Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces
Abstract
:1. Introduction
2. Materials and Methods
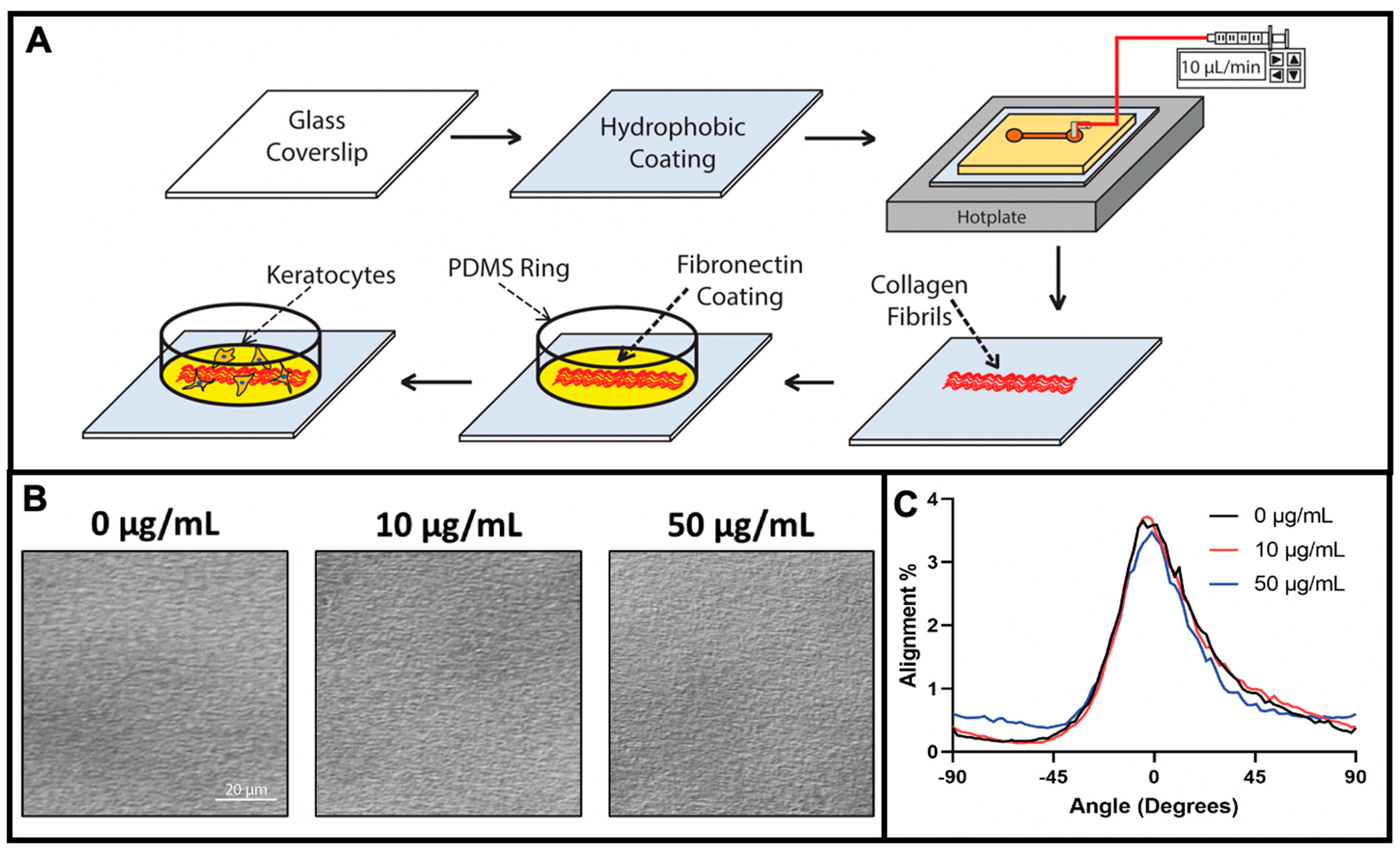
2.1. Fabrication of Aligned Collagen Fibrils
2.2. Fabrication and Coating of PDMS Ring—Microwell Culture System
2.3. Primary Keratocyte Extraction and Cell Culture
2.4. Immunohistochemistry
2.5. Keratocyte Alignment
2.6. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of Fibronectin Coated Aligned Collagen Fibrils
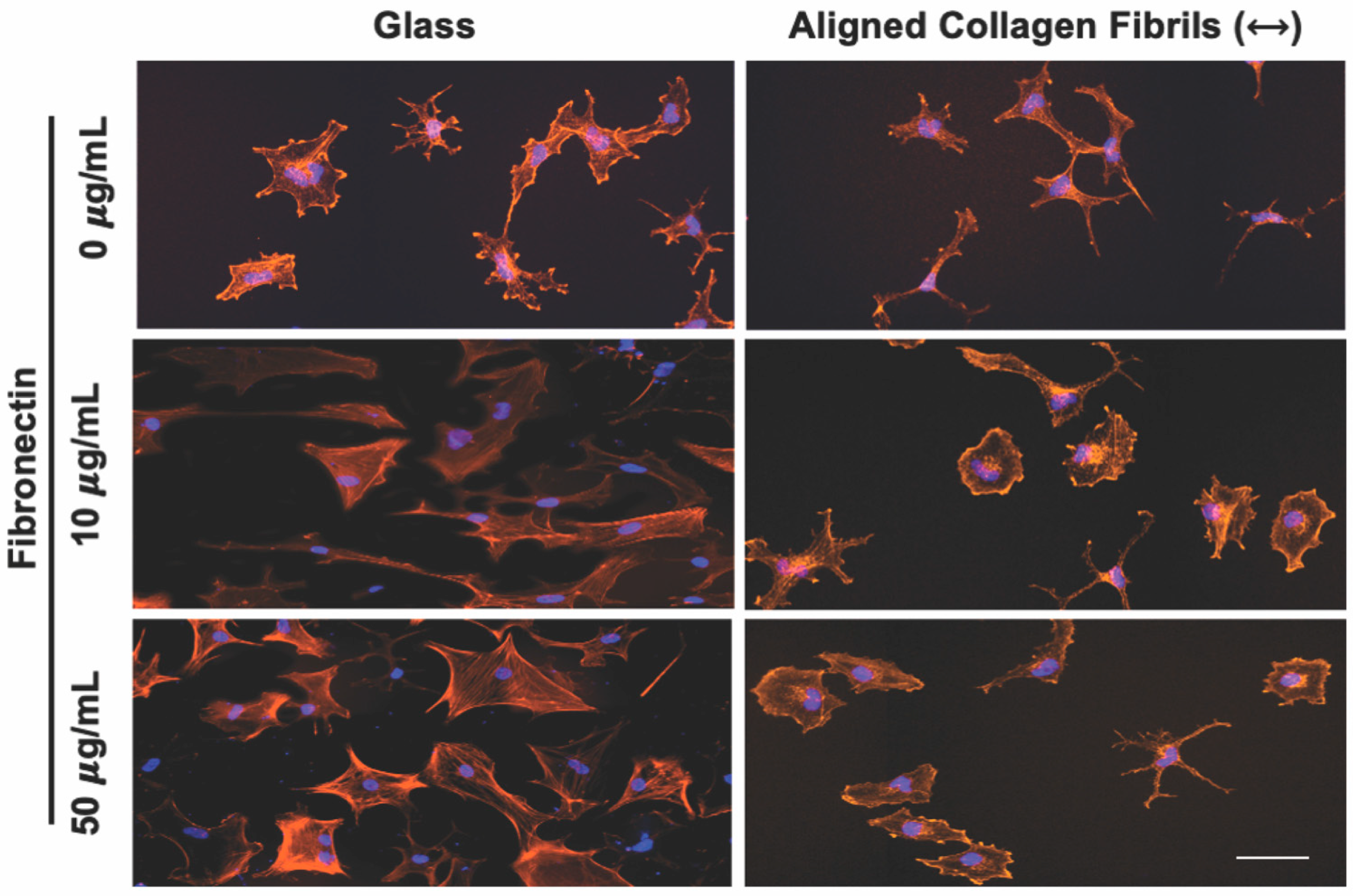
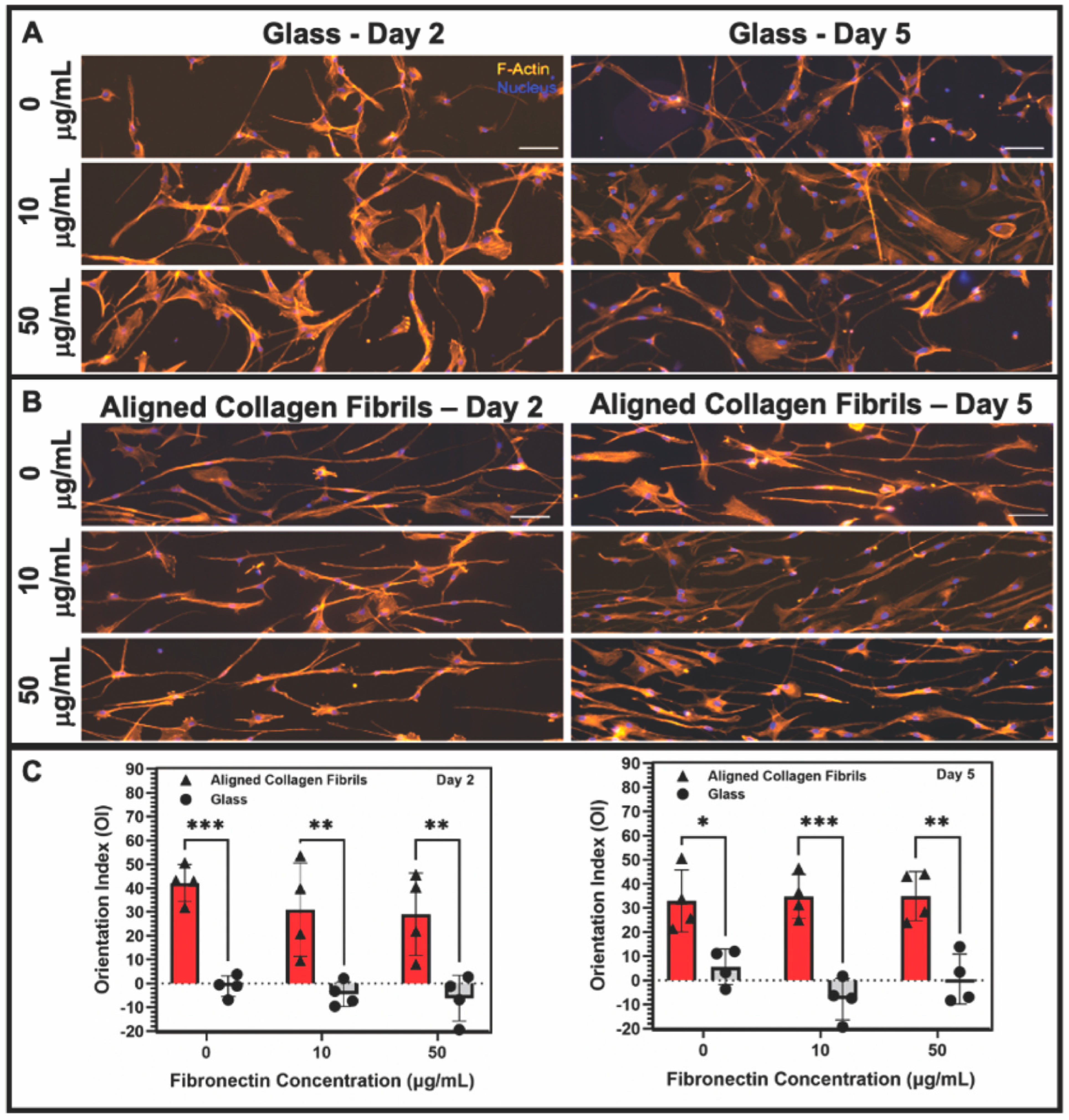
3.2. Effect of Topographical and ECM Cues on Keratocyte Behavior in the Absence of Growth Factors
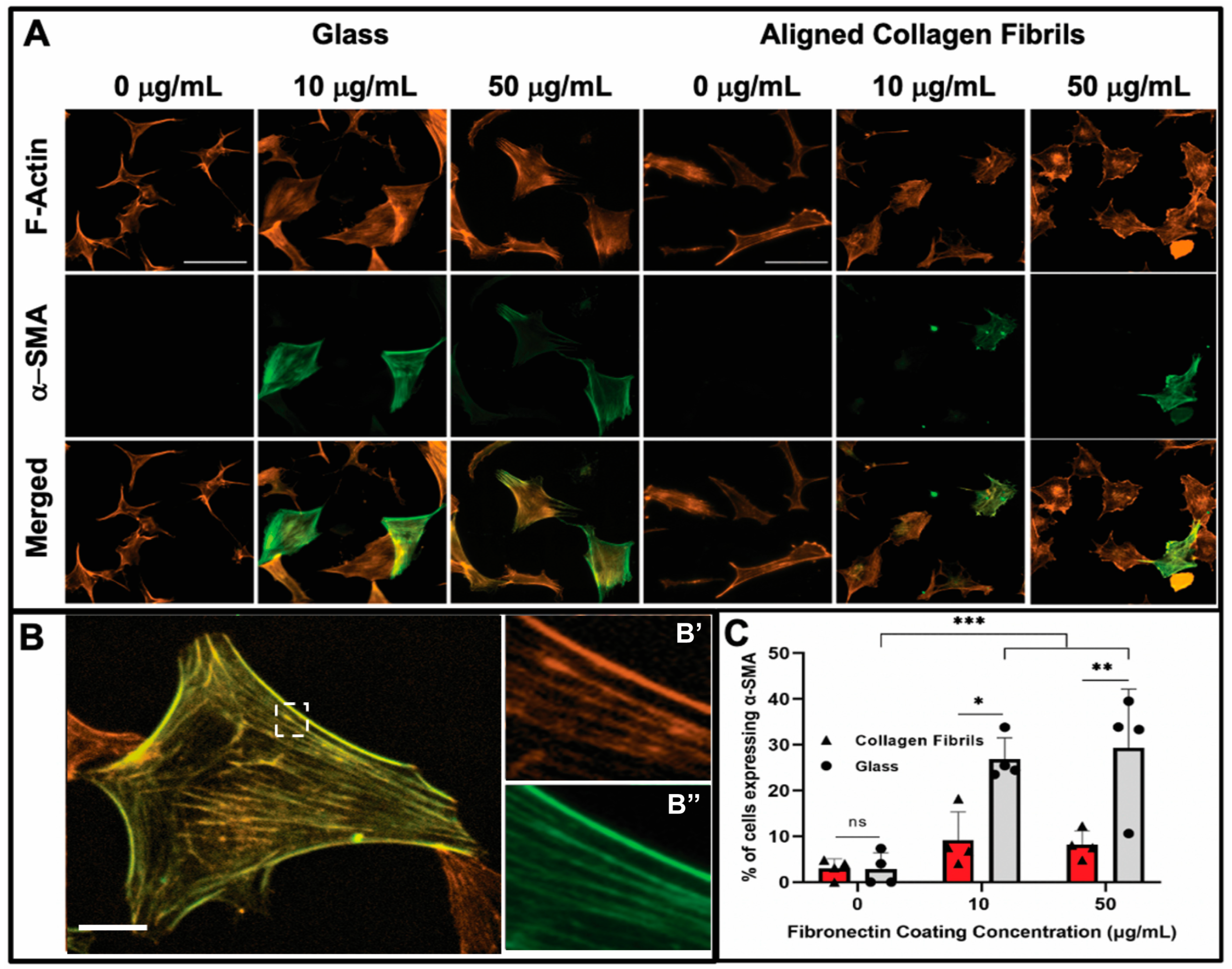
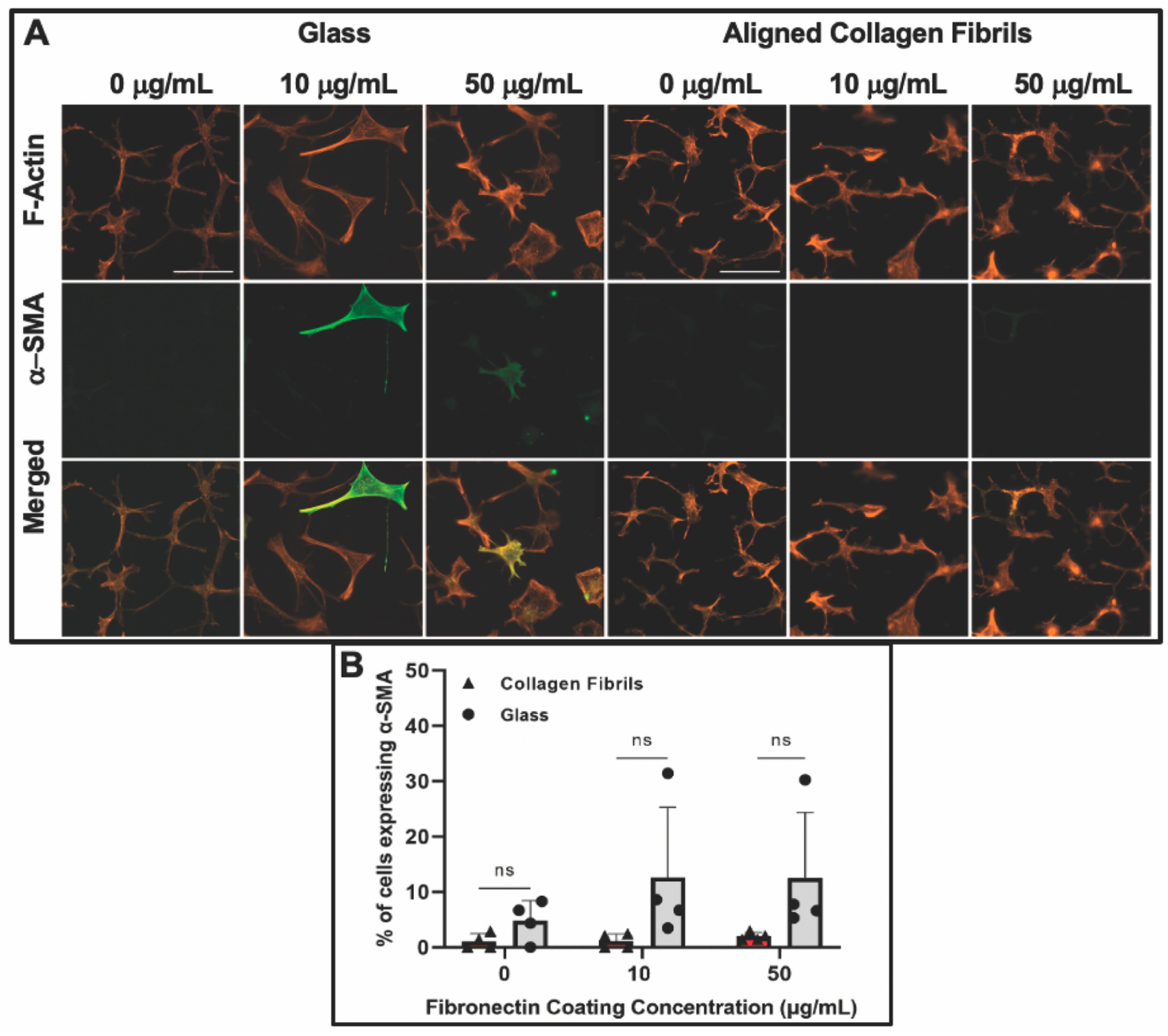
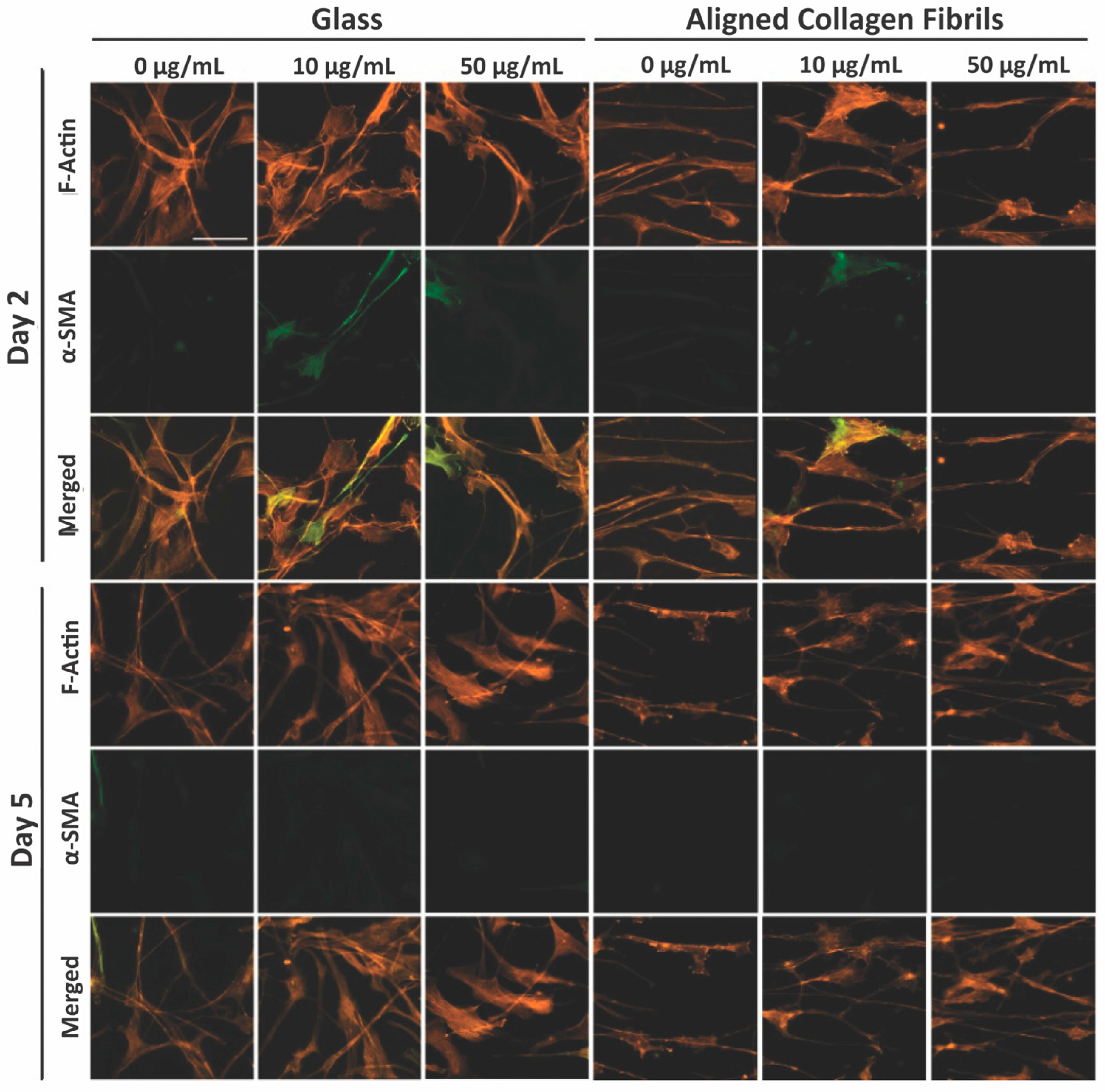
3.3. Keratocyte Response to ECM Composition and Topographical Cues in the Presence of PDGF-BB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassell, J.R.; Birk, D.E. The molecular basis of corneal transparency. Exp. Eye Res. 2010, 91, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.S.X.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Vesaluoma, M.; Teppo, A.M.; Grönhagen-Riska, C.; Tervo, T. Platelet-derived growth factor-BB (PDGF-BB) in tear fluid: A potential modulator of corneal wound healing following photorefractive keratectomy. Curr. Eye Res. 1997, 16, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Mohan, R.R.; Mohan, R.R.; Wilson, S.E. Effect of PDGF, IL-1a, and BMP2/4 on corneal fibroblast chemotaxis: Expression of the platelet-derived growth factor system in the cornea. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1364–1372. [Google Scholar]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrósio, R.; Hong, J.W.; Lee, J.S. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Kamiyama, K.; Iguchi, I.; Wang, X.; Imanishi, J. Effects of PDGF on the migration of rabbit corneal fibroblasts and epithelial cells. Cornea 1998, 17, 315. [Google Scholar] [CrossRef] [PubMed]
- Denk, P.O.; Knorr, M. The in vitro effect of platelet-derived growth factor isoforms on the proliferation of bovine corneal stromal fibroblasts depends on cell density. Graefe’s Arch. Clin. Exp. Ophthalmol. 1997, 235, 530–534. [Google Scholar] [CrossRef]
- Andresen, J.L.; Ehlers, N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr. Eye Res. 1998, 17, 79–87. [Google Scholar] [CrossRef]
- Iyer, K.S.; Maruri, D.P.; Peak, K.E.; Schmidtke, D.W.; Petroll, W.M.; Varner, V.D. ECM stifness modulates the proliferation but not the motility of primary corneal keratocytes in response to PDGF-BB. Exp. Eye Res. 2022, 220, 109112. [Google Scholar] [CrossRef]
- Kim, A.; Lakshman, N.; Karamichos, D.; Matthew Petroll, W. Growth factor regulation of corneal keratocyte differentiation and migration in compressed collagen matrices. Investig. Ophthalmol. Vis. Sci. 2010, 51, 864–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jester, J.V.; Ho-Chang, J. Modulation of cultured keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp. Eye Res. 2003, 77, 581–592. [Google Scholar] [CrossRef]
- Wachtlin, J.; Langenbeck, K.; Schründer, S.; Zhang, E.; Hoffmann, F. Immunohistology of corneal wound healing after photorefractive keratectomy and laser in situ keratomileusis. J. Refract. Surg. 1999, 15, 451–458. [Google Scholar]
- van Setten, G.B.; Koch, J.W.; Tervo, K.; Lang, G.K.; Tervo, T.; Naumann, G.O.H.; Kolkmeier, J.; Virtanen, I.; Tarkkanen, A. Expression of tenascin and fibronectin in the rabbit cornea after excimer laser surgery. Graefe’s Arch. Clin. Exp. Ophthalmol. 1992, 230, 178–183. [Google Scholar] [CrossRef]
- Kivanany, P.B.; Grose, K.C.; Petroll, W.M. Temporal and spatial analysis of stromal cell and extracellular matrix patterning following lamellar keratectomy. Exp. Eye Res. 2016, 153, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Jester, J.V.; Petroll, W.M.; Cavanagh, H.D. Corneal stromal wound healing in refractive surgery: The role of myofibroblasts. Prog. Retin. Eye Res. 1999, 18, 311–356. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Barry-Lane, P.A.; Petroll, W.M.; Olsen, D.R.; Cavanagh, H.D. Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF(β) in the rabbit. Cornea 1997, 16, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [Green Version]
- Andresen, J.L.; Ledet, T.; Hager, H.; Josephsen, K.; Ehlers, N. The influence of corneal stromal matrix proteins on the migration of human corneal fibroblasts. Exp.Eye Res. 2000, 71, 33–43. [Google Scholar] [CrossRef]
- Jester, J.V.; Huang, J.; Barry-Lane, P.A.; Kao, W.W.Y.; Petroll, W.M.; Cavanagh, H.D. Transforming growth factor(beta)-mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1959–1967. [Google Scholar]
- Sandbo, N.; Dulin, N. Actin cytoskeleton in myofibroblast differentiation: Ultrastructure defining form and driving function. Transl. Res. 2011, 158, 181–196. [Google Scholar] [CrossRef] [Green Version]
- Miron-Mendoza, M.; Graham, E.; Manohar, S.; Petroll, W.M. Fibroblast-fibronectin patterning and network formation in 3D fibrin matrices. Matrix Biol. 2017, 64, 69–80. [Google Scholar] [CrossRef]
- Miron-Mendoza, M.; Lin, X.; Ma, L.; Ririe, P.; Petroll, W.M. Individual versus collective fibroblast spreading and migration: Regulation by matrix composition in 3D culture. Exp. Eye Res. 2012, 99, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, T.; Nakagawa, S.; Nishibayashi, C.; Tanaka, H.; Manabe, R. Fibronectin Enhancement of Corneal Epithelial Wound Healing of Rabbits in Vivo. Arch. Ophthalmol. 1984, 102, 455–456. [Google Scholar] [CrossRef]
- Somasundaram, R.; Schuppan, D. Type I, II, III, IV, V, and VI Collagens Serve as Extracellular Ligands for the Isoforms of Platelet-derived Growth Factor (AA, BB, and AB). J. Biol. Chem. 1996, 271, 26884–26891. [Google Scholar] [CrossRef] [Green Version]
- Somasundaram, R.; Ruehl, M.; Tiling, N.; Ackermann, R.; Schmid, M.; Riecken, E.O.; Schuppan, D. Collagens serve as an extracellular store of bioactive interleukin 2. J. Biol. Chem. 2000, 275, 38170–38175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuppan, D.; Schmid, M.; Somasundaram, R.; Ackermann, R.; Ruehl, M.; Nakamura, T.; Riecken, E.-O. Collagens in the Liver Extracellular Matrix Bind Hepatocyte Growth Factor. Gastroenterology 1998, 114, 139–152. [Google Scholar] [CrossRef]
- Mooradian, D.L.; Lucas, R.C.; Weatherbee, J.A.; Furcht, L.T. Transforming growth factor-β1 binds to immobilized fibronectin. J. Cell. Biochem. 1989, 41, 189–200. [Google Scholar] [CrossRef]
- Rahman, S.; Patel, Y.; Murray, J.; Patel, K.V.; Sumathipala, R.; Sobel, M.; Wijelath, E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Rep. Reg. 2008, 17, 153–162. [Google Scholar] [CrossRef]
- Martino, M.M.; Hubbell, J.A. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010, 24, 4711–4721. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Zhu, J.; Tonnesen, M.G.; Taira, B.R.; McClain, S.A.; Singer, A.J.; Clark, R.A.F. Fibronectin peptides that bind PDGF-BB enhance survival of cells and tissue under stress. J. Investig. Dermatol. 2014, 134, 1119–1127. [Google Scholar] [CrossRef] [Green Version]
- Brizzi, M.F.; Tarone, G.; Defilippi, P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr. Opin. Cell Biol. 2012, 24, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, J.; Chen, M.; Guan, S.; Sawcer, D.; Bokoch, G.M.; Woodley, D.T. Mechanism of Human Dermal Fibroblast Migration Driven by Type I Collagen and Platelet-derived Growth Factor-BB. Mol. Biol. Cell 2004, 15, 294–309. [Google Scholar] [CrossRef] [Green Version]
- Jester, J.V.; Huang, J.; Petroll, W.M.; Cavanagh, H.D. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp. Eye Res. 2002, 75, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Kivanany, P.B.; Grose, K.; Yonet-Tanyeri, N.; Alsmadi, N.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. A high-throughput microfluidic method for fabricating aligned collagen fibrils to study Keratocyte behavior. Biomed. Microdevices 2019, 21, 99. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, N.; Sander, E.A.; Zareian, R.; Ruberti, J.W. Production of highly aligned collagen lamellae by combining shear force and thin film confinement. Acta Biomater. 2011, 7, 2437–2447. [Google Scholar] [CrossRef] [Green Version]
- Kivanany, P.; Grose, K.; Yonet-Tanyeri, N.; Manohar, S.; Sunkara, Y.; Lam, K.; Schmidtke, D.; Varner, V.; Petroll, W. An In Vitro Model for Assessing Corneal Keratocyte Spreading and Migration on Aligned Fibrillar Collagen. J. Funct. Biomater. 2018, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Lam, K.H. Development of Novel Aligned Collagen Fibril Coated Substrates for Mimicking Corneal Tissue Structures. Ph.D. Thesis, University of Texas at Dallas, Richardson, TX, USA, 2020. [Google Scholar]
- Di Lullo, G.A.; Sweeney, S.M.; Körkkö, J.; Ala-Kokko, L.; San Antonio, J.D. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J. Biol. Chem. 2002, 277, 4223–4231. [Google Scholar] [CrossRef] [Green Version]
- Jester, J.V.; Barry, P.A.; Lind, G.J.; Petroll, W.M.; Garana, R.; Cavanagh, H.D. Corneal keratocytes: In situ and in vitro organization of cytoskeletal contractile proteins. Investig. Ophthalmol. Vis. Sci. 1994, 35, 730–743. [Google Scholar]
- Yam, G.H.F.; Yusoff, N.Z.B.M.; Kadaba, A.; Tian, D.; Myint, H.H.; Beuerman, R.W.; Zhou, L.; Mehta, J.S. Ex vivo propagation of human corneal stromal “Activated Keratocytes” for tissue engineering. Cell Transplant. 2015, 24, 1845–1861. [Google Scholar] [CrossRef] [PubMed]
- Jester, J.V.; Huang, J.; Fisher, S.; Spiekerman, J.; Chang, J.H.; Wright, W.E.; Shay, J.W. Myofibroblast Differentiation of Normal Human Keratocytes and hTERT, Extended-Life. Investig. Ophthalmol. Vis. Sci. 2002, 44, 1850–1858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papers, J.B.C.; Plow, E.F.; Haas, T.A.; Zhang, L.; Loftus, J.; Smith, J.W.; Red, A.; Holland, C. Ligand Binding to Integrins. J. Biol. Chem. 2000, 275, 21785–21788. [Google Scholar] [CrossRef] [Green Version]
- Schmidinger, G.; Hanselmayer, G.; Pieh, S.; Lackner, B.; Kaminski, S.; Ruhswurm, I.; Skorpik, C. Effect of tenascin and fibronectin on the migration of human corneal fibroblasts. J. Cataract Refract. Surg. 2003, 29, 354–360. [Google Scholar] [CrossRef] [PubMed]
- To, W.S.; Midwood, K.S. Plasma and cellular fibronectin: Distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair 2011, 4, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Nakagawa, S.; Nishida, T. Chemotactic and haptotactic activities of fibronectin for cultered rabbit corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 1988, 29, 572–577. [Google Scholar]
- Thannickal, V.J.; Lee, D.Y.; White, E.S.; Cui, Z.; Larios, J.M.; Chacon, R.; Horowitz, J.C.; Day, R.M.; Thomas, P.E. Myofibroblast differentiation by TGF-beta 1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 2003, 278, 12384–12389. [Google Scholar] [CrossRef] [Green Version]
- de Jong-Hesse, Y.; Kampmeier, J.; Lang, G.K.; Lang, G.E. Effect of extracellular matrix on proliferation and differentiation of porcine lens epithelial cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2005, 243, 695–700. [Google Scholar] [CrossRef]
- Gamulescu, M.A.; Chen, Y.; He, S.; Spee, C.; Jin, M.; Ryan, S.J.; Hinton, D.R. Transforming growth factor β2-induced myofibroblastic differentiation of human retinal pigment epithelial cells: Regulation by extracellular matrix proteins and hepatocyte growth factor. Exp. Eye Res. 2006, 83, 212–222. [Google Scholar] [CrossRef]
- Muthusubramaniam, L.; Peng, L.; Zaitseva, T.; Paukshto, M.; Martin, G.R.; Desai, T.A. Collagen fibril diameter and alignment promote the quiescent keratocyte phenotype. J. Biomed. Mater. Res. Part A 2012, 100, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Phu, D.; Wray, L.S.; Warren, R.V.; Haskell, R.C.; Orwin, E.J. Effect of Substrate Composition and Alignment on Corneal Cell Phenotype. Tissue Eng. Part A 2011, 17, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wray, L.S.; Orwin, E.J. Recreating the Microenvironment of the Native Cornea for Tissue Engineering Applications. Tissue Eng. Part A 2009, 15, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Haber, M.; Cao, Z.; Panjwani, N.; Bedenice, D.; Li, W.W.; Provost, P.J. Effects of growth factors (EGF, PDGF-BB and TGF-β1) on cultured equine epithelial cells and keratocytes: Implications for wound healing. Vet. Ophthalmol. 2003, 6, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Chaurasia, S.S.; de Medeiros, F.W.; Agrawal, V.; Salomao, M.Q.; Singh, N.; Ambati, B.K.; Wilson, S.E. Corneal stroma PDGF blockade and myofibroblast development. Exp. Eye Res. 2009, 88, 960–965. [Google Scholar] [CrossRef] [Green Version]
- Gallego-Muñoz, P.; Ibares-Frías, L.; Valsero-Blanco, M.C.; Cantalapiedra-Rodriguez, R.; Merayo-Lloves, J.; Martinez-Garcia, M. Cytokine Effects of TGFβ1, PDGF-BB, and bFGF, on human corneal fibroblasts proliferation and differentiation during stromal repair. Cytokine 2017, 96, 94–101. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, K.H.; Shihabeddin, T.Z.; Awkal, J.A.; Najjar, A.M.; Miron-Mendoza, M.; Maruri, D.P.; Varner, V.D.; Petroll, W.M.; Schmidtke, D.W. Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces. J. Funct. Biomater. 2023, 14, 217. https://doi.org/10.3390/jfb14040217
Lam KH, Shihabeddin TZ, Awkal JA, Najjar AM, Miron-Mendoza M, Maruri DP, Varner VD, Petroll WM, Schmidtke DW. Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces. Journal of Functional Biomaterials. 2023; 14(4):217. https://doi.org/10.3390/jfb14040217
Chicago/Turabian StyleLam, Kevin H., Tarik Z. Shihabeddin, Jacob A. Awkal, Alex M. Najjar, Miguel Miron-Mendoza, Daniel P. Maruri, Victor D. Varner, W. Matthew Petroll, and David W. Schmidtke. 2023. "Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces" Journal of Functional Biomaterials 14, no. 4: 217. https://doi.org/10.3390/jfb14040217
APA StyleLam, K. H., Shihabeddin, T. Z., Awkal, J. A., Najjar, A. M., Miron-Mendoza, M., Maruri, D. P., Varner, V. D., Petroll, W. M., & Schmidtke, D. W. (2023). Effects of Topography and PDGF on the Response of Corneal Keratocytes to Fibronectin-Coated Surfaces. Journal of Functional Biomaterials, 14(4), 217. https://doi.org/10.3390/jfb14040217





