Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrogel Lap/GelMA
2.3. Characterizations
2.4. Artemia Salina Assay
2.5. Cytotoxicity
2.6. Osteogenic Differentiation Assssement
2.7. Statistical Analysis
3. Results and Discussion
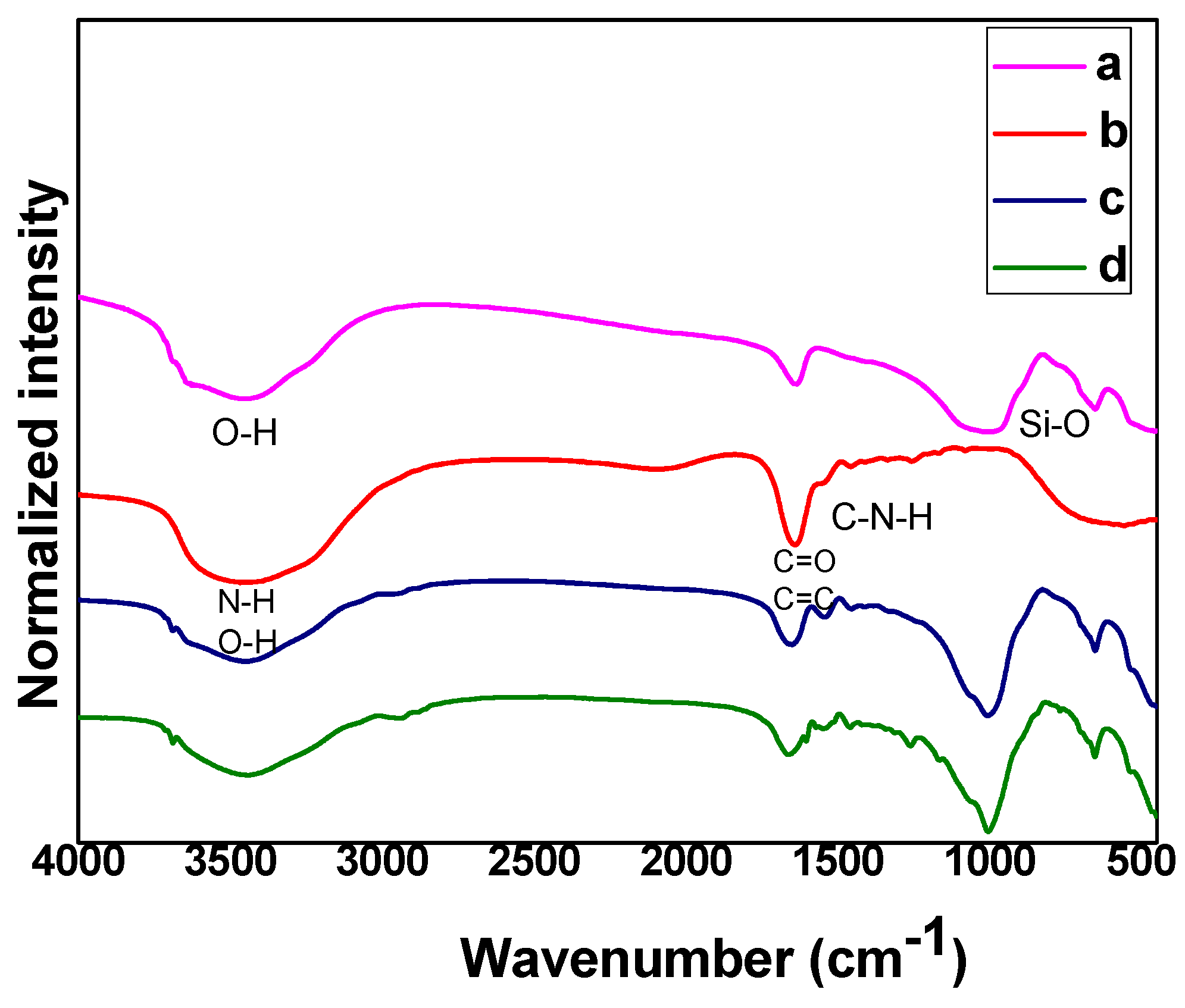
3.1. Structural Analysis of Hydrogel
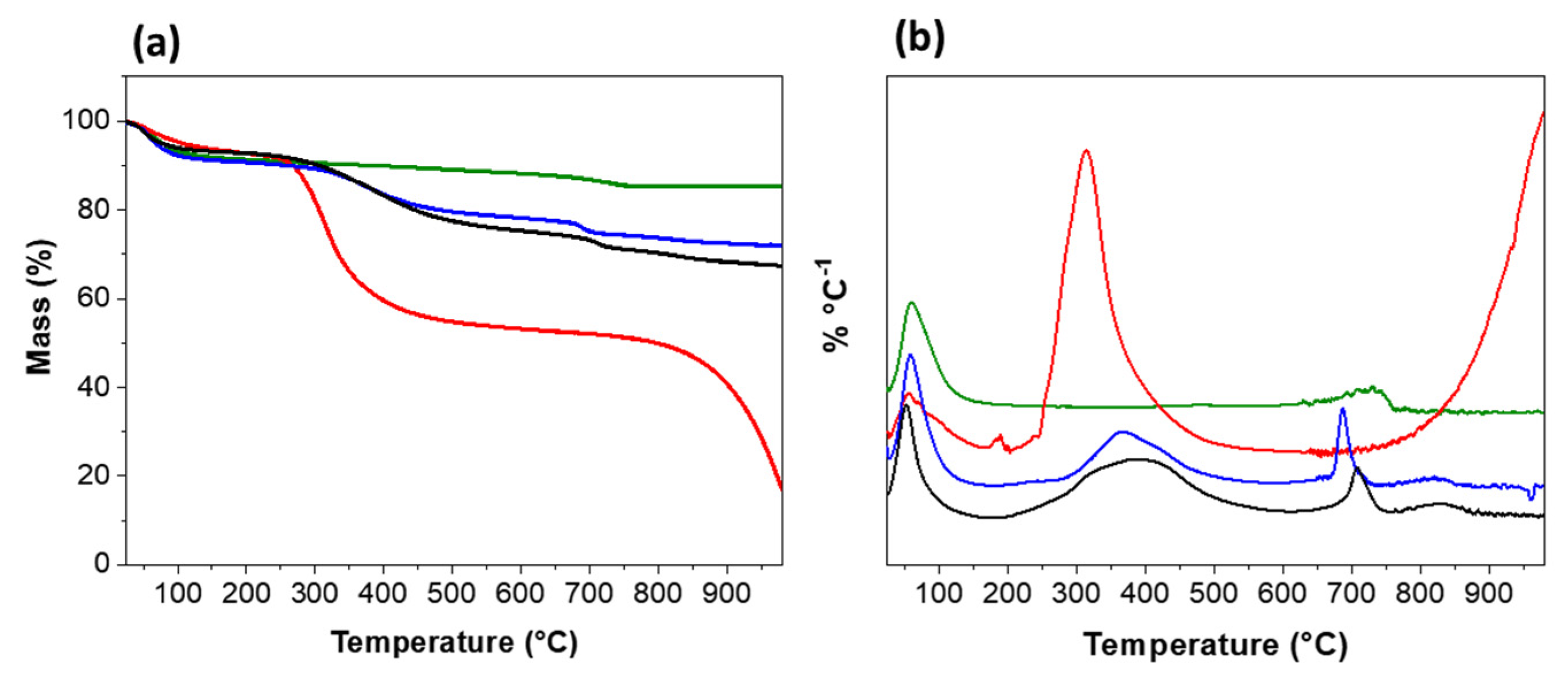
3.2. Thermogravimetric Analysis
3.3. Rheology
3.4. Morphology
3.5. Assay Toxicity
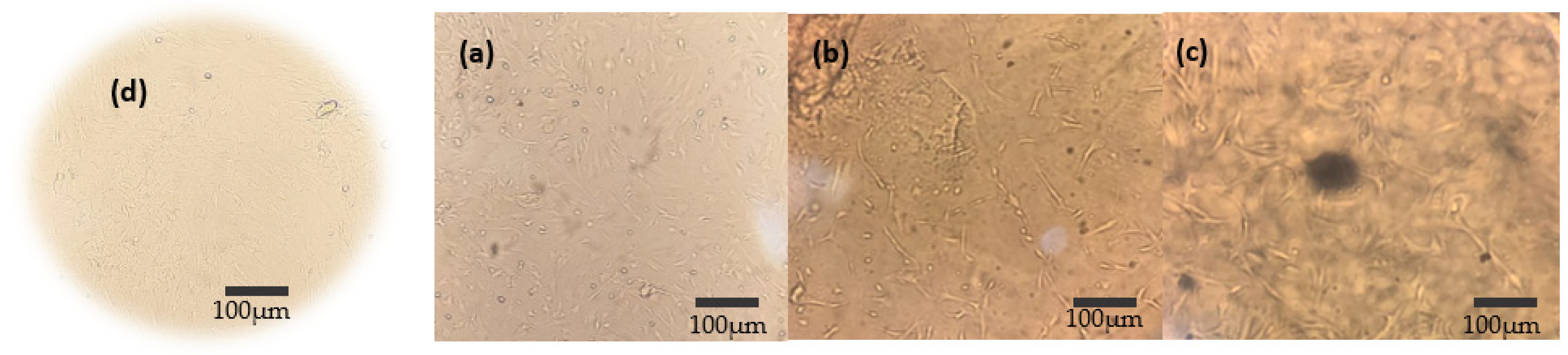
3.6. In Vitro Cytotoxicity
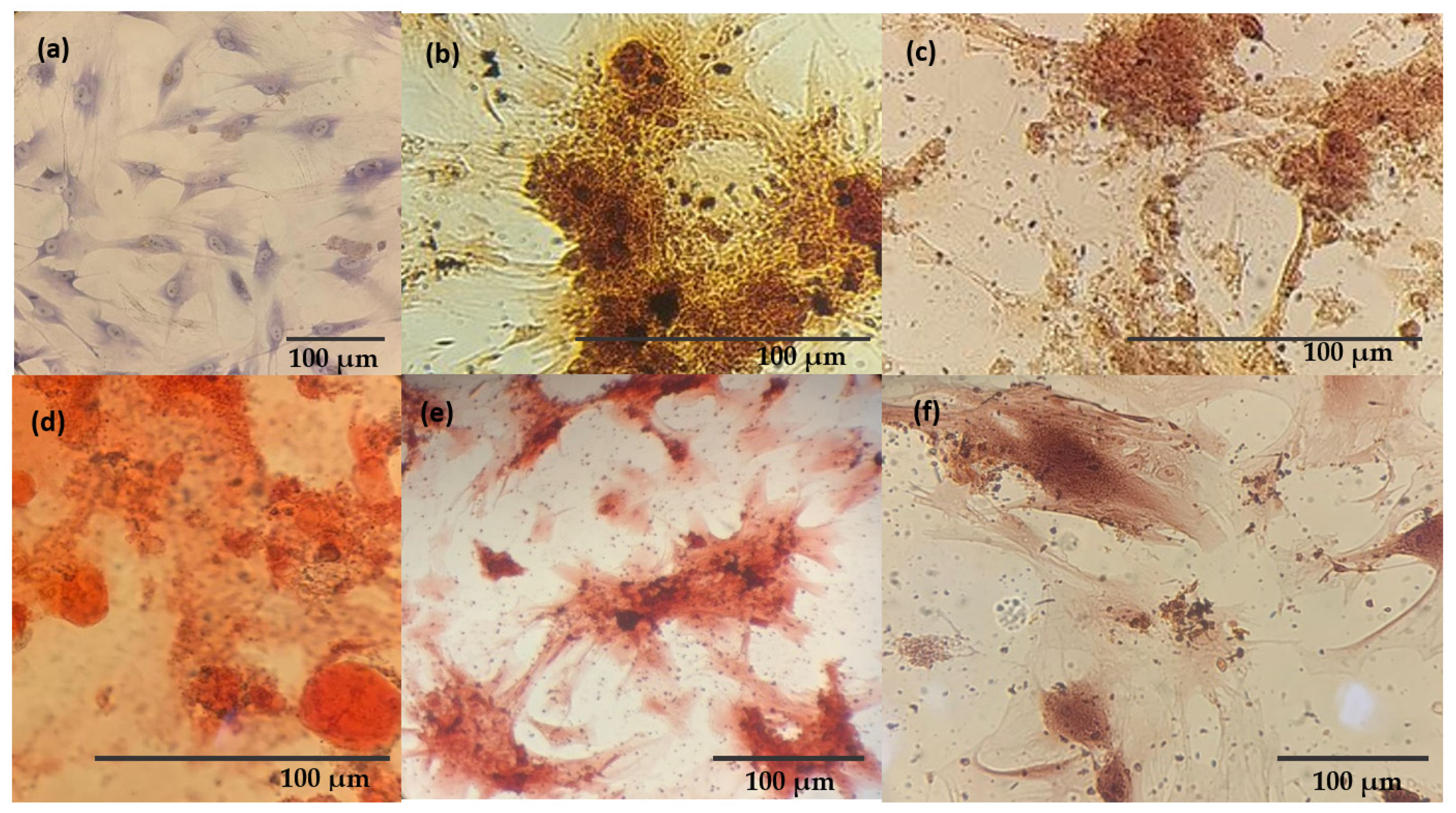
3.7. Osteogenic Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoppe, A.; Guldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactiveglasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Choi, D.; Heo, J.; Milan, J.A.; Oreffo, R.O.C.; Dawson, J.I.; Hong, J.; Kim, Y.-H. Structured nanofilms comprising Laponite® and bone extracellular matrixfor osteogenic differentiation of skeletal progenitor cells. Mater. Sci. Eng. C 2021, 118, 111440. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, Y.; Chen, F.; Liu, J.; Wang, T.; Luo, Y.; Jia, S.; Wang, P.; Tan, S.; Lu, B.; et al. The hydroxyapatite microtubes enhanced GelMA hydrogel scaffold with inner “pipeline framework” structure for bone tissue regeneration. Compos. B Eng. 2022, 228, 109396. [Google Scholar] [CrossRef]
- Hsu, C.-C.; George, J.H.; Waller, S.; Besnard, C.; Nagel, D.A.; Hill, E.J.; Coleman, M.D.; Korsunsky, A.M.; Cui, Z.; Ye, H. Increased connectivity of hiPSC-derived neural networks in multiphase granular hydrogel scaffolds. Bioact. Mater. 2022, 9, 358–372. [Google Scholar] [CrossRef]
- Rehman, S.R.; Augustine, R.; Zahid, A.A.; Ahmed, R.; Tariq, M.; Hasan, A. Reduced Graphene Oxide Incorporated GelMA Hydrogel Promotes Angiogenesis For Wound Healing Applications. Int. J. Nanomed. 2019, 14, 9603–9617. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a Biomaterial for Bone TissueEngineering: A Review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef]
- Ziminska, M.; Wilson, J.J.; McERlean, E.; Dunne, N.; McCarthy, H. Synthesis and Evaluation of a Thermoresponsive Degradable Chitosan-Grafted PNIPAAm Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, T.; Ning, C. Biomimetic mineralization of anionic gelatin hydrogels: Effect of degree of methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Kurian, A.M.; Singh, R.K.; Patel, K.D.; Lee, J.-H.; Kim, H.-W. Multifunctional GelMA platforms with nanomaterials for advancedtissue therapeutics. Bioact. Mater. 2022, 8, 267–295. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, T.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem. Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Dong, L.; Bu, Z.; Yiong, Y.; Zhang, H.; Fang, J.; Hu, H.; Liu, Z.; Li, X. Facile extrusion 3D printing of gelatine methacrylate/Laponitenanocomposite hydrogel with high concentration nanoclay for bone tissue regeneration. Int. J. Biol. Macromol. 2021, 188, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mousa, M.; Evans, N.D.; Oreffo, R.O.C.; Dawson, J.J. Clay nanoparticles for regenerative medicine and biomaterial design: A review of clay bioactivity. Biomaterials 2018, 159, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Afewerki, S.; Magalhães, L.S.S.M.; Silva, A.D.R.; Stocco, T.D.; Siva Filho, E.C.; Marciano, F.R.; Lobo, A.O. Bioprinting a synthetic smectic clay for orthopedic applications. Adv. Healthc. Mater 2019, 8, 1900158. [Google Scholar] [CrossRef]
- Tang, L.; Wei, W.; Wang, X.; Qian, J.; Li, J.; He, A.; Yang, L.; Jiang, X.; Li, X.; Wei, J. LAPONITE® nanorods regulating degradability, acidic-alkaline microenvironment, apatite mineralization and MC3T3-E1 cells responses to poly (butylene succinate) based bio-nanocomposite scaffolds. RSC Adv. 2018, 8, 10794–10805. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Yin, C.; Mo, A.; Hong, G. Applications of Hydrogel with Special Physical Properties inBone and Cartilage Regeneration. Materials 2021, 14, 235. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, J.; Zhang, N.; Chen, Y.; Chen, Y.; Li, H.; Liu, H. Dual-network sodium alginate/polyacrylamide/laponite nanocomposite hydrogels with high toughness and cyclic mechano-responsiveness. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127867. [Google Scholar] [CrossRef]
- Shi, P.; Kim, Y.-H.; Mousa, M.; Sanchez, R.R.; Oreffo, R.O.; Dawson, J.J. Self-Assembling Nanoclay Diffusion Gels for Bioactive Osteogenic Microenvironments. Adv. Healthc. Mater. 2018, 7, 1800331. [Google Scholar] [CrossRef]
- Valencia, G.A.; Luciano, C.G.; Lourenço, R.V.; Sobral, P.J.A. Microstructure and physical properties of nano-biocomposite films based on cassava starch and laponite. Int. J. Biol. Macromol. 2018, 107, 1576–1583. [Google Scholar] [CrossRef]
- Tomás, H.; Alves, C.S.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. Biol. Med. 2017, 14, 2407–2420. [Google Scholar] [CrossRef]
- Cidonio, G.; Alecula-Orozco, C.R.; Lin, K.S.; Glinka, M.; Mutreja, I.; Kim, Y.-H.; Dawson, J.J.; Woodfield, T.B.F.; Oreffo, R.O.C. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar] [CrossRef]
- Das, S.S.; Hussian, K.; Singh, S.; Hussian, A.; Faruk, A.; Tebyetekerwa, M. Laponite-based Nanomaterials for Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.S.S.M.; Andrade, D.B.; Bezerra, R.D.S.; Moaris, A.I.S.; Oliveira, F.C.; Rizzo, M.S.; Silva-Filho, E.C.; Lobo, A.O. Nanocomposite Hydrogel Produced from PEGDA and Laponite for bone regeneration. J. Funct. Biomater. 2022, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, A.; Afewerki, S.; Oklu, R.; Gaharwar, A.K.; Khademhosseini, A. Effect of ionic strength on shear-thinning nanoclay-polymer composite hydrogels. Biomater. Sci. 2018, 6, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca, C.Y.C.; Afewerki, S.; Stocco, T.A.; Corat, M.A.F.; Machado de Paula, M.M.; Marciano, F.R.; Lobo, A.O. Oxygen-generating smart hydrogels supporting chondrocytes survival in oxygen-free environments. Colloids Surf. B Biointerfaces 2020, 194, 111192. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; Mc Laughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices. Part 5: Tests for Cytotoxicity: In Vitro Methods, 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2009.
- Rajabi, N.; Kharaziha, M.; Emadi, R.; Zarrabi, A.; Mokhtari, H.; Salehi, S. An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant. J. Colloid Interface Sci. 2020, 564, 155–169. [Google Scholar] [CrossRef]
- Choi, J.; Kim, Y.-K.; Byeon, S.-M.; Park, J.-E.; Bae, T.-S.; Jang, Y.-S.; Lee, M.-H. Fabrication and Characterization of Biodegradable Gelatin Methacrylate/Biphasic Calcium Phosphate Composite Hydrogel for Bone Tissue Engineering. Nanomaterials 2021, 11, 617. [Google Scholar] [CrossRef]
- Colleti, C.G.; Massaro, M.; Lazzara, G.; Cavallaro, G.; Milioto, S.; Pibiri, I.; Noto, R.; Riela, S. Synthesis, characterization and study of covalently modified triazole LAPONITE® edges. Appl. Clay Sci. 2020, 187, 105489. [Google Scholar] [CrossRef]
- Li, H.; Ren, W.; Zhu, J.; Xu, S.; Wang, J. Rheology and Processing of Laponite/PolymerNanocomposites. Rheol. Process. Polym. Nanocomposites 2016, 383–404. [Google Scholar] [CrossRef]
- Shanbhag, S.; Pandis, N.; Mustafa, K.; Nyengaard, J.R.; Stavropoulos, A. Bone tissue engineering in oral peri-implant defects in preclinical in vivo research: A systematic review and meta-analysis. J. Tissue Eng. Regen. Med. 2018, 12, 336–349. [Google Scholar] [CrossRef]
- Ou, Q.; Huang, K.; Fu, C.; Huang, C.; Fang, Y.; Gu, Z.; Wu, J.; Wang, Y. Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybridhydrogel with osteoimmunomodulatory and antibacterial activity for boneregeneration. Chem. Eng. J. 2020, 382, 123019. [Google Scholar] [CrossRef]
- Nguta, J.N.; Mbaria, J.M.; Gakuya, A.W.; Gathumbi, P.K.; Kabasa, J.D.; Kiama, S.G. Biological screenig of keya medicial plants using Artemia salina L. (Artemiidae). Pharmacologyonline 2011, 2, 458–478. [Google Scholar]
- Orafa, Z.; Bakhshi, H.; Arab-Ahmadi, S.; Irani, S. Laponite/amoxicillin-functionalized PLA nanofbrous as osteoinductive and antibacterial scafolds. Sci. Rep. 2022, 12, 6583. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.S.; Chauhan, G.; Goyal, M.; Sarvepalli, S.; Gupta, V. Development of gelatina methacrylate (GelMA) hydrogels for versatile intracavitary applications. Biomater. Sci. 2022, 10, 4492–4507. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, D.B.; Soares, L.L.S.; Cardoso, F.L.A.; Lima, I.S.; Silva, J.G.V.; Carvalho, M.A.M.; Fonseca, M.G.; Brito, G.d.C.; Santos, F.E.P.; Osajima, J.A.; et al. Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application. J. Funct. Biomater. 2023, 14, 74. https://doi.org/10.3390/jfb14020074
Andrade DB, Soares LLS, Cardoso FLA, Lima IS, Silva JGV, Carvalho MAM, Fonseca MG, Brito GdC, Santos FEP, Osajima JA, et al. Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application. Journal of Functional Biomaterials. 2023; 14(2):74. https://doi.org/10.3390/jfb14020074
Chicago/Turabian StyleAndrade, Danielle B., Leticya L. S. Soares, Francisca L. A. Cardoso, Idglan S. Lima, Jhaemely G. V. Silva, Maria A. M. Carvalho, Maria G. Fonseca, Guilherme de C. Brito, Francisco Eroni P. Santos, Josy A. Osajima, and et al. 2023. "Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application" Journal of Functional Biomaterials 14, no. 2: 74. https://doi.org/10.3390/jfb14020074
APA StyleAndrade, D. B., Soares, L. L. S., Cardoso, F. L. A., Lima, I. S., Silva, J. G. V., Carvalho, M. A. M., Fonseca, M. G., Brito, G. d. C., Santos, F. E. P., Osajima, J. A., Lobo, A. O., & Silva-Filho, E. C. (2023). Hydrogel Based on Nanoclay and Gelatin Methacrylate Polymeric Matrix as a Potential Osteogenic Application. Journal of Functional Biomaterials, 14(2), 74. https://doi.org/10.3390/jfb14020074









