Research Progress on Nanomaterials for Tissue Engineering in Oral Diseases
Abstract
1. The Clinical Demand, Development and Delivery Challenges of Nanomaterials for Oral Medicine
2. Nanotechnology Platforms
3. Nanomaterials in Prevention of Oral Diseases
3.1. Prevention of Bacterial Growth in the Mouth
3.2. Prevention of Demineralization and Promotion of Hard Tissue Mineralization to Control Caries
4. Diagnosis of Oral Diseases
5. Disease Treatments and Tissue Engineering Applications
5.1. Oral Cancer Treatment
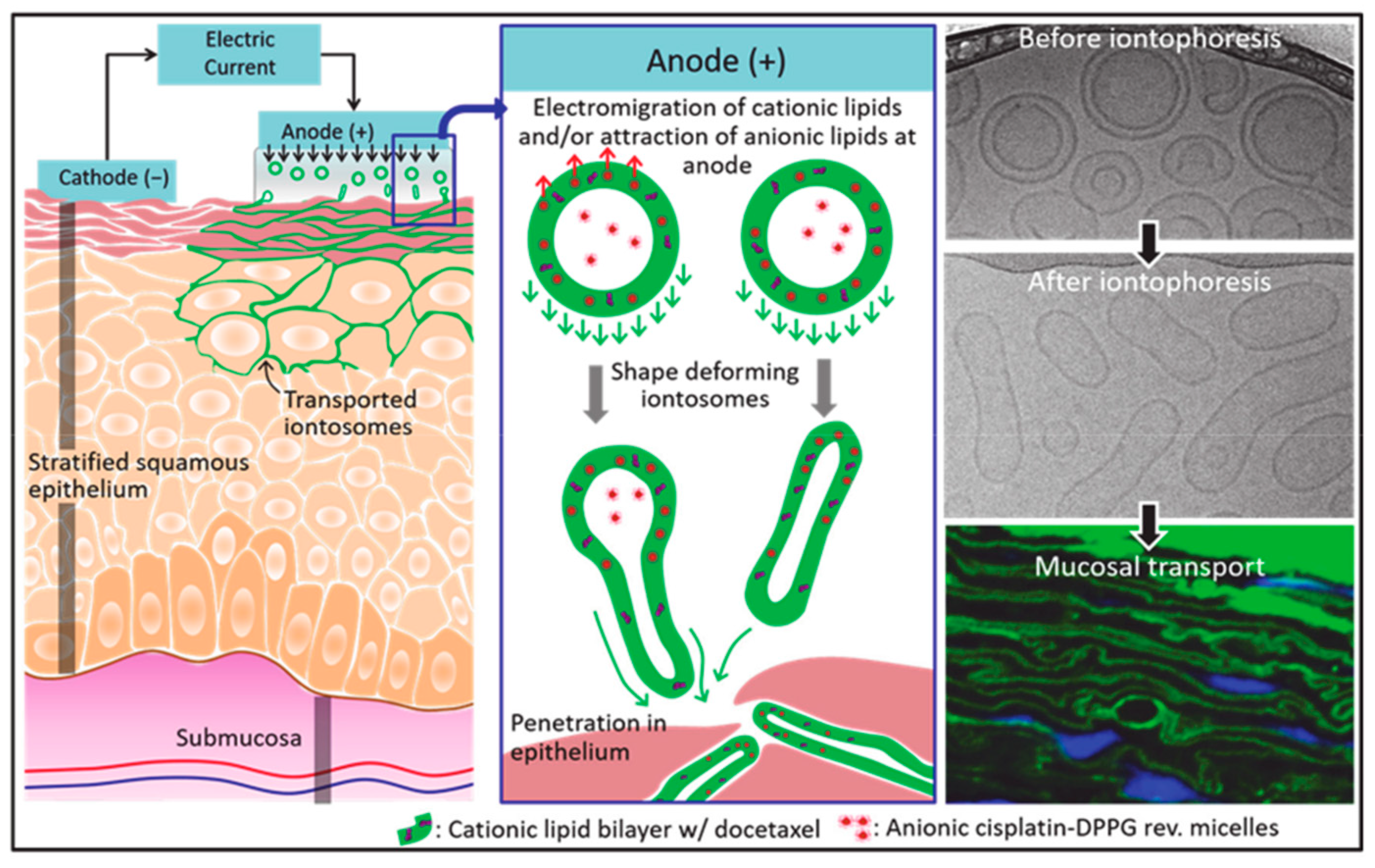
5.2. Treatment of Oral Mucosal Diseases
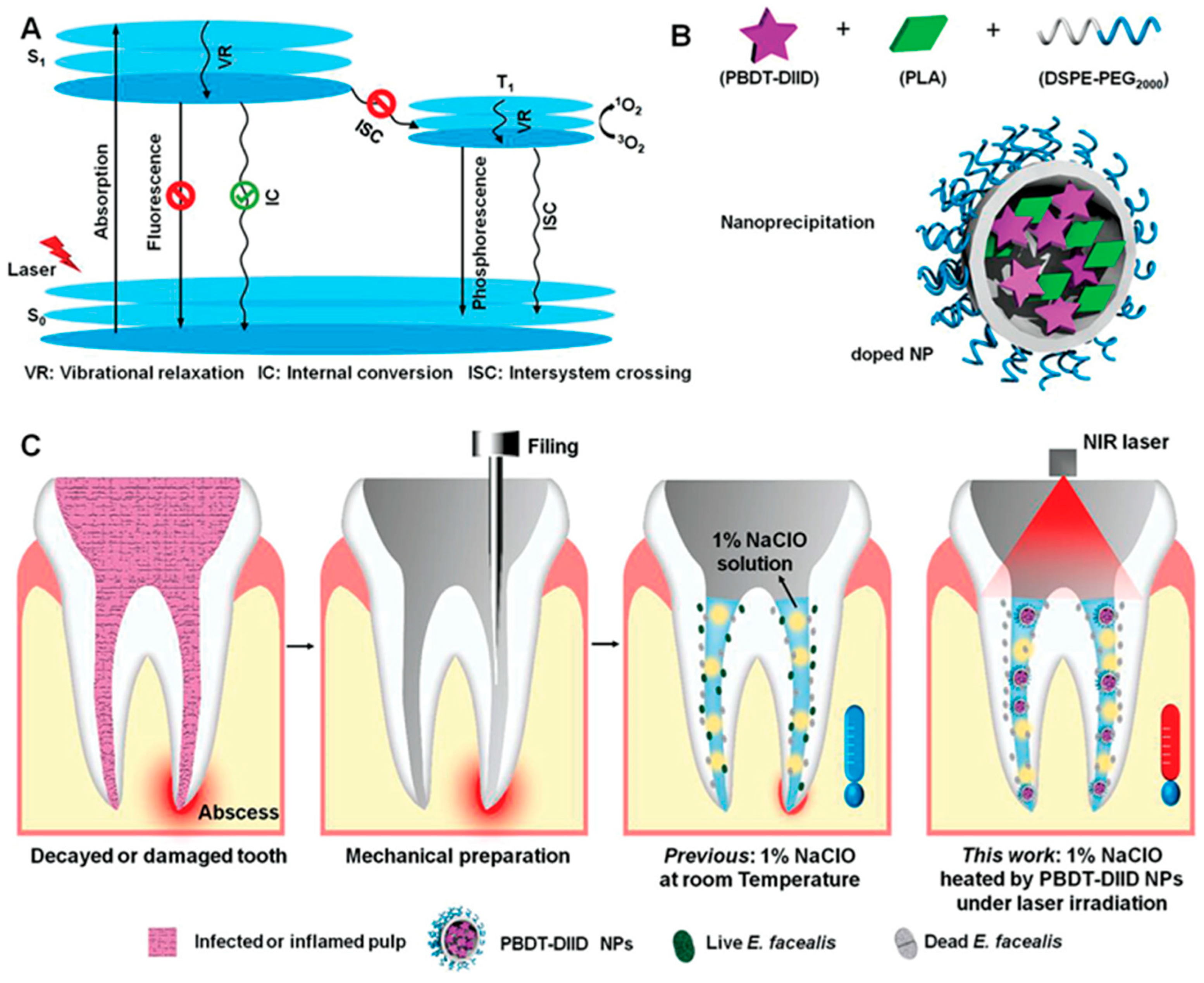
5.3. Treatment of Dental Pulp Disease
5.4. Treatment of Periodontal Disease
5.5. Tissue Engineering Applications
5.5.1. Application in Oral and Maxillofacial Bone Repair
5.5.2. Implant Restoration
5.5.3. Application of 3D Bioprinting Technology in Stomatology
Regeneration of Cranial and Maxillofacial Bone and Cartilage
Periodontal Tissue Regeneration
Regeneration of Pulp Nerve and Blood Vessel
6. Safety and Technical Analyses
6.1. Safety Analyses
6.2. Technical Difficulties Limit Further Developments
7. Discussions and Problems Faced
8. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Jiang, R.; Lei, L.; Yang, Y.; Hu, T. Drug delivery systems for oral disease applications. J. Appl. Oral. Sci. 2022, 30, e20210349. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Dong, J.; Li, W.; Wang, Q.; Chen, J.; Zu, Y.; Zhou, X.; Guo, Q. Relationships Between Oral Microecosystem and Respiratory Diseases. Front. Mol. Biosci. 2022, 8, 718222. [Google Scholar] [CrossRef]
- Nomura, Y.; Okada, A.; Hanada, N. Future Prospective of Oral Microbiome Research. Appl. Sci. 2022, 12, 55. [Google Scholar] [CrossRef]
- Cao, L.; Su, H.; Si, M.; Xu, J.; Chang, X.; Lv, J.; Zhai, Y. Tissue Engineering in Stomatology: A Review of Potential Approaches for Oral Disease Treatments. Front. Bioeng. Biotechnol. 2021, 9, 662418. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Duarte, D.; Panariello, B.H.D.; Santiago, S.L.; Pavarina, A.C. Advances and challenges in oral biofilm control. Curr. Oral Health Rep. 2017, 4, 29–33. [Google Scholar]
- Liu, H.; Jie, Y.; Han, X. 181 Anlyasis of therapeutic effect and safty of PD-1 inhibitors in clinical treatment of oral and maxillofacial malignant tumors. J. ImmunoTherapy Cancer 2020, 8, A108. [Google Scholar] [CrossRef]
- Sanz, M.; Marco Del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef] [PubMed]
- Anusuya, G.S.; Kandasamy, M.; Jacob Raja, S.A.; Sabarinathan, S.; Ravishankar, P.; Kandhasamy, B. Bone morphogenetic proteins: Signaling periodontal bone regeneration and repair. J. Pharm. Bioallied Sci. 2016, 8, S39–S41. [Google Scholar] [CrossRef] [PubMed]
- Abedi, N.; Sajadi-Javan, Z.S.; Kouhi, M.; Ansari, L.; Khademi, A.; Ramakrishna, S. Antioxidant Materials in Oral and Maxillofacial Tissue Regeneration: A Narrative Review of the Literature. Antioxidants 2023, 12, 594. [Google Scholar] [CrossRef]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Cui, H.; You, Y.; Cheng, G.W.; Lan, Z.; Zou, K.L.; Mai, Q.Y.; Han, Y.H.; Chen, H.; Zhao, Y.Y.; Yu, G.T. Advanced materials and technologies for oral diseases. Sci. Technol. Adv. Mater. 2023, 24, 2156257. [Google Scholar] [CrossRef] [PubMed]
- Hugo, F.N.; Kassebaum, N.J.; Marcenes, W.; Bernabé, E. Role of Dentistry in Global Health: Challenges and Research Priorities. J. Dent. Res. 2021, 100, 681–685. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Feng, Z.; Dong, Y.; Zhou, W.; Li, B.; Bai, S.; Zhao, Y. Advanced biomaterials and their potential applications in the treatment of periodontal disease. Crit. Rev. Biotechnol. 2016, 36, 760–775. [Google Scholar] [CrossRef]
- Eid, A.; Mancino, D.; Rekab, M.S.; Haikel, Y.; Kharouf, N. Effectiveness of Three Agents in Pulpotomy Treatment of Permanent Molars with Incomplete Root Development: A Randomized Controlled Trial. Healthcare 2022, 10, 431. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Schwartz, S., Jr. Unmet needs in developing nanoparticles for precision medicine. Nanomedicine 2017, 12, 271–274. [Google Scholar] [CrossRef] [PubMed]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking Down the Barriers to Precision Cancer Nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, K.A.; Dahlman, J.E.; Langer, R.S.; Anderson, D.G. Silencing or stimulation? siRNA delivery and the immune system. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 77–96. [Google Scholar] [CrossRef]
- Jiang, T.; Gonzalez, K.M.; Cordova, L.E.; Lu, J. Nanotechnology-enabled gene delivery for cancer and other genetic diseases. Expert. Opin. Drug. Deliv. 2023, 20, 523–540. [Google Scholar] [CrossRef]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Sen Gupta, A. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Riebensahm, C.; Mohme, M.; Joosse, S.A.; Velthaus, J.L.; Berger, L.A.; Bernreuther, C.; Glatzel, M.; Loges, S.; Lamszus, K.; et al. Frequency of Circulating Tumor Cells (CTC) in Patients with Brain Metastases: Implications as a Risk Assessment Marker in Oligo-Metastatic Disease. Cancers 2018, 10, 527. [Google Scholar] [CrossRef]
- Jiang, T.; Qiao, Y.; Ruan, W.; Zhang, D.; Yang, Q.; Wang, G.; Chen, Q.; Zhu, F.; Yin, J.; Zou, Y.; et al. Cation-Free siRNA Micelles as Effective Drug Delivery Platform and Potent RNAi Nanomedicines for Glioblastoma Therapy. Adv. Mater. 2021, 33, e2104779. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Josic, U.; Delfi, M.; Pinelli, F.; Jahed, V.; Kaya, E.; Ashrafizadeh, M.; Zarepour, A.; Rossi, F.; Zarrabi, A.; et al. Drug Delivery (Nano)Platforms for Oral and Dental Applications: Tissue Regeneration, Infection Control, and Cancer Management. Adv. Sci. 2021, 8, 2004014. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Pasch, S.; Schamberger, T.; Maneck, M.; Mohlendick, B.; Schumacher, S.; Brockhoff, G.; Knoefel, W.T.; Izbicki, J.; Polzer, B.; et al. Diagnostic pathology of early systemic cancer: ERBB2 gene amplification in single disseminated cancer cells determines patient survival in operable esophageal cancer. Int. J. Cancer 2018, 142, 833–843. [Google Scholar] [CrossRef]
- Singh, A.; Trivedi, P.; Jain, N.K. Advances in siRNA delivery in cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 274–283. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Liu, D.Y.; Guo, Z.J. Endogenous Stimuli-responsive Nanocarriers for Drug Delivery. Chem. Lett. 2016, 45, 242–249. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Solans, C. Characterization of Polymeric Nanoparticle Dispersions for Biomedical Applications: Size, Surface Charge and Stability. Pharm. Nanotechnol. 2018, 6, 147–164. [Google Scholar] [CrossRef]
- Madeira, S.; Barbosa, A.; Moura, C.G.; Buciumeanu, M.; Silva, F.S.; Carvalho, O. Aunps and Agμps-functionalized zirconia surfaces by hybrid laser technology for dental implants. Ceram. Int. 2020, 46, 7109–7121. [Google Scholar] [CrossRef]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release Off. J. Control. Release Soc. 2011, 156, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Bozec, L.; Perez, R.A.; Kim, H.W.; Knowles, J.C. Nanotechnology in dentistry: Prevention, diagnosis, and therapy. Int. J. Nanomed. 2015, 10, 6371–6394. [Google Scholar] [CrossRef]
- Friedman, A.J.; Phan, J.; Schairer, D.O.; Champer, J.; Qin, M.; Pirouz, A.; Blecher-Paz, K.; Oren, A.; Liu, P.T.; Modlin, R.L.; et al. Antimicrobial and Anti-Inflammatory Activity of Chitosan–Alginate Nanoparticles: A Targeted Therapy for Cutaneous Pathogens. J. Investig. Dermatol. 2013, 133, 1231–1239. [Google Scholar] [CrossRef]
- Blecher, K.; Nasir, A.; Friedman, A. The growing role of nanotechnology in combating infectious disease. Virulence 2011, 2, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Hannig, C. Nanotechnology and its role in caries therapy. Adv. Dent. Res. 2012, 24, 53–57. [Google Scholar] [CrossRef]
- Lopez-Piriz, R.; Sola-Linares, E.; Rodriguez-Portugal, M.; Malpica, B.; Diaz-Guemes, I.; Enciso, S.; Esteban-Tejeda, L.; Cabal, B.; Granizo, J.J.; Moya, J.S.; et al. Evaluation in a Dog Model of Three Antimicrobial Glassy Coatings: Prevention of Bone Loss around Implants and Microbial Assessments. PLoS ONE 2015, 10, e0140374. [Google Scholar] [CrossRef]
- Botelho, M.A.; Martins, J.G.; Ruela, R.S.; Queiroz, D.B.; Ruela, W.S. Nanotechnology in ligature-induced periodontitis: Protective effect of a doxycycline gel with nanoparticules. J. Appl. Oral. Sci. 2010, 18, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, X.; Huang, X.; Zhu, C.; Weir, M.D.; Melo, M.A.S.; Bonavente, A.; Lynch, C.D.; Imazato, S.; Oates, T.W.; et al. Antibacterial and remineralizing nanocomposite inhibit root caries biofilms and protect root dentin hardness at the margins. J. Dent. 2020, 97, 103344. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M. The oral microbiome—Friend or foe? Eur. J. Oral. Sci. 2018, 126 (Suppl. S1), 5–12. [Google Scholar] [CrossRef]
- Zafar, M.S.; Khurshid, Z.; Almas, K. Oral Tissue Engineering Progress and Challenges. Tissue Eng. Regen. Med. 2015, 12, 387–397. [Google Scholar] [CrossRef]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Sanz, Y.; Rastmanesh, R.; Agostoni, C. Corrigendum to “Understanding the role of gut microbes and probiotics in obesity: How far are we?” [Pharmacol. Res. 69 (2013) 144–155]. Pharmacol. Res. 2013, 71, 69. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Darveau, R.P. Periodontitis: A polymicrobial disruption of host homeostasis. Nat. Rev. Microbiol. 2010, 8, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bisht, B.; Dutta, S.; Paul, M.K. Current advances in the use of exosomes, liposomes, and bioengineered hybrid nanovesicles in cancer detection and therapy. Acta Pharmacol. Sin. 2022, 43, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zhu, Z.D.; Gu, Y.C.; Li, J.; Zhu, W.L.; Guo, Y.W. Further New Diterpenoids as PTP1B Inhibitors from the Xisha Soft Coral Sinularia polydactyla. Mar. Drugs 2018, 16, 103. [Google Scholar] [CrossRef]
- Garcia-Contreras, R.; Argueta-Figueroa, L.; Mejia-Rubalcava, C.; Jimenez-Martinez, R.; Cuevas-Guajardo, S.; Sanchez-Reyna, P.A.; Mendieta-Zeron, H. Perspectives for the use of silver nanoparticles in dental practice. Int. Dent. J. 2011, 61, 297–301. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar]
- Yan, Q.; Zhi, N.; Yang, L.; Xu, G.; Feng, Q.; Zhang, Q.; Sun, S. A highly sensitive uric acid electrochemical biosensor based on a nano-cube cuprous oxide/ferrocene/uricase modified glassy carbon electrode. Sci. Rep. 2020, 10, 10607. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, A.; Della Ventura, B.; Lenyk, B.; Gentile, F.; Tanner, J.A.; Offenhausser, A.; Mayer, D.; Velotta, R. Ultrasensitive antibody-aptamer plasmonic biosensor for malaria biomarker detection in whole blood. Nat. Commun. 2020, 11, 6134. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Vacic, A.; Rajan, N.K.; Criscione, J.M.; Park, J.; Ilic, B.R.; Mooney, D.J.; Reed, M.A.; Fahmy, T.M. Label-free biomarker detection from whole blood. Nat. Nanotechnol. 2010, 5, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, R.; Bhargava, A.; Bunkar, N.; Tiwari, R.; Goryacheva, I.Y.; Mishra, P.K. Nanobiosensors: Point-of-care approaches for cancer diagnostics. Biosens. Bioelectron. 2019, 130, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chu, C.; Cheng, Y.; Zhang, Y.; Pang, X.; Li, D.; Wang, X.; Ren, E.; Xie, F.; Bai, Y.; et al. In Situ Formation of Nanotheranostics to Overcome the Blood-Brain Barrier and Enhance Treatment of Orthotopic Glioma. ACS Appl. Mater. Interfaces 2020, 12, 26880–26892. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, J.C.; Sultan, L.R.; Hunt, S.J.; Gade, T.P.; Karmacharya, M.B.; Schultz, S.M.; Brice, A.K.; Wood, A.K.W.; Sehgal, C.M. Microbubble-enhanced ultrasound for the antivascular treatment and monitoring of hepatocellular carcinoma. Nanotheranostics 2019, 3, 331–341. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Yuan, C.; Li, M.; Wang, T.; Chen, B.; Jin, J.; Zhao, P.; Tong, J.; Luo, S.; et al. Magnetic Nanoliposomes as in Situ Microbubble Bombers for Multimodality Image-Guided Cancer Theranostics. ACS Nano 2017, 11, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Fatima, T.; Khurshid, Z.; Rehman, A.; Imran, E.; Srivastava, K.C.; Shrivastava, D. Gingival Crevicular Fluid (GCF): A Diagnostic Tool for the Detection of Periodontal Health and Diseases. Molecules 2021, 26, 1208. [Google Scholar] [CrossRef]
- Koregol, A.C.; More, S.P.; Nainegali, S.; Kalburgi, N.; Verma, S. Analysis of inorganic ions in gingival crevicular fluid as indicators of periodontal disease activity: A clinico-biochemical study. Contemp. Clin. Dent. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Totu, E.E.; Isildak, I.; Nechifor, A.C.; Cristache, C.M.; Enachescu, M. New sensor based on membranes with magnetic nano-inclusions for early diagnosis in periodontal disease. Biosens. Bioelectron. 2018, 102, 336–344. [Google Scholar] [CrossRef]
- Abiodun-Solanke, I.; Ajayi, D.; Arigbede, A. Nanotechnology and its application in dentistry. Ann. Med. Health Sci. Res. 2014, 4, S171–S177. [Google Scholar] [CrossRef]
- Shetty, N.J.; Swati, P.; David, K. Nanorobots: Future in dentistry. Saudi Dent. J. 2013, 25, 49–52. [Google Scholar] [CrossRef]
- Datta, M.; Desai, D.; Kumar, A. Gene Specific DNA Sensors for Diagnosis of Pathogenic Infections. Indian J. Microbiol. 2017, 57, 139–147. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Bhardwaj, A.; Misuriya, A.; Maroli, S.; Manjula, S.; Singh, A.K. Nanotechnology in dentistry: Present and future. J. Int. Oral. Health 2014, 6, 121–126. [Google Scholar]
- Ankri, R.; Ashkenazy, A.; Milstein, Y.; Brami, Y.; Olshinka, A.; Goldenberg-Cohen, N.; Popovtzer, A.; Fixler, D.; Hirshberg, A. Gold Nanorods Based Air Scanning Electron Microscopy and Diffusion Reflection Imaging for Mapping Tumor Margins in Squamous Cell Carcinoma. ACS Nano 2016, 10, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Sudri, S.; Duadi, H.; Altman, F.; Allon, I.; Ashkenazy, A.; Chakraborty, R.; Novikov, I.; Fixler, D.; Hirshberg, A. Diffusion Reflection Method for Early Detection of Oral Squamous Cell Carcinoma Specifically Targeted by Circulating Gold-Nanorods Bio-Conjugated to Anti-Epidermal Growth Factor Receptor. Int. J. Nanomed. 2021, 16, 2237–2246. [Google Scholar] [CrossRef]
- Amit, D.; Hochberg, A. Development of targeted therapy for bladder cancer mediated by a double promoter plasmid expressing diphtheria toxin under the control of H19 and IGF2-P4 regulatory sequences. J. Transl. Med. 2010, 8, 134. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Mao, C.P.; He, L.; Tsai, Y.C.; Liu, K.; La, V.; Wu, T.C.; Hung, C.F. Tumor-targeted delivery of IL-2 by NKG2D leads to accumulation of antigen-specific CD8+ T cells in the tumor loci and enhanced anti-tumor effects. PLoS ONE 2012, 7, e35141. [Google Scholar] [CrossRef]
- Wei, L.; Wen, X.S.; Xian, C.J. Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.R.; Steinmetz, N.F.; Zhu, H. New Directions for Drug Delivery in Cancer Therapy. Mol. Pharm. 2018, 15, 3601–3602. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, T.; Yang, W.; Zou, Y.; Wu, H.; Liu, X.; Zhu, F.; Qian, R.; Ling, D.; McDonald, K.; et al. The siRNAsome: A cation-free and versatile nanostructure for siRNA and drug co-delivery. Angew. Chem. 2019, 58, 4938–4942. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Zhu, B.; Xu, Q. Development of a multifunctional gold nanoplatform for combined chemo-photothermal therapy against oral cancer. Nanomedicine 2020, 15, 661–676. [Google Scholar] [CrossRef]
- Su, J.; Lu, S.; Jiang, S.; Li, B.; Liu, B.; Sun, Q.; Li, J.; Wang, F.; Wei, Y. Engineered Protein Photo-Thermal Hydrogels for Outstanding In Situ Tongue Cancer Therapy. Adv. Mater. 2021, 33, e2100619. [Google Scholar] [CrossRef]
- Schulz, D.; Streller, M.; Piendl, G.; Brockhoff, G.; Reichert, T.E.; Menevse, A.N.; Beckhove, P.; Hautmann, M.G.; Bauer, R.J.; Ettl, T. Differential localization of PD-L1 and Akt-1 involvement in radioresistant and radiosensitive cell lines of head and neck squamous cell carcinoma. Carcinogenesis 2020, 41, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, N.; Mizuno-Kamiya, M.; Umemura, N.; Takayama, E.; Kawaki, H.; Mitsudo, K.; Muramatsu, Y.; Sumitomo, S. Immunomodulatory aspects in the progression and treatment of oral malignancy. Jpn. Dent. Sci. Rev. 2019, 55, 113–120. [Google Scholar] [CrossRef]
- Lenouvel, D.; Gonzalez-Moles, M.A.; Talbaoui, A.; Ramos-Garcia, P.; Gonzalez-Ruiz, L.; Ruiz-Avila, I.; Gil-Montoya, J.A. An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral. Dis. 2020, 26, 511–526. [Google Scholar] [CrossRef]
- Naruse, T.; Yanamoto, S.; Okuyama, K.; Ohmori, K.; Tsuchihashi, H.; Furukawa, K.; Yamada, S.I.; Umeda, M. Immunohistochemical Study of PD-1/PD-L1 Axis Expression in Oral Tongue Squamous Cell Carcinomas: Effect of Neoadjuvant Chemotherapy on Local Recurrence. Pathol. Oncol. Res. 2020, 26, 735–742. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; Porta, C.L.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef] [PubMed]
- Sonaje, K.; Tyagi, V.; Chen, Y.; Kalia, Y.N. Iontosomes: Electroresponsive Liposomes for Topical Iontophoretic Delivery of Chemotherapeutics to the Buccal Mucosa. Pharmaceutics 2021, 13, 88. [Google Scholar] [CrossRef] [PubMed]
- Bilginaylar, K.; Aykac, A.; Sayiner, S.; Ozkayalar, H.; Sehirli, A.O. Evaluation of the antiapoptotic and anti-inflammatory properties of chitosan in methotrexate-induced oral mucositis in rats. Mol. Biol. Rep. 2022, 49, 3237–3245. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, S.H.; Hyun, C.; Yoo, H.S. Buccal adhesive nanofibers containing human growth hormone for oral mucositis. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Potrč, T.; Baumgartner, S.; Roškar, R.; Planinšek, O.; Lavrič, Z.; Kristl, J.; Kocbek, P. Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur. J. Pharm. Sci. 2015, 75, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Enkel, B.; Dupas, C.; Armengol, V.; Akpe Adou, J.; Bosco, J.; Daculsi, G.; Jean, A.; Laboux, O.; LeGeros, R.Z.; Weiss, P. Bioactive materials in endodontics. Expert Rev. Med. Devices 2008, 5, 475–494. [Google Scholar] [CrossRef]
- Keller, L.; Offner, D.; Schwinté, P.; Morand, D.; Wagner, Q.; Gros, C.; Bornert, F.; Bahi, S.; Musset, A.-M.; Benkirane-Jessel, N.; et al. Active Nanomaterials to Meet the Challenge of Dental Pulp Regeneration. Materials 2015, 8, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Yuan, Z.; Wang, Y.; Wan, Z.; Wang, X.; Yu, S.; Han, J.; Huang, J.; Xiong, C.; Ge, L.; et al. Vascularized pulp regeneration via injecting simvastatin functionalized GelMA cryogel microspheres loaded with stem cells from human exfoliated deciduous teeth. Mater. Today Bio 2022, 13, 100209. [Google Scholar] [CrossRef]
- Yi, B.; Ding, T.; Jiang, S.; Gong, T.; Chopra, H.; Sha, O.; Dissanayaka, W.L.; Ge, S.; Zhang, C. Conversion of stem cells from apical papilla into endothelial cells by small molecules and growth factors. Stem Cell. Res. Ther. 2021, 12, 266. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, Q.; Jiang, Y.; Wu, X.; Yue, X.; Geng, Y.; Shen, J.; Ding, D. Semiconducting Polymer Nanoparticles with Intramolecular Motion-Induced Photothermy for Tumor Phototheranostics and Tooth Root Canal Therapy. Adv. Mater. 2022, 34, e2200179. [Google Scholar] [CrossRef]
- Baras, B.H.; Melo, M.A.S.; Sun, J.; Oates, T.W.; Weir, M.D.; Xie, X.; Bai, Y.; Xu, H.H.K. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent. Mater. 2019, 35, 1117–1129. [Google Scholar] [CrossRef]
- Imura, K.; Hashimoto, Y.; Okada, M.; Yoshikawa, K.; Yamamoto, K. Application of hydroxyapatite nanoparticle-assembled powder using basic fibroblast growth factor as a pulp-capping agent. Dent. Mater. J. 2019, 38, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Sugii, H.; Itoyama, T.; Kadowaki, M.; Hasegawa, D.; Tomokiyo, A.; Hamano, S.; Ipposhi, K.; Yamashita, K.; Maeda, H. Development of a novel direct dental pulp-capping material using 4-META/MMA-TBB resin with nano hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112426. [Google Scholar] [CrossRef]
- Bao, X.; Zhao, J.; Sun, J.; Hu, M.; Yang, X. Polydopamine Nanoparticles as Efficient Scavengers for Reactive Oxygen Species in Periodontal Disease. ACS Nano 2018, 12, 8882–8892. [Google Scholar] [CrossRef]
- Hirschfeld, J.; White, P.C.; Milward, M.R.; Cooper, P.R.; Chapple, I.L.C. Modulation of Neutrophil Extracellular Trap and Reactive Oxygen Species Release by Periodontal Bacteria. Infect. Immun. 2017, 85, e00297-17. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Li, J.; Cochran, D.L. Inflammation and uncoupling as mechanisms of periodontal bone loss. J. Dent. Res. 2011, 90, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Preem, L.; Kristl, J.; Putrinš, M.; Tenson, T.; Kocbek, P.; Kogermann, K. Impact of PCL nanofiber mat structural properties on hydrophilic drug release and antibacterial activity on periodontal pathogens. Eur. J. Pharm. Sci. 2018, 122, 347–358. [Google Scholar] [CrossRef]
- Beck, J.D.; Papapanou, P.N.; Philips, K.H.; Offenbacher, S. Periodontal Medicine: 100 Years of Progress. J. Dent. Res. 2019, 98, 1053–1062. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol. 2000 2020, 83, 213–233. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontol. 2000 2020, 84, 202–214. [Google Scholar] [CrossRef]
- Jain, P.; Mirza, M.A.; Iqbal, Z. A 4-D approach for amelioration of periodontitis. Med. Hypotheses 2019, 133, 109392. [Google Scholar] [CrossRef]
- Jain, P.; Mirza, M.A.; Talegaonkar, S.; Nandy, S.; Dudeja, M.; Sharma, N.; Anwer, M.K.; Alshahrani, S.M.; Iqbal, Z. Design and in vitro/in vivo evaluations of a multiple-drug-containing gingiva disc for periodontotherapy. RSC Adv. 2020, 10, 8530–8538. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nanomicro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Zhang, Z.; Zhang, X.; Saunders, L.; Zhou, Y.; Ma, P.X. Nanofibrous Spongy Microspheres To Distinctly Release miRNA and Growth Factors To Enrich Regulatory T Cells and Rescue Periodontal Bone Loss. ACS Nano 2018, 12, 9785–9799. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Nuzzo, M.; Yang, X.; Yang, Y.; Zhang, X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials 2018, 182, 279–288. [Google Scholar] [CrossRef]
- Mitsiadis, T.A.; Woloszyk, A.; Jiménez-Rojo, L. Nanodentistry: Combining nanostructured materials and stem cells for dental tissue regeneration. Nanomedicine 2012, 7, 1743–1753. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef]
- Dejan, M.; Bojan, P.; Vukoman, J.; Tamara, P.; Bozana, C.; Ivana, K. Nanomaterials as scaffolds in bone tissue engineering in dental medicine. Appl. Nanobiomater. 2016, 4, 413–442. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Zhai, Y.; Schilling, K.; Wang, T.; El Khatib, M.; Vinogradov, S.; Brown, E.B.; Zhang, X. Spatiotemporal blood vessel specification at the osteogenesis and angiogenesis interface of biomimetic nanofiber-enabled bone tissue engineering. Biomaterials 2021, 276, 121041. [Google Scholar] [CrossRef]
- Beaman, F.D.; Bancroft, L.W.; Peterson, J.J.; Kransdorf, M.J. Bone graft materials and synthetic substitutes. Radiol. Clin. N. Am. 2006, 44, 451–461. [Google Scholar] [CrossRef]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Ma, P.; Sutrisno, L.; Luo, Z.; Hu, Y.; Yu, Y.; Tao, B.; Li, C.; Cai, K. Fabrication of magnesium/zinc-metal organic framework on titanium implants to inhibit bacterial infection and promote bone regeneration. Biomaterials 2019, 212, 1–16. [Google Scholar] [CrossRef]
- Pan, Y.; Zhao, Y.; Kuang, R.; Liu, H.; Sun, D.; Mao, T.; Jiang, K.; Yang, X.; Watanabe, N.; Mayo, K.H.; et al. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111158. [Google Scholar] [CrossRef]
- Hu, H.; Wang, D.; Li, L.; Yin, H.; He, G.; Zhang, Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell. Death Dis. 2021, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Meng, C.X.; Lv, Z.Y.; Zhang, Y.J.; Li, J.; Li, K.Y.; Liu, F.Z.; Zhang, B.; Cui, F.Z. Enhancement of BMP-2 and VEGF carried by mineralized collagen for mandibular bone regeneration. Regen. Biomater. 2020, 7, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual Delivery of Alendronate and E7-BMP-2 Peptide via Calcium Chelation to Mineralized Nanofiber Fragments for Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143s–152s. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Zhai, Y.; Zhou, Z.; Zhou, B.; Brown, E.; Zhang, X. High-resolution imaging of the osteogenic and angiogenic interface at the site of murine cranial bone defect repair via multiphoton microscopy. Elife 2022, 11, e83146. [Google Scholar] [CrossRef]
- Smith, B.T.; Shum, J.; Wong, M.; Mikos, A.G.; Young, S. Bone Tissue Engineering Challenges in Oral & Maxillofacial Surgery. Adv. Exp. Med. Biol. 2015, 881, 57–78. [Google Scholar] [CrossRef]
- Perez, R.A.; Ginebra, M.P. Injectable collagen/α-tricalcium phosphate cement: Collagen-mineral phase interactions and cell response. J. Mater. Sci. Mater. Med. 2013, 24, 381–393. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Sun, X.; Zhan, C.; Li, Z.; Qiu, L.; Luo, R.; Liu, H.; Sun, X.; Li, R.; et al. Silk Fibroin/Collagen/Hydroxyapatite Scaffolds Obtained by 3D Printing Technology and Loaded with Recombinant Human Erythropoietin in the Reconstruction of Alveolar Bone Defects. ACS Biomater. Sci. Eng. 2022, 8, 5245–5256. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40. [Google Scholar] [CrossRef]
- Rajapakse, S.; Ahmed, N.; Sidebottom, A.J. Current thinking about the management of dysfunction of the temporomandibular joint: A review. Br. J. Oral. Maxillofac. Surg. 2017, 55, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Park, H.K.; Auh, Q.S.; Nah, H.; Lee, J.S.; Moon, H.J.; Heo, D.N.; Kim, I.S.; Kwon, I.K. Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1541. [Google Scholar] [CrossRef]
- Cui, D.; Li, H.; Xu, X.; Ye, L.; Zhou, X.; Zheng, L.; Zhou, Y. Mesenchymal Stem Cells for Cartilage Regeneration of TMJ Osteoarthritis. Stem Cells Int. 2017, 2017, 5979741. [Google Scholar] [CrossRef] [PubMed]
- Heirani-Tabasi, A.; Hosseinzadeh, S.; Rabbani, S.; Ahmadi Tafti, S.H.; Jamshidi, K.; Soufizomorrod, M.; Soleimani, M. Cartilage tissue engineering by co-transplantation of chondrocyte extracellular vesicles and mesenchymal stem cells, entrapped in chitosan-hyaluronic acid hydrogel. Biomed. Mater. 2021, 16, 055003. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Z.; Wu, H.; Li, W.; Zheng, L.; Zhao, J. Collagen-alginate as bioink for three-dimensional (3D) cell printing based cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Daghrery, A.; Ferreira, J.A.; de Souza Araújo, I.J.; Clarkson, B.H.; Eckert, G.J.; Bhaduri, S.B.; Malda, J.; Bottino, M.C. A Highly Ordered, Nanostructured Fluorinated CaP-Coated Melt Electrowritten Scaffold for Periodontal Tissue Regeneration. Adv. Healthc. Mater. 2021, 10, e2101152. [Google Scholar] [CrossRef]
- Latimer, J.M.; Maekawa, S.; Yao, Y.; Wu, D.T.; Chen, M.; Giannobile, W.V. Regenerative Medicine Technologies to Treat Dental, Oral, and Craniofacial Defects. Front. Bioeng. Biotechnol. 2021, 9, 704048. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Sowmya, S.; Mony, U.; Jayachandran, P.; Reshma, S.; Kumar, R.A.; Arzate, H.; Nair, S.V.; Jayakumar, R. Tri-Layered Nanocomposite Hydrogel Scaffold for the Concurrent Regeneration of Cementum, Periodontal Ligament, and Alveolar Bone. Adv. Healthc. Mater. 2017, 6, 1601251. [Google Scholar] [CrossRef]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef]
- Ganguly, A.; Ian, C.K.; Sheshala, R.; Sahu, P.S.; Al-Waeli, H.; Meka, V.S. Application of diverse natural polymers in the design of oral gels for the treatment of periodontal diseases. J. Mater. Sci. Mater. Med. 2017, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Chao, P.G.; Tai, W.C.; Chang, P.C. 3D-Printed Collagen-Based Waveform Microfibrous Scaffold for Periodontal Ligament Reconstruction. Int. J. Mol. Sci. 2021, 22, 7725. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Fawzy El-Sayed, K.M.; Elsalawy, R.; Ibrahim, N.; Gadalla, M.; Albargasy, H.; Zahra, N.; Mokhtar, S.; El Nahhas, N.; El Kaliouby, Y.; Dörfer, C.E. The Dental Pulp Stem/Progenitor Cells-Mediated Inflammatory-Regenerative Axis. Tissue Eng. Part. B Rev. 2019, 25, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Yu, V.S.H.; Lim, K.C.; Tan, B.C.K.; Neo, C.L.J.; Shen, L.; Messer, H.H. Long-term Pulpal and Restorative Outcomes of Pulpotomy in Mature Permanent Teeth. J. Endod. 2020, 46, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef]
- Moreira, M.S.; Sarra, G.; Carvalho, G.L.; Gonçalves, F.; Caballero-Flores, H.V.; Pedroni, A.C.F.; Lascala, C.A.; Catalani, L.H.; Marques, M.M. Physical and Biological Properties of a Chitosan Hydrogel Scaffold Associated to Photobiomodulation Therapy for Dental Pulp Regeneration: An In Vitro and In Vivo Study. Biomed. Res. Int. 2021, 2021, 6684667. [Google Scholar] [CrossRef]
- El Ashiry, E.A.; Alamoudi, N.M.; El Ashiry, M.K.; Bastawy, H.A.; El Derwi, D.A.; Atta, H.M. Tissue Engineering of Necrotic Dental Pulp of Immature Teeth with Apical Periodontitis in Dogs: Radiographic and Histological Evaluation. J. Clin. Pediatr. Dent. 2018, 42, 373–382. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Song, W.; Pan, T.; Wang, H.; Ning, T.; Wei, Q.; Xu, H.H.K.; Wu, B.; Ma, D. Effects of 3-dimensional Bioprinting Alginate/Gelatin Hydrogel Scaffold Extract on Proliferation and Differentiation of Human Dental Pulp Stem Cells. J. Endod. 2019, 45, 706–715. [Google Scholar] [CrossRef]
- Patil, M.; Mehta, D.S.; Guvva, S. Future impact of nanotechnology on medicine and dentistry. J. Indian Soc. Periodontol. 2008, 12, 34–40. [Google Scholar] [CrossRef]
- Izzetti, R.; Gennai, S.; Nisi, M.; Gulia, F.; Miceli, M.; Giuca, M.R. Clinical Applications of Nano-Hydroxyapatite in Dentistry. Appl. Sci. 2022, 12, 10762. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Biological and Medical Applications of Calcium Phosphate Nanoparticles. Chemistry 2021, 27, 7471–7488. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral. Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative Evaluation of Effect of Nano-hydroxyapatite and 8% Arginine Containing Toothpastes in Managing Dentin Hypersensitivity: Double Blind Randomized Clinical Trial. Acta Med. 2017, 60, 114–119. [Google Scholar] [CrossRef]
- Malekpour, B.; Ajami, S.; Salehi, P.; Hamedani, S. Use of nano-hydroxyapatite serum and different finishing/polishing techniques to reduce enamel staining of debonding after orthodontic treatment: A randomized clinical trial. J. Orofac. Orthop. 2022, 83, 205–214. [Google Scholar] [CrossRef]
- Khaled, H.; Atef, M.; Hakam, M. Maxillary sinus floor elevation using hydroxyapatite nano particles vs tenting technique with simultaneous implant placement: A randomized clinical trial. Clin. Implant. Dent. Relat. Res. 2019, 21, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Gelinsky, M.; Worch, H.; Hanke, T. Resorbable bone substitution materials: An overview of commercially available materials and new approaches in the field of composites. Orthopade 2011, 40, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Kaur, H.; Jain, S.; Kapoor, D.; Nanda, T.; Jain, M. Comparison of Nano-Sized Hydroxyapatite and β-Tricalcium Phosphate in the Treatment of Human Periodontal Intrabony Defects. J. Clin. Diagn. Res. 2014, 8, ZC74–ZC78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, L.; Liu, X.; Jiang, X.; Yu, Z.; Zhao, D.; Zhang, L.; Zhang, C.; Huang, F. In vitro and in vivo evaluation of xenogeneic bone putty with the carrier of hydrogel derived from demineralized bone matrix. Cell Tissue Bank. 2018, 19, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Soicher, M.A.; Christiansen, B.A.; Stover, S.M.; Leach, J.K.; Yellowley, C.E.; Griffiths, L.G.; Fyhrie, D.P. Remineralized bone matrix as a scaffold for bone tissue engineering. J. Biomed. Mater. Res. A 2014, 102, 4480–4490. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shi, J.; Sun, J.; Zhang, W.; Liang, H.; Shi, Q.; Li, X.; Chen, Y.; Zhuang, Y.; Dai, J. Demineralized Bone Matrix Scaffolds Modified by CBD-SDF-1α Promote Bone Regeneration via Recruiting Endogenous Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 27511–27522. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef] [PubMed]
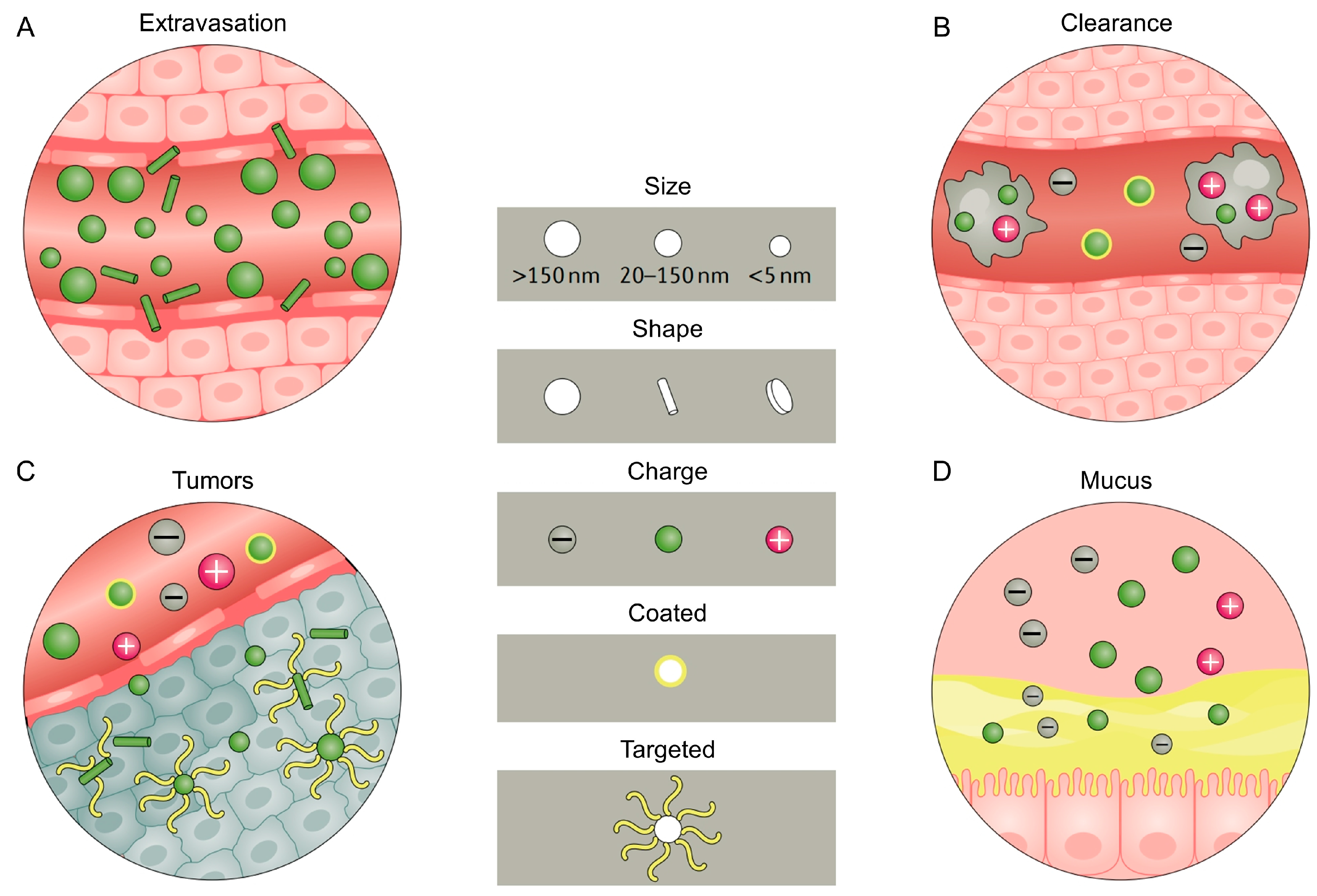



| Disease Intervention Types | Regulation Types | Nanomaterial Types (Part) | Materials (Part) | Refs. | |
|---|---|---|---|---|---|
| Nanomaterials in prevention of oral diseases | Prevention of bacterial growth | Metals, polymers, hybrid nanomaterials, nanocomposites | Au, Ag, Zn NPs, ZnG, chitosan, dextran | [35,37,40] | |
| Demineralization prevention and tissue mineralization promotion | inorganic systems, nanogels, hybrid nanomaterials | Ag, CaF2, NACP, Si/Ca/SrNPs | [43,46] | ||
| Diagnosis of oral diseases | Polymers, inorganic systems, nanocomposites, quantum dots | Au, Fe3O4 CdTe, ZnS, CdSe | [58,59,63,68] | ||
| Oral disease treatments | Oral cancer treatment | Metal/polymer NPs, vesicle/micelle, lipid-based NPs, hydrogels, nanogels, dendrimers, exosomes | Au, Ag, silica, Fe3O4, PLGA, PLL, dextran, chitosan, liposomes | [81,83,84,86] | |
| Treatment of oral mucosal diseases | Metal/polymer NPs, ionizable lipid NPs, nano-emulsifiers, suspensions, nanofibers, nanogels | liposomes, PLGA, PNIPAM, dextran, PCL | [90,91,93] | ||
| Treatment of dental pulp disease | Metal/polymer NPs, hybrid nanomaterials, nanofibers, nanogels | Au, Ag, NACP | [95,96,98,99] | ||
| Treatment of periodontal disease | Metals, polymers, hybrid nanomaterials, nanocomposites | Ag, Zn+, PLA, PEG, PLGA | [103,107,112,114] | ||
| Tissue engineering applications | Application in oral and maxillofacial bone repair | Metals, bioactive ceramics, natural polymers, synthetic polymers, hydrogels, nanogels, nanofibers, hybrid nanomaterials, nanocomposites | HA, collagen, PLGA, chitosan, gelatin, hydroxyapatite | [117,120,121,129,130] | |
| Implant restoration | |||||
| 3D printing technology | Regeneration of cranial and maxillofacial bone and cartilage | 3D-printed scaffolds, tissue/organ analogue materials, polylactic acid–collagen scaffolds | Polylactic acid, collagen, chitosan, HA | [132,133,137,138,148,150,152,155] | |
| Periodontal tissue regeneration | |||||
| Regeneration of pulp nerve and blood vessel | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, T.; Su, W.; Li, Y.; Jiang, M.; Zhang, Y.; Xian, C.J.; Zhai, Y. Research Progress on Nanomaterials for Tissue Engineering in Oral Diseases. J. Funct. Biomater. 2023, 14, 404. https://doi.org/10.3390/jfb14080404
Jiang T, Su W, Li Y, Jiang M, Zhang Y, Xian CJ, Zhai Y. Research Progress on Nanomaterials for Tissue Engineering in Oral Diseases. Journal of Functional Biomaterials. 2023; 14(8):404. https://doi.org/10.3390/jfb14080404
Chicago/Turabian StyleJiang, Tong, Wen Su, Yan Li, Mingyuan Jiang, Yonghong Zhang, Cory J. Xian, and Yuankun Zhai. 2023. "Research Progress on Nanomaterials for Tissue Engineering in Oral Diseases" Journal of Functional Biomaterials 14, no. 8: 404. https://doi.org/10.3390/jfb14080404
APA StyleJiang, T., Su, W., Li, Y., Jiang, M., Zhang, Y., Xian, C. J., & Zhai, Y. (2023). Research Progress on Nanomaterials for Tissue Engineering in Oral Diseases. Journal of Functional Biomaterials, 14(8), 404. https://doi.org/10.3390/jfb14080404










