poly(L-lactic acid) fibres | nanofiber | N/A | Rabbit model of critical alveolar defects | promote bone formation in load bearing mandibular region | Manju et al., 2018 [32] | |
| Metformin hydrochloride, citric acid | carbon dots | Rat BMSCs | Rat model of ligature-induced periodontitis | promote BMSCs osteogenesis under normal and inflammatory conditions, and regenerate the lost alveolar bone in rats | Ren et al., 2021 [74] | |
| Gold nanoparticles, adenovirus-mediated human β-defensin 3 gene | nanoparticle | hPDLCs | Rat model of ligature-induced periodontitis | promote hPDLCs osteogenic differentiation and periodontal regeneration, via the p38 MAPK pathway | Li et al., 2021 [59] | |
| CaCO3/miR-200c | nanoparticle | Human embryonic palate mesenchymal cells | Rat model of alveolar defects | increase bone formation in rat alveolar bone defects | Remy et al., 2022 [75] | |
| CaP@P-fiber | nanofiber | Rat BMSCs and murine-derived macrophage cell line | Rat model of artificial alveolar bone defect | enhance the osteo-immunomodulatory and osteo-inductive functions, result in an excellent bone repair | He et al., 2022 [76] | |
| Polydopamine structure, bone-forming peptide-1, ascular endothelial growth factor-mimicking peptide | nanocomposite | hPDLSCs | Rat model of cranial defect | boost the proliferation and osteogenic differentiation of PDLSCs with improved cytocompatibility, regenerate of periodontal bone dramatically | Xiang et al., 2020 [77] | |
| PLGA-collagen-gelatin | nanofiber | Murine fibroblasts | Rat model of critical maxillary alveolar bone defect | reveal ~3 times greater new bone volume and bone mineral density compared to the unfilled control defects over 4 weeks | Boda et al., 2019 [41] | |
| gelatin/nano-HA/metformin | nanocomposite | Human MSCs | Rat model of critical maxillary alveolar bone defect | show bone regenerations with greater alveolar ridge preservation, and bone formation with less connective tissue and residual scaffold | Fang et al., 2022 [33] | |
| Alendronate, heptaglutamate conjugated BMP-2 mimicking peptide, PLGA-collagen-gelatin | nanofiber | N/A | Rat model of critical maxillary alveolar bone defect | elevate new bone volume fraction and bone mineral density | Boda et al., 2020 [42] | |
| Tetrahedral framework nucleic acids | nanoparticle | hPDLSCs | Rat model of ligature-induced periodontitis | promote osteogenic differentiation, reduce bone absorption by decreasing inflammatory infiltration and inhibiting osteoclast formation | Zhou et al., 2021 [78] | |
| Zeolitic imidazolate framework-8 nanoparticle loaded hydrophilic PVP, FK506 loaded PCL | nanofiber | Rat BMSCs | Rat model of ligature-induced periodontitis | exert antibacterial function benefiting the microenvironment for the osteogenesis process | Sun et al., 2022 [51] | |
| Gelatin/nano-HA microsphere embedded with stromal cell-derived factor-1 | nanoparticle | Human MSCs | Rat model of mandible alveolar bone defect | enhance the alveolar bone regeneration | Fang et al., 2019 [34] | |
| Superparamagnetic iron oxide nanoparticles loaded gelatin sponge | nanoparticle | N/A | Rat model of mandible incisor sockets | obtain good visibility on MRI and enhanced bone regeneration without using an external magnetic field. | Hu et al., 2018 [79] | |
| HA nanowires modified polylactic acid membrane | nanowire | N/A | Rat model of mansible bone defect | promote the expression of multiple bone-related markers and form more bones with higher quality | Han et al., 2018 [35] | |
| Alginate encapsulated minocycline-loaded nanocrystalline carbonated HA | nanoparticle | Murine femur osteoblast cells | Rat model of maxillary alveolar bone defect | inhibit the growth of Enterococcus faecalis with cyto-compatibility and osteo-conduction properties | Calasans-Maia et al., 2019 [36] | |
| Nanostructured carbonated HA/sodium alginate containing strontium microspheres | nanoparticle | N/A | Rat model of maxillary alveolar bone defect | form new bone | Carmo et al., 2018 [80] | |
| Enamel matrix derivatives-loaded chitosan nanospheres embedded poly(D,L-lactic acid)-doxycycline | nanofiber | Murine osteoblasts | Rat model of maxillary alveolar ridge defects | accelerate wound healing and facilitate osteogenesis | Ho et al., 2022 [81] | |
| MXene (Ti3 C2 Tx), | nanoflake | hPDLCs | Rat model of maxillary periodontal fenestration defects | exhibit biocompatibility and induce osteogenic differentiation, form new bone and inhibit osteoclast with enhanced expression of β-catenin, RUNX2, HIF-1α | Cui et al., 2021 [82] | |
| BMP-2 plasmid DNA-loaded chitosan nanoparticles | nanoparticle | N/A | Rat model of muscle pouches and calvarial defects and beagle model of ligature-induced periodontitis | increase new bone formation in rat calvarial defects and enhance bone healing in beagle dog, with non-specific inflammation | Li et al., 2017 [65] | |
| Cerium oxide nanoparticles | nanofiber | hPDLSCs | Rat model of periodontal bone defects | promote hPDLSCs osteogenic differentiation and accelerate new bone formation | Ren et al., 2021 [83] | |
| Chitosan, nano-HA and amnion membrane | nanofiber | N/A | Patient with gingival recession defects | enhance bone growth while prevent the gingival tissue downgrowth | Dhawan et al., 2021 [37] | |
| PLGA nanoparticles, CS nanoparticles and silver nanoparticles | nanoparticle | hPDLCs | Rabbit model of mandible bone defects | have an optimal proportion, show no cytotoxicity and contribute to cell mineralization. | Xue et al., 2019 [49] |
4.2. PDL Regeneration
The nanomaterials applied in PDL, cementum and periodontal complex regeneration are listed in Table 2. Three of the studies included in this review are for PDL and alveolar bone regeneration [38,70,84]. It is important to remark that they all shared several common limitations: firstly, the study design lacked GTR or gold-standard bone grafts as controls. Secondly, they all used an animal model of experimental bone defect, which could neither simulate a chronic inflammatory microenvironment nor reflect the regenerative effect of the nanomaterials in complex periodontal defects. Thirdly, the underlying mechanisms of PDL and bone regeneration were not fully investigated. Moreover, Zhang et al. [84] stated that large animals should be used for more robust experiments and a variety of AuNPs should be constructed for further investigations. El-Sayed et al. [70] achieved a significant increase in functional PDL length but failed to increase the alveolar bone height, which might attribute to the deficiencies of material properties coupled with the possible peptide degradation in situ. The persistence of the peptides in the wound site was possibly too short, but the degradation profile of the peptide was not examined over time.
The study of Li et al. [85] was excluded from this review as it did not report new-formed PDL or alveolar bone after the application of nanoparticles. However, it is worth to mention that it describes periodontal tissue healing and reduced root resorption after tooth autotransplantation by applying nuclear factor-κB -PLGA nanospheres. PDL and alveolar bone regeneration were not directly detected in this study but were implied by a decrease in the expression of inflammatory markers and an increase in growth factors in PDL.
PDL is a specialized connective tissue surrounding and attaching teeth to the alveolar bone. There are several specific challenges in PDL regeneration. Firstly, the space that the PDL occupies, which spans approximately 150–400 μm from the alveolar bone to the cementum, is extremely small and thus limits the possibilities of applying medication or large transplants [86]. Although nanomaterials may present advantages in this point, it is still very difficult to regenerate soft tissue between two mineralized surfaces and specifically anchor it to them, while respecting the PDL space [87]. Notably, the PDL periodically undergoes various combinations of mechanical loading (i.e., compression, stretch, fluid-induced shear stress), contributing to maintaining the homeostasis [88]. Natural PDL fibers have a self-repair mechanism to preserve their integrity. They are aligned according to the magnitude and direction of loading, which increases their mechanical strength in this direction [89]. Tissue engineering technology combining biomimetic scaffolds and stem cells aims to mimic the properties of natural PDL and have opened a range of new therapeutic strategies in periodontal regeneration. However, there is a great challenge in the functional alignment of engineered nanofibers.
Three-dimensional bioprinting techniques have been widely used in craniofacial tissue engineering [90] and three-dimensional-printed scaffolds can aid complex periodontal reconstruction [91]. Nevertheless, such techniques only control the external properties of the scaffolds and not their internal architecture. The distribution of the seeded cells has also been reported to be heterogeneous [92]. Electrospinning has also been suggested as an effective approach to regenerate PDL [93], as it can improve the orientation of collagen fibers and reconstruct directional fiber bundles [94]. Combining 3D bioprinting techniques and electrospinning may overcome the shortcomings described above and produce effective biomimetic nanomaterials for PDL regeneration.

Table 2.
Nanomaterials used for PDL, cementum and periodontal complex regeneration.
Table 2.
Nanomaterials used for PDL, cementum and periodontal complex regeneration.
| Application | Nanomaterials | Morphology | In Vitro Experiments | In Vivo Experiments | Outcomes | References |
|---|---|---|---|---|---|---|
| Alveolar bone and PDL regeneration | Polycaprolactone (PCL), cross-linked alginate, nano-HA (HA) | nanofiber | Dog adipose-derived MSCs | Dog model of class II furcation defects | yield periodontal wound healing, type I collagen of newly formed bone and PDL, and enhance the expression of VEGF and osteopontin | Mansour et al., 2022 [38] |
| L/D-cysteine-anchored AuNPs | nanoparticle | Human periodontal ligament cells (PDLCs) | Rat model of mandible periodontal fenestration defect | show a better performance in cellular internalization, autophagy regulation, osteogenic differentiation and periodontal tissue regeneration | Zhang et al., 2021 [84] | |
| Self-assembling peptide (SAP; P11-4) | nanoparticle | N/A | Rat model of maxillary periodontal defects | increase functional PDL length and reduce epithelial down growth after 4 weeks, with a significant increase in osteocalcin and OPG and higher OPG/RANKL ratio | El-Sayed et al., 2020 [70] | |
| Alveolar bone and cementum regeneration | Chitosan, type I collagen, Poly(ethylene oxide), (Arg-Gly-Asp) peptide, acetic acid solution, HA nanoparticles | nanoparticle | Human dental pulp stem cells (DPSCs) | Nude mice, rat model of mandible periodontal defect, mini-swine model of mandible periodontal defect | facilitate the regeneration of dentin, cementum and alveolar bone | Yan et al., 2022 [39] |
| Cementum-ligament-bone complex regeneration | Intrafibrillarly mineralized collagen and unmineralized parallel-aligned fibrils | biphasic scaffold | hPDLSCs | Rat model of complete periodontal defect model | reconstruct native periodontium with the insertion of PDL fibers into newly formed cementum and alveolar bone by recruiting host MSCs | Yu et al., 2021 [43] |
| ε-aminocaproic acid-releasing chitosan particles-incorporated fibrin | nanoparticle | Cementoblasts and MC3T3-E1 | Beagle model of mandible Class II furcation defect | promote alveolar bone and cementum formation, develop structural integrations of the cementum-PDL-bone complex by the Sharpey’s fiber insertion, | Park et al., 2017 [95] | |
| Enamel matrix derivatives and bone morphogenetic protein-2 loaded biphasic cryogel scaffold | nanofiber | MSCs | Beagle model of mandibular periodontal intrabony defect | have potential for the reconstruction of alveolar ridge, PDL and cementum | Huang et al., 2020 [96] | |
| chitin–PLGA/nBGC/CEMP1, chitin–PLGA/FGF2 and chitin–PLGA/nBGC/PRP | nanocomposite | Human dental follicle stem cells | Rabbit model of maxillary periodontal defects | achieve simultaneous and complete periodontal regeneration | Sowmya et al., 2017 [47] | |
| 15-deoxy-Δ12,14-prostaglandin J2, PCL/GE | nanoparticle | decellularized hPDLC sheets | Rat model of mandible periodontal fenestration defect | form new bone, cementum and PDL | Jiang et al., 2021 [52] | |
| Dimethyloxalylglycine, nanosilicate, PLGA | nanoplatelet | hPDLSCs | Rat model of mandibular buccal bone defect | promote the recruitment of CD90+/CD34− stromal cells, induce angiogenesis and osteogenesis and regenerate cementum-ligament-bone complex | Shang et al., 2021 [48] | |
| Gold nanoparticles | nanoparticle | hPDLCs and human macrophages | Rat models of both fenestration and ligature-induced periodontitis | increase newly formed periodontal attachment, bone and cementum in periodontal defect with less tissue breakdown in periodontitis | Ni et al., 2019 [60] |
4.3. Cementum Regeneration
The tooth cementum is a calcified connective tissue covering the outer surface of the dental root, which provides a medium for attachment and insertion of PDL fibers. The cementum can become damaged or deteriorate over time, especially in cases of advanced periodontal disease or aggressive tooth brushing. This can lead to tooth sensitivity, root decay and other oral health problems. Treatment options for cementum loss may include scaling and root planning, gingival grafting and other procedures to restore the tooth and surrounding tissues.
Only one article in this review reported alveolar bone and cementum regeneration, which was based on the grafting of exogenous DPSCs embedded in an osteogenic scaffold [39]. This article uses a patch to deliver DPSCs with differentiation potential and paracrine functions to suppress the local inflammatory reaction. Moreover, this patch inhibited epithelial invasion, providing necessary space for periodontal regeneration and satisfying the requirements for GTR membranes. However, PDL regeneration was not mentioned in this study. The regeneration of cementum with PDL attachment remains difficult.
4.4. Regeneration of the Cementum-Ligament-Bone Complex
Seven of the included articles reported regeneration of the cementum–ligament–bone complex [43,47,48,52,60,95,96]. The periodontium exhibits a typical “sandwich structure” composed of the cementum, alveolar bone and PDL with fiber bundles in various directions. Recently, the key to periodontal regeneration by tissue engineering has become the reconstruction of polyphase scaffolds and different periodontal fiber orientations, mimicking the interaction between the layers and the distinctive alignment of periodontal fibers [97,98]. It is crucial to highlight that all of the aforementioned studies show that nanomaterials alone might not be the only solution for producing successful bone regenerative scaffolds. A hierarchical design would also be needed to build the optimal biomimetic nanomaterials. Eventually, it might be possible to develop an ideal scaffold by combining various components and methods [28].
Since the regeneration of the cementum–ligament–bone complex usually requires a hierarchical design with multiple components, the biomedical safety of the used formulations brings great concern. However, as those nanomaterials are still in the experimental stage, very little attention has been paid to this point, which is reviewed and discussed in the last chapter of this review, ‘Biomedical safety.’ In addition, the synchronized or well-organized biodegradation time among different components is also crucial to the function of the nanomaterials. A too-fast biodegradation of the scaffold may not provide enough mechanical strength or necessary time for drug release, while a too-slow biodegradation can induce a host reaction to the synthetic materials [52]. Finally, some other problems are still to be solved. For example, can the nanomaterials still promote complete regeneration when the periodontal defect exceeds the critical size? Can the nanomaterials be used in an environment of microbial contamination?
4.5. Gingiva Regeneration
The gingiva is part of the oral mucosa that extends in the form of a collar around the enamel–cement junction of the tooth and covers the alveolar extensions of the maxilla and mandible. It adheres to the enamel via the junctional epithelium and attaches to the cementum and the bone through the collagen fibers of the lamina. The interdental gingiva occupies the space between two adjacent teeth and its flexible shape adapts to this space [99]. Gingival recession is a mucogingival defect defined as the apical shifting of the gingival margin in relation to its physiological position, located 1–2 mm coronally to the cementoenamel junction (CEJ) [100]. Patient-contributed trauma and iatrogenic interventions, such as improper toothbrushing technique, deep cervical restorative margins and orthodontic tooth movement, have all been associated with gingival recession [101].
None of the studies included in this review used nanomaterials to promote gingival regeneration due to a lack of in vivo studies. However, there are two in vitro studies that might be useful for gingival tissue engineering [102,103]. The reasons for the lack of studies are probably as follows: Firstly, up until now, therapeutic approaches for gingival recession have been oriented towards periodontal plastic surgery for root coverage and CEJ reconstruction [100,104]. Secondly, unlike alveolar bone defects, which can be covered and protected by gingiva after applying the nanomaterial-based transplant, the gingival wound or recessive gingiva itself is directly exposed to the complex microenvironment of the oral cavity. Third, applying nanomaterials may change the color, shape and texture of the gingiva, which can greatly influence smile aesthetics, especially when it happens in anterior teeth.
4.6. Biomedical Safety
Toxicity is an important consideration for the biomedical applications of nanomaterials and nanotechnology. Due to their extensive interactions with biological environments following their in vivo administration, nanomaterials can potentially have different degrees of toxicity to the body. First, nanoparticles interact with the blood cells and components once they enter the bloodstream, which can lead to hematological toxicity. Second, extensive amounts of nanoparticles can be accumulated in organs, especially those of the reticular endothelial system, which can result in toxicity to specific organs, such as the lungs, liver, spleen, kidneys or induce endocrine and immunotoxicity. Most of the intracellular and in vivo toxicity induced by nanoparticles arises from the production of excess reactive oxygen species (ROS). High ROS levels can damage cells by peroxidizing lipids, altering proteins, disrupting DNA, interfering with signaling functions and modulating gene transcription. These can finally result in cancer, neurodegeneration, cardiovascular, renal or pulmonary disease [105].
The toxicity of nanomaterials has been largely decreased with the application of biodegradable materials [106]. However, it needs to be noted that not all biodegradable materials are deemed safe for application in humans. Thus, to avoid misuse of nanomaterials, the main aspects involved in their toxicity, including their size, shape and surface chemistry [15], are summarized in this review as follows:
The size directly determines the surface area of nanomaterials available to interact with biological environments. Therefore, size is a critical physicochemical property influencing the cellular response and in vivo fate of nanomaterials [107]. The cellular response includes cytotoxicity, penetration of the biological barrier and immune response. For example, nanomaterials of smaller size have a higher ratio of surface area to volume, which leads to an increase in their reactivity [108]. Additionally, a decrease in the size is known to positively affect their vascular permeation [109]. A smaller size seems to result in a longer circulation time and reduced accumulation in the liver and spleen [110]. Particularly, small nanoparticles are even able to pass through the blood-brain barrier. Thus, their toxicity must be taken into full consideration and the potential beneficial function of any new nanomaterials must always be weighed against the risk prior to their clinical application.
The shape is also a pivotal physicochemical property of nanomaterials that largely determines their in vivo fate, including macrophage uptake, blood circulation and biodistribution, margination, extravasation and disease targeting [107]. Spherical particles have been more extensively studied in circulation due to their simple geometry. For example, the drug delivery efficiency was significantly higher in spherical particles than rod and elliptical disks [111]. However, these particles are often associated with filtration into off-target organs. For instance, the slits in the spleen that allow for blood filtration are asymmetrical, which makes it difficult for spherical particles to pass through [112]. Other asymmetric nanoparticles have been observed to offer benefits in bloodstream circulation, target tissue penetration and drug release [113]. Nanostructures of disks, large compound vesicles and staggered lamellae exhibited a significantly more extended circulation than that of spheres, while staggered lamellae and disks had longer circulation half-life than large compound vesicles and spheres [114]. Interestingly, smart nanoparticles have been developed recently with shape-switching and adjustable stiffness properties [115]. At first, the rod-like profile of nanoparticles prolongs blood circulation time and enhances extravasation into tumor tissues. When exposed to the acidic tumor microenvironment, nanoparticles decompose to transform into a spherical shape with higher uptake and cytotoxicity to breast cancer cells. Such shape and stiffness-adjustable design might be prospectively applied in periodontal regeneration to acquire higher drug accumulation in situ, providing a stronger engineering scaffold and alleviating systemic toxicities to the blood, liver, kidney and heart tissues.
The surface properties of nanoparticles, such as charge, hydrophobicity and targeting ligands, have a significant impact on their ability to circulate and subsequently be internalized by cells [107]. Surfaces with net positive charges or grafting with targeting ligand/peptide promote binding with negatively charged cell membranes and thus enhance cellular uptake. In contrast, surfaces with neutral or negatively charges promote longer-term circulation but typically have lower internalization potential [112,116]. Stimuli-responsive nanoparticles with switchable negative/positive charges or hidden/exposed target ligands might prevent off-target interactions during circulation while promoting immobilization and cell uptake at the site of the endogenous or exogenously applied stimulus. For example, the tunable surface charge of lipid nanoparticles contributes to form stabilization in the physiological environment and enhances internalization/drug release in the slightly acidic tumor microenvironment, respectively [117]. Furthermore, surface hydrophobicity can also influence the internalization of nanoparticles. For example, the translocation abilities of hydrophilic nanoparticles can be enhanced by increasing their stiffness, while the penetrability of hydrophobic nanoparticles is weakened by increasing their stiffness [118]. These strategies of well-designed surface chemistry could also be utilized to inspire specific targeting of periodontal tissue regeneration.
5. Conclusive Remarks
The use of nanomaterials for periodontal tissue regeneration is still in the experimental stage. Further research is needed to optimize the design and application of nanomaterials and enhance their biomedical safety, especially for the use of (large) animal models mimicking chronic inflammation and testing the regeneration of cementum–ligament–bone complex. In spite of this, they have shown the potential to significantly improve the outcomes of periodontal tissue regeneration.
Author Contributions
Conceptualization, C.Z. and M.C.d.L.-P.; methodology, C.Z.; software, C.Z.; validation, C.Z. and M.C.d.L.-P.; resources, M.C.d.L.-P., G.W. and H.H.; data curation, C.Z.; writing—original draft preparation, C.Z.; writing—review and editing, C.Z., M.C.d.L.-P., G.W., A.B. and H.H.; visualization, C.Z.; supervision, M.C.d.L.-P., G.W. and A.B.; project administration, C.Z. and M.C.d.L.-P.; funding acquisition, C.Z. and M.C.d.L.-P. All authors have read and agreed to the published version of the manuscript.
Funding
C.Z. was supported by the China Scholarship Council (File No. 201806270252). M.C.d.L.-P. was supported by the ‘Start up Grant for ZAP’ of KU Leuven (EGW-D4244-BLOZ/17/014).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced Biomaterials and Techniques for Oral Tissue Engineering and Regeneration—A Review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules 2020, 25, 5330. [Google Scholar] [CrossRef] [PubMed]
- Hollý, D.; Klein, M.; Mazreku, M.; Zamborský, R.; Polák, Š.; Danišovič, Ľ.; Csöbönyeiová, M. Stem Cells and Their Derivatives-Implications for Alveolar Bone Regeneration: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11746. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xia, X.; Huang, J.; Yuan, C.; Zuo, Y.; Li, Y.; Li, J. Recent advances in PLGA-based biomaterials for bone tissue regeneration. Acta Biomater. 2021, 127, 56–79. [Google Scholar] [CrossRef]
- Vouvoudi, E.C. Overviews on the Progress of Flowable Dental Polymeric Composites: Their Composition, Polymerization Process, Flowability and Radiopacity Aspects. Polymers 2022, 14, 4182. [Google Scholar] [CrossRef]
- Valandro, L.F.; Cadore-Rodrigues, A.C.; Dapieve, K.S.; Machry, R.V.; Pereira, G.K.R. A brief review on fatigue test of ceramic and some related matters in Dentistry. J. Mech. Behav. Biomed. Mater. 2023, 138, 105607. [Google Scholar] [CrossRef]
- Albeshir, E.G.; Alsahafi, R.; Albluwi, R.; Balhaddad, A.A.; Mitwalli, H.; Oates, T.W.; Hack, G.D.; Sun, J.; Weir, M.D.; Xu, H.H.K. Low-Shrinkage Resin Matrices in Restorative Dentistry-Narrative Review. Materials 2022, 15, 2951. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, X.-J.; You, B.-A.; Ren, N.; Liu, H. Application of Nanomaterials in Stem Cell-Based Therapeutics for Cardiac Repair and Regeneration. Small 2023, 19, 2206487. [Google Scholar] [CrossRef]
- Hu, T.; Gu, Z.; Williams, G.R.; Strimaite, M.; Zha, J.; Zhou, Z.; Zhang, X.; Tan, C.; Liang, R. Layered double hydroxide-based nanomaterials for biomedical applications. Chem. Soc. Rev. 2022, 51, 6126–6176. [Google Scholar] [CrossRef] [PubMed]
- Armenia, I.; Cuestas Ayllón, C.; Torres Herrero, B.; Bussolari, F.; Alfranca, G.; Grazú, V.; Martínez de la Fuente, J. Photonic and magnetic materials for on-demand local drug delivery. Adv. Drug Deliv. Rev. 2022, 191, 114584. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Van Holm, W.; Bronckaers, A.; Zhao, Z.; Čokić, S.; Aktan, M.K.; Castro, A.B.; Van Meerbeek, B.; Braem, A.; Willems, G.; et al. Biomimetic Periodontal Ligament Transplantation Activated by Gold Nanoparticles Protects Alveolar Bone. Adv. Healthc. Mater. 2023, 2300328. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cui, J.; Shen, H.; He, C.; Wang, X.; Shen, S.G.F.; Lin, K. Advances of nanomaterial applications in oral and maxillofacial tissue regeneration and disease treatment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 13, e1669. [Google Scholar] [CrossRef]
- Feng, W.; Chen, Y. Chemoreactive nanomedicine. J. Mater. Chem. B 2020, 8, 6753–6764. [Google Scholar] [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: Ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046–5089. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem. Int. Ed. Engl. 2022, 61, e202107960. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Brannon, E.R.; Guevara, M.V.; Pacifici, N.J.; Lee, J.K.; Lewis, J.S.; Eniola-Adefeso, O. Polymeric particle-based therapies for acute inflammatory diseases. Nat. Rev. Mater. 2022, 7, 796–813. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ortiz, G.; Elangovan, S.; Kramer, K.W.; Blanchette, D.; Dawson, D.V. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis. J. Dent. Res. 2014, 93, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Hamdan, N.; Ikeda, Y.; Grynpas, M.; Ganss, B.; Glogauer, M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: A review. Biomater. Res. 2017, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Y.; Fu, Y.; Zou, D.; Lu, J.; Lyu, C. Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry. APL Bioeng. 2022, 6, 031503. [Google Scholar] [CrossRef]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum proteins: Role in cementogenesis, biomineralization, periodontium formation and regeneration. Periodontol. 2000 2015, 67, 211–233. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef]
- Zakrzewski, W.; Dobrzynski, M.; Rybak, Z.; Szymonowicz, M.; Wiglusz, R.J. Selected Nanomaterials’ Application Enhanced with the Use of Stem Cells in Acceleration of Alveolar Bone Regeneration during Augmentation Process. Nanomaterials 2020, 10, 1216. [Google Scholar] [CrossRef]
- Funda, G.; Taschieri, S.; Bruno, G.A.; Grecchi, E.; Paolo, S.; Girolamo, D.; Del Fabbro, M. Nanotechnology Scaffolds for Alveolar Bone Regeneration. Materials 2020, 13, 201. [Google Scholar] [CrossRef]
- Iviglia, G.; Kargozar, S.; Baino, F. Biomaterials, Current Strategies, and Novel Nano-Technological Approaches for Periodontal Regeneration. J. Funct. Biomater. 2019, 10, 3. [Google Scholar] [CrossRef]
- Al-Ahmady, H.H.; Abd Elazeem, A.F.; Bellah Ahmed, N.E.; Shawkat, W.M.; Elmasry, M.; Abdelrahman, M.A.; Abderazik, M.A. Combining autologous bone marrow mononuclear cells seeded on collagen sponge with Nano Hydroxyapatite, and platelet-rich fibrin: Reporting a novel strategy for alveolar cleft bone regeneration. J. Craniomaxillofac. Surg. 2018, 46, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Manju, V.; Anitha, A.; Menon, D.; Iyer, S.; Nair, S.V.; Nair, M.B. Nanofibrous yarn reinforced HA-gelatin composite scaffolds promote bone formation in critical sized alveolar defects in rabbit model. Biomed. Mater. 2018, 13, 065011. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.H.; Sun, C.K.; Lin, Y.W.; Hung, M.C.; Lin, H.Y.; Li, C.H.; Lin, I.P.; Chang, H.C.; Sun, J.S.; Chang, J.Z. Metformin-Incorporated Gelatin/Nano-Hydroxyapatite Scaffolds Promotes Bone Regeneration in Critical Size Rat Alveolar Bone Defect Model. Int. J. Mol. Sci. 2022, 23, 558. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.H.; Lin, Y.W.; Lin, F.H.; Sun, J.S.; Chao, Y.H.; Lin, H.Y.; Chang, Z.C. Biomimetic Synthesis of Nanocrystalline Hydroxyapatite Composites: Therapeutic Potential and Effects on Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 6002. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, B.; Liu, H.; Wang, T.; Wang, F.; Xie, C.; Li, M.; Liu, H.; Ge, S. Hydroxyapatite nanowires modified polylactic acid membrane plays barrier/osteoinduction dual roles and promotes bone regeneration in a rat mandible defect model. J. Biomed. Mater. Res. A 2018, 106, 3099–3110. [Google Scholar] [CrossRef]
- Calasans-Maia, M.D.; Barboza, C.A.B., Jr.; Soriano-Souza, C.A.; Alves, A.; Uzeda, M.J.P.; Martinez-Zelaya, V.R.; Mavropoulos, E.; Rocha Leão, M.H.; de Santana, R.B.; Granjeiro, J.M.; et al. Microspheres of alginate encapsulated minocycline-loaded nanocrystalline carbonated hydroxyapatite: Therapeutic potential and effects on bone regeneration. Int. J. Nanomed. 2019, 14, 4559–4571. [Google Scholar] [CrossRef]
- Dhawan, S.; Takiar, M.; Manocha, A.; Dhawan, R.; Malhotra, R.; Gupta, J. Functionally graded membrane: A novel approach in the treatment of gingival recession defects. J. Indian Soc. Periodontol. 2021, 25, 411–417. [Google Scholar] [CrossRef]
- Mansour, A.M.; Yahia, S.; Elsayed, H.R.H.; El-Attar, S.A.E.; Grawish, M.E.; El-Hawary, Y.M.; El-Sherbiny, I.M. Efficacy of biocompatible trilayers nanofibrous scaffold with/without allogeneic adipose-derived stem cells on class II furcation defects of dogs’ model. Clin. Oral Investig. 2022, 26, 2537–2553. [Google Scholar] [CrossRef]
- Yan, N.; Hu, B.; Xu, J.; Cai, R.; Liu, Z.; Fu, D.; Huo, B.; Liu, Z.; Zhao, Y.; Chen, C.; et al. Stem cell Janus patch for periodontal regeneration. Nano Today 2022, 42, 101336. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual Delivery of Alendronate and E7-BMP-2 Peptide via Calcium Chelation to Mineralized Nanofiber Fragments for Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Luo, D.; Qiao, J.; Guo, J.; He, D.; Jin, S.; Tang, L.; Wang, Y.; Shi, X.; Mao, J.; et al. A hierarchical bilayer architecture for complex tissue regeneration. Bioact. Mater. 2022, 10, 93–106. [Google Scholar] [CrossRef]
- Shaikh, M.S.; Zafar, M.S.; Alnazzawi, A. Comparing Nanohydroxyapatite Graft and Other Bone Grafts in the Repair of Periodontal Infrabony Lesions: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2021, 22, 12021. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.T.; Munguia-Lopez, J.G.; Cho, Y.W.; Ma, X.; Song, V.; Zhu, Z.; Tran, S.D. Polymeric Scaffolds for Dental, Oral, and Craniofacial Regenerative Medicine. Molecules 2021, 26, 7043. [Google Scholar] [CrossRef]
- Xing, D.; Zuo, W.; Chen, J.; Ma, B.; Cheng, X.; Zhou, X.; Qian, Y. Spatial Delivery of Triple Functional Nanoparticles via an Extracellular Matrix-Mimicking Coaxial Scaffold Synergistically Enhancing Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 37380–37395. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Ma, B.; Shao, J.; Wang, B.; Ma, C.; Ge, S. Dimethyloxallyl glycine/nanosilicates-loaded osteogenic/angiogenic difunctional fibrous structure for functional periodontal tissue regeneration. Bioact. Mater. 2021, 6, 1175–1188. [Google Scholar] [CrossRef]
- Xue, Y.; Hong, X.; Gao, J.; Shen, R.; Ye, Z. Preparation and biological characterization of the mixture of poly(lactic-co-glycolic acid)/chitosan/Ag nanoparticles for periodontal tissue engineering. Int. J. Nanomed. 2019, 14, 483–498. [Google Scholar] [CrossRef]
- Stutz, C.; Strub, M.; Clauss, F.; Huck, O.; Schulz, G.; Gegout, H.; Benkirane-Jessel, N.; Bornert, F.; Kuchler-Bopp, S. A New Polycaprolactone-Based Biomembrane Functionalized with BMP-2 and Stem Cells Improves Maxillary Bone Regeneration. Nanomaterials 2020, 10, 1774. [Google Scholar] [CrossRef]
- Sun, M.; Liu, Y.; Jiao, K.; Jia, W.; Jiang, K.; Cheng, Z.; Liu, G.; Luo, Y. A periodontal tissue regeneration strategy via biphasic release of zeolitic imidazolate framework-8 and FK506 using a uniaxial electrospun Janus nanofiber. J. Mater. Chem. B 2022, 10, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.M.; Huang, J.P.; Lu, K.X.; Sun, W.L.; Tan, J.Y.; Li, B.X.; Chen, L.L.; Wu, Y.M. Regeneration potential of decellularized periodontal ligament cell sheets combined with 15-deoxy-Δ(12,14)-prostaglandin J(2) nanoparticles in a rat periodontal defect. Biomed. Mater. 2021, 16, 045008. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Strub, M.; Petit, C.; Bugueno, I.M.; Bornert, F.; Clauss, F.; Huck, O.; Kuchler-Bopp, S.; Benkirane-Jessel, N. Periodontal Tissues, Maxillary Jaw Bone, and Tooth Regeneration Approaches: From Animal Models Analyses to Clinical Applications. Nanomaterials 2018, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020, 110, 110698. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Zong, C.; Bronckaers, A.; Vande Velde, G.; Willems, G.; Cadenas de Llano-Pérula, M. In Vivo Micro-Computerized Tomography Tracking of Human Periodontal Ligament Stem Cells Labeled with Gold Nanocomplexes. Adv. Healthc. Mater. 2022, 11, e2101133. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Madheswaran, T.; Tharmalingam, N.; Bose, R.J.; Park, H.; Ha, D.H. Labeling and tracking cells with gold nanoparticles. Drug Discov. Today 2021, 26, 94–105. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Wang, M.; Zhou, J.; Zhang, Q.; Yang, W.; Li, Y.; Yan, F. Gold Nanoparticles Combined Human β-Defensin 3 Gene-Modified Human Periodontal Ligament Cells Alleviate Periodontal Destruction via the p38 MAPK Pathway. Front. Bioeng. Biotechnol. 2021, 9, 631191. [Google Scholar] [CrossRef]
- Ni, C.; Zhou, J.; Kong, N.; Bian, T.; Zhang, Y.; Huang, X.; Xiao, Y.; Yang, W.; Yan, F. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials 2019, 206, 115–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, N.; Zhang, Y.; Yang, W.; Yan, F. Size-dependent Effects of Gold Nanoparticles on Osteogenic Differentiation of Human Periodontal Ligament Progenitor Cells. Theranostics 2017, 7, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Liu, D.; Fong, C.-C.; Zhang, J.; Yang, M. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano 2010, 4, 6439–6448. [Google Scholar] [CrossRef]
- Zhan, X.; Yan, J.; Tang, H.; Xia, D.; Lin, H. Antibacterial Properties of Gold Nanoparticles in the Modification of Medical Implants: A Systematic Review. Pharmaceutics 2022, 14, 2654. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Wang, Y.; Chen, Y.; Xie, J.; Su, J.; Huang, C. One-step treatment of periodontitis based on a core-shell micelle-in-nanofiber membrane with time-programmed drug release. J. Control. Release 2020, 320, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ji, Q.; Chen, X.; Sun, Y.; Xu, Q.; Deng, P.; Hu, F.; Yang, J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J. Biomed. Mater. Res. A 2017, 105, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.M.d.; Spin-Neto, R.; Marcantonio, E., Jr.; Pereira, L.A.V.D.; Wikesjö, U.M.E.; Susin, C. Alveolar ridge and maxillary sinus augmentation using rhBMP-2: A systematic review. Clin. Implant Dent. Relat. Res. 2015, 17, e192–e201. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yu, D.; Wei, L.; Su, N.; Liu, Y. Preclinical application of recombinant human bone morphogenetic protein 2 on bone substitutes for vertical bone augmentation: A systematic review and meta-analysis. J. Prosthet. Dent. 2019, 122, 355–363. [Google Scholar] [CrossRef]
- Huang, R.-L.; Sun, Y.; Ho, C.-K.; Liu, K.; Tang, Q.-Q.; Xie, Y.; Li, Q. IL-6 potentiates BMP-2-induced osteogenesis and adipogenesis via two different BMPR1A-mediated pathways. Cell Death Dis. 2018, 9, 144. [Google Scholar] [CrossRef]
- Zhou, L.-L.; Liu, W.; Wu, Y.-M.; Sun, W.-L.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020, 2020, 1327405. [Google Scholar] [CrossRef]
- El-Sayed, B.; Davies, R.P.W.; El-Zehery, R.R.; Ibrahim, F.M.; Grawish, M.E.; Kirkham, J.; El-Gendy, R. An In-Vivo Intraoral Defect Model for Assessing the Use of P(11)-4 Self-Assembling Peptide in Periodontal Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 559494. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Y.; Bai, Y.; Wang, Y.; Wang, J.; Heng, B.; Wei, J.; Jiang, X.; Gao, M.; Zheng, X.; et al. Restoring the electrical microenvironment using ferroelectric nanocomposite membranes to enhance alveolar ridge regeneration in a mini-pig preclinical model. J. Mater. Chem. B 2023, 11, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, W.I.; El Ashry, S.H.; Ali, G.W.; Hamid, M.A.A.; El-Din, A.G.; El-Ashry, B. Regeneration of periapical lesions post-endodontic treatment and periapical surgeries in experimental animals utilizing thermo-responsive nano-β-tricalcium phosphate/chitosan hydrogel: A proof of concept. Biomed. Mater. 2017, 12, 045007. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.H.; Chang, H.C.; Chang, Y.C.; Claudia, J.; Lin, T.C.; Chang, P.C. Pdgf-metronidazole-encapsulated nanofibrous functional layers on collagen membrane promote alveolar ridge regeneration. Int. J. Nanomed. 2017, 12, 5525–5535. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Hao, X.; Wang, L.; Hu, Y.; Meng, L.; Zheng, S.; Ren, F.; Bu, W.; Wang, H.; Li, D.; et al. Metformin Carbon Dots for Promoting Periodontal Bone Regeneration via Activation of ERK/AMPK Pathway. Adv. Healthc. Mater. 2021, 10, e2100196. [Google Scholar] [CrossRef] [PubMed]
- Remy, M.T.; Ding, Q.; Krongbaramee, T.; Hu, J.; Mora Mata, A.V.; Haes, A.J.; Amendt, B.A.; Sun, H.; Buchakjian, M.R.; Hong, L. Plasmid encoding miRNA-200c delivered by CaCO(3)-based nanoparticles enhances rat alveolar bone formation. Nanomedicine 2022, 17, 1339–1354. [Google Scholar] [CrossRef]
- He, Y.; Tian, M.; Li, X.; Hou, J.; Chen, S.; Yang, G.; Liu, X.; Zhou, S. A Hierarchical-Structured Mineralized Nanofiber Scaffold with Osteoimmunomodulatory and Osteoinductive Functions for Enhanced Alveolar Bone Regeneration. Adv. Healthc. Mater. 2022, 11, e2102236. [Google Scholar] [CrossRef]
- Xiang, M.; Zhu, M.; Yang, Z.; He, P.; Wei, J.; Gao, X.; Song, J. Dual-Functionalized Apatite Nanocomposites with Enhanced Cytocompatibility and Osteogenesis for Periodontal Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 1704–1714. [Google Scholar] [CrossRef]
- Zhou, M.; Gao, S.; Zhang, X.; Zhang, T.; Zhang, T.; Tian, T.; Li, S.; Lin, Y.; Cai, X. The protective effect of tetrahedral framework nucleic acids on periodontium under inflammatory conditions. Bioact. Mater. 2021, 6, 1676–1688. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; Zhao, Y.; Xu, Y.; Zhang, F.; Gu, N.; Ma, J.; Reynolds, M.A.; Xia, Y.; Xu, H.H.K. Enhanced bone regeneration and visual monitoring via superparamagnetic iron oxide nanoparticle scaffold in rats. J. Tissue Eng. Regen. Med. 2018, 12, e2085–e2098. [Google Scholar] [CrossRef]
- Carmo, A.; Sartoretto, S.C.; Alves, A.; Granjeiro, J.M.; Miguel, F.B.; Calasans-Maia, J.; Calasans-Maia, M.D. Alveolar bone repair with strontium—containing nanostructured carbonated hydroxyapatite. J. Appl. Oral Sci. 2018, 26, e20170084. [Google Scholar] [CrossRef]
- Ho, M.H.; Huang, K.Y.; Tu, C.C.; Tai, W.C.; Chang, C.H.; Chang, Y.C.; Chang, P.C. Functionally graded membrane deposited with PDLLA nanofibers encapsulating doxycycline and enamel matrix derivatives-loaded chitosan nanospheres for alveolar ridge regeneration. Int. J. Biol. Macromol. 2022, 203, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Kong, N.; Ding, L.; Guo, Y.; Yang, W.; Yan, F. Ultrathin 2D Titanium Carbide MXene (Ti(3) C(2) T(x) ) Nanoflakes Activate WNT/HIF-1α-Mediated Metabolism Reprogramming for Periodontal Regeneration. Adv. Healthc. Mater. 2021, 10, e2101215. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhou, Y.; Zheng, K.; Xu, X.; Yang, J.; Wang, X.; Miao, L.; Wei, H.; Xu, Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact. Mater. 2022, 7, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, H.; Kong, N.; Wang, Z.; Fu, H.; Zhang, Y.; Xiao, Y.; Yang, W.; Yan, F. l-cysteine-modified chiral gold nanoparticles promote periodontal tissue regeneration. Bioact. Mater. 2021, 6, 3288–3299. [Google Scholar] [CrossRef]
- Li, K.; Ishida, Y.; Hatano-Sato, K.; Ongprakobkul, N.; Hosomichi, J.; Usumi-Fujita, R.; Kaneko, S.; Yamaguchi, H.; Ono, T. Nuclear factor-kappa B decoy oligodeoxynucleotide-loaded poly lactic-co-glycolic acid nanospheres promote periodontal tissue healing after tooth replantation in rats. J. Periodontol. 2022, 93, 458–470. [Google Scholar] [CrossRef]
- Berkovitz, B.K.; Moxham, B.J.; Newman, H.N. The Periodontal Ligament in Health and Disease; Mosby-Wolfe: Maryland Heights, MO, USA, 1995. [Google Scholar]
- O’Brien, E.J.O.; Frank, C.B.; Shrive, N.G.; Hallgrímsson, B.; Hart, D.A. Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. Int. J. Exp. Pathol. 2012, 93, 319–331. [Google Scholar] [CrossRef]
- Roato, I.; Masante, B.; Putame, G.; Massai, D.; Mussano, F. Challenges of Periodontal Tissue Engineering: Increasing Biomimicry through 3D Printing and Controlled Dynamic Environment. Nanomaterials 2022, 12, 3878. [Google Scholar] [CrossRef]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Emara, A.; Shah, R. Recent update on craniofacial tissue engineering. J. Tissue Eng. 2021, 12, 20417314211003735. [Google Scholar] [CrossRef]
- Mei, N.; Wu, Y.; Chen, B.; Zhuang, T.; Yu, X.; Sui, B.; Ding, T.; Liu, X. 3D-printed mesoporous bioactive glass/GelMA biomimetic scaffolds for osteogenic/cementogenic differentiation of periodontal ligament cells. Front. Bioeng. Biotechnol. 2022, 10, 950970. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol. Bioeng. 2019, 116, 452–468. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Wang, H.; Bai, J.; Miao, X.; Lin, Q.; Zheng, J.; Ding, S.; Li, X.; Tang, Y. Multidrug-loaded electrospun micro/nanofibrous membranes: Fabrication strategies, release behaviors and applications in regenerative medicine. J. Control. Release 2021, 330, 1264–1287. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Yan, J.; Zhang, K.; Lin, F.; Xiang, L.; Deng, L.; Guan, Z.; Cui, W.; Zhang, H. Pharmaceutical electrospinning and 3D printing scaffold design for bone regeneration. Adv. Drug Deliv. Rev. 2021, 174, 504–534. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Oh, J.H.; Jung, H.M.; Choi, Y.; Rahman, S.U.; Kim, S.; Kim, T.I.; Shin, H.I.; Lee, Y.S.; Yu, F.H.; et al. Effects of the incorporation of ε-aminocaproic acid/chitosan particles to fibrin on cementoblast differentiation and cementum regeneration. Acta Biomater. 2017, 61, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Tai, W.C.; Ho, M.H.; Chang, P.C. Combination of a biomolecule-aided biphasic cryogel scaffold with a barrier membrane adhering PDGF-encapsulated nanofibers to promote periodontal regeneration. J. Periodontal Res. 2020, 55, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Amrollahi, P.; Shah, B.; Seifi, A.; Tayebi, L. Recent advancements in regenerative dentistry: A review. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016, 69, 1383–1390. [Google Scholar] [CrossRef]
- Uskoković, V.; Pejčić, A.; Koliqi, R.; Anđelković, Z. Polymeric nanotechnologies for the treatment of periodontitis: A chronological review. Int. J. Pharm. 2022, 625, 122065. [Google Scholar] [CrossRef]
- Cortellini, P.; Bissada, N.F. Mucogingival conditions in the natural dentition: Narrative review, case definitions, and diagnostic considerations. J. Periodontol. 2018, 89, S204–S213. [Google Scholar] [CrossRef]
- Kim, D.M.; Bassir, S.H.; Nguyen, T.T. Effect of gingival phenotype on the maintenance of periodontal health: An American Academy of Periodontology best evidence review. J. Periodontol. 2020, 91, 311–338. [Google Scholar] [CrossRef]
- Wright, M.E.E.; Wong, A.T.; Levitt, D.; Parrag, I.C.; Yang, M.; Santerre, J.P. Influence of ciprofloxacin-based additives on the hydrolysis of nanofiber polyurethane membranes. J. Biomed. Mater. Res. A 2018, 106, 1211–1222. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, G.; Whitescarver, R.A.; Nunes, L.; Palmer, X.L.; Skrtic, D.; Tutak, W. Effects of protein-coated nanofibers on conformation of gingival fibroblast spheroids: Potential utility for connective tissue regeneration. Biomed. Mater. 2018, 13, 025006. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F. Periodontal plastic surgery of gingival recessions at single and multiple teeth. Periodontol. 2000 2017, 75, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-Nano Approach: Enabling the Translation of Metal Nanomaterials to Clinics. Bioconjug. Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Saifi, M.A.; Khan, W.; Godugu, C. Cytotoxicity of Nanomaterials: Using Nanotoxicology to Address the Safety Concerns of Nanoparticles. Pharm. Nanotechnol. 2018, 6, 3–16. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, R.; Wu, J.; Fan, Q.; Yung, B.C.; Niu, G.; Jacobson, O.; Wang, Z.; Liu, G.; Yu, G.; et al. Impact of Semiconducting Perylene Diimide Nanoparticle Size on Lymph Node Mapping and Cancer Imaging. ACS Nano 2017, 11, 4247–4255. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Kaplan, M.; Öztürk, K.; Öztürk, S.C.; Tavukçuoğlu, E.; Esendağlı, G.; Calis, S. Effects of Particle Geometry for PLGA-Based Nanoparticles: Preparation and In Vitro/In Vivo Evaluation. Pharmaceutics 2023, 15, 175. [Google Scholar] [CrossRef]
- Li, X.; Montague, E.C.; Pollinzi, A.; Lofts, A.; Hoare, T. Design of Smart Size-, Surface-, and Shape-Switching Nanoparticles to Improve Therapeutic Efficacy. Small 2022, 18, e2104632. [Google Scholar] [CrossRef]
- Wang, W.; Gaus, K.; Tilley, R.D.; Gooding, J.J. The impact of nanoparticle shape on cellular internalisation and transport: What do the different analysis methods tell us? Mater. Horiz. 2019, 6, 1538–1547. [Google Scholar] [CrossRef]
- Hu, X.; Hu, J.; Tian, J.; Ge, Z.; Zhang, G.; Luo, K.; Liu, S. Polyprodrug amphiphiles: Hierarchical assemblies for shape-regulated cellular internalization, trafficking, and drug delivery. J. Am. Chem. Soc. 2013, 135, 17617–17629. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Xue, G.; Cao, W.; Zhang, Z.; Wang, C.; Li, X. Shape switching of CaCO3-templated nanorods into stiffness-adjustable nanocapsules to promote efficient drug delivery. Acta Biomater. 2021, 128, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Lee, N.; Arifin, D.R.; Shats, I.; Janowski, M.; Walczak, P.; Hyeon, T.; Bulte, J.W.M. In Vivo Micro-CT Imaging of Human Mesenchymal Stem Cells Labeled with Gold-Poly-L-Lysine Nanocomplexes. Adv. Funct. Mater. 2017, 27, 1604213. [Google Scholar] [CrossRef]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Misra, A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone-marrow mesenchymal stem cells for osteoinduction. Int. J. Pharm. 2019, 563, 324–336. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Li, Y.; Li, X. Penetration of nanoparticles across a lipid bilayer: Effects of particle stiffness and surface hydrophobicity. Nanoscale 2019, 11, 4025–4034. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).