Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tooth Specimen Preparation
2.2. Bonding Procedure
2.3. Micro-Tensile Bond Strength Testing
2.4. Failure Mode Analysis
2.5. Scanning Electron Microscopy
2.6. Statistical Analysis
3. Results
3.1. Micro-Tensile Bond Strength Testing
3.2. Failure Mode Analysis
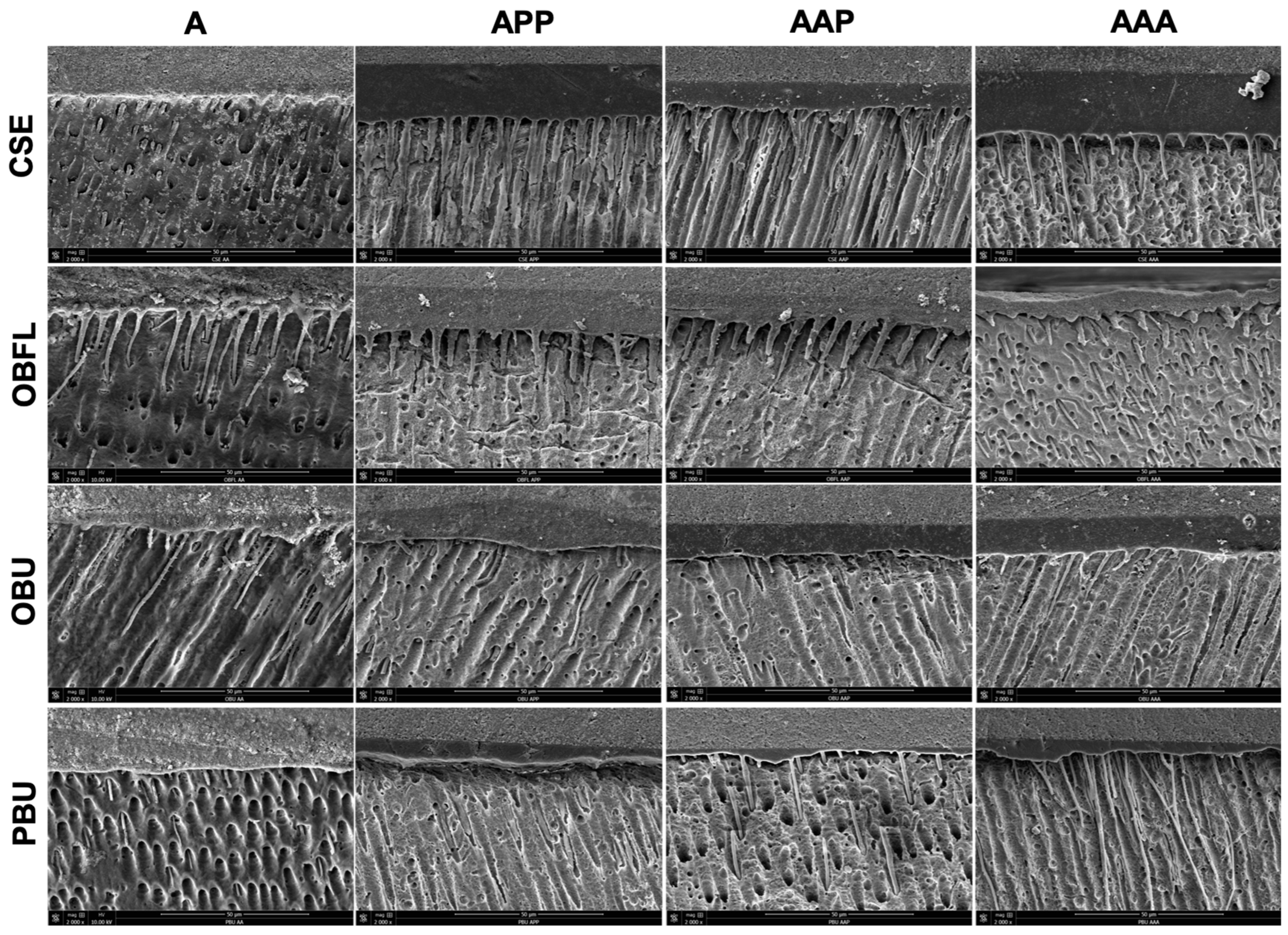
3.3. Scanning Electron Microscopy of Resin–Dentin Interface
4. Discussion
5. Conclusions
- The laboratory adhesive properties were mostly material-dependent.
- For better bond strength performance, CSE, OBU, and PBU were benefited by the A technique; for OBFL, the use of the AAA technique could be recommended to achieve stability in the adhesive layer.
- The combination of ABT with an MLA was a good choice for the highly filled OBFL. This explains that the LR-MTLA was considered a novel approach in the field of adhesive dentistry with an acceptable bond stability after 6 months.
- Clinicians must consider the chemistry and the physical features of each adhesive system in an attempt to determine its ideal performance before applying the MTLA.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reis, A.; Loguercio, A.; Favoreto, M.; Chibinski, A. Some Myths in Dentin Bonding: An Evidence-Based Perspective. J. Dent. Res. 2023, 102, 376–382. [Google Scholar] [CrossRef]
- Saikaew, P.; Sattabanasuk, V.; Harnirattisai, C.; Chowdhury, A.F.M.A.; Carvalho, R.; Sano, H. Role of the Smear Layer in Adhesive Dentistry and the Clinical Applications to Improve Bonding Performance. Jpn. Dent. Sci. Rev. 2022, 58, 59–66. [Google Scholar] [CrossRef]
- Bourgi, R.; Hardan, L.; Cuevas-Suárez, C.E.; Devoto, W.; Kassis, C.; Kharma, K.; Harouny, R.; Ashi, T.; Mancino, D.; Kharouf, N.; et al. Effectiveness of Different Application Modalities on the Bond Performance of Four Polymeric Adhesive Systems to Dentin. Polymers 2023, 15, 3924. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Zarow, M.; Kharouf, N.; Mancino, D.; Villares, C.F.; Skaba, D.; Lukomska-Szymanska, M. The Bond Strength and Antibacterial Activity of the Universal Dentin Bonding System: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 1230. [Google Scholar] [CrossRef]
- Cadenaro, M.; Josic, U.; Maravić, T.; Mazzitelli, C.; Marchesi, G.; Mancuso, E.; Breschi, L.; Mazzoni, A. Progress in Dental Adhesive Materials. J. Dent. Res. 2023, 102, 254–262. [Google Scholar] [CrossRef]
- Hardan, L.; Daood, U.; Bourgi, R.; Cuevas-Suárez, C.E.; Devoto, W.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderón, J.E.; Radwanski, M.; Orsini, G.; et al. Effect of Collagen Crosslinkers on Dentin Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis. Cells 2022, 11, 2417. [Google Scholar] [CrossRef]
- Yoshihara, K.; Yoshida, Y.; Hayakawa, S.; Nagaoka, N.; Irie, M.; Ogawa, T.; Van Landuyt, K.L.; Osaka, A.; Suzuki, K.; Minagi, S. Nanolayering of Phosphoric Acid Ester Monomer on Enamel and Dentin. Acta Biomater. 2011, 7, 3187–3195. [Google Scholar] [CrossRef]
- Ito, S.; Tay, F.R.; Hashimoto, M.; Yoshiyama, M.; Saito, T.; Brackett, W.W.; Waller, J.L.; Pashley, D.H. Effects of Multiple Coatings of Two All-in-One Adhesives on Dentin Bonding. J. Adhes. Dent. 2005, 7, 133–141. [Google Scholar]
- Cardoso, M.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current Aspects on Bonding Effectiveness and Stability in Adhesive Dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef]
- Aljifan, M.K.; Alshehri, A.M.; Alharbi, R.M.; Al Alhareth, M.S.; Alharbi, Z.A.; Alamri, S.M.; Alshahrani, N.S.; Alyamani, A.E.; Alzahrani, A.A.; Alqarni, A.A. An Overview of Dentin Conditioning and Its Effect on Bond Strength. Int. J. Community Med. Public Health 2023, 10, 1. [Google Scholar] [CrossRef]
- Zheng, L.; Pereira, P.N.; Nakajima, M.; Sano, H.; Tagami, J. Relationship between Adhesive Thickness and Microtensile Bond Strength. Oper. Dent. 2001, 26, 97–104. [Google Scholar] [PubMed]
- Hardan, L.; Bourgi, R.; Cuevas-Suárez, C.E.; Devoto, W.; Zarow, M.; Monteiro, P.; Jakubowicz, N.; Zoghbi, A.E.; Skaba, D.; Mancino, D. Effect of Different Application Modalities on the Bonding Performance of Adhesive Systems to Dentin: A Systematic Review and Meta-Analysis. Cells 2023, 12, 190. [Google Scholar] [CrossRef]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Saikaew, P.; Alam, A.; Sun, J.; Carvalho, R.M.; Sano, H. Effects of Double Application of Contemporary Self-Etch Adhesives on Their Bonding Performance to Dentin with Clinically Relevant Smear Layers. J. Adhes. Dent. 2019, 21, 59–66. [Google Scholar]
- Pinto, M.V.; Pires, S.; Marto, C.M.; Amaro, I.; Coelho, A.; Sousa, J.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E.; Abrantes, A.M. Microleakage Study of a Bulk Fill over an Uncured Adhesive System. J. Compos. Sci. 2023, 7, 40. [Google Scholar] [CrossRef]
- Al-Nabulsi, M.; Daud, A.; Yiu, C.; Omar, H.; Sauro, S.; Fawzy, A.; Daood, U. Co-Blend Application Mode of Bulk Fill Composite Resin. Materials 2019, 12, 2504. [Google Scholar] [CrossRef]
- Zecin-Deren, A.; Sokolowski, J.; Szczesio-Wlodarczyk, A.; Piwonski, I.; Lukomska-Szymanska, M.; Lapinska, B. Multi-Layer Application of Self-Etch and Universal Adhesives and the Effect on Dentin Bond Strength. Molecules 2019, 24, 345. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials Guidance on in Vitro Testing of Dental Composite Bonding Effectiveness to Dentin/Enamel Using Micro-Tensile Bond Strength (μTBS) Approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef]
- Kharouf, N.; Arntz, Y.; Eid, A.; Zghal, J.; Sauro, S.; Haikel, Y.; Mancino, D. Physicochemical and Antibacterial Properties of Novel, Premixed Calcium Silicate-Based Sealer Compared to Powder–Liquid Bioceramic Sealer. J. Clin. Med. 2020, 9, 3096. [Google Scholar] [CrossRef]
- Al-Abd Al, M.A.F.; Al-Badr, R.J.; Abbas, M.S. Modern Adhesive Dentistry: A Review of Literatures. Med. J. Ahl Al-Bayt Univ. 2023, 2, 6–13. [Google Scholar]
- Rocha Gomes Torres, C.; Barcellos, D.C.; Pucci, C.R.; de Morais Gouvêa Lima, G.; Rodrigues, C.M.; Siviero, M. Influence of Methods of Application of Self-Etching Adhesive Systems on Adhesive Bond Strength to Enamel. J. Adhes. Dent. 2009, 11, 279–286. [Google Scholar]
- Kumagai, R.Y.; Takagaki, T.; Sato, T.; Nikaido, T.; Giannini, M.; Reis, A.; Tagami, J. Resin Cement/Enamel Interface: A Morphological Evaluation of the Acid-Base Resistant Zone, Enamel Etching Pattern, and Effect of Thermocycling on the Microshear Bond Strength. J. Adhes. Dent. 2023, 25, 71–78. [Google Scholar]
- Itou, K.; Torii, Y.; Takimura, T.; Chikami, K.; Ishikawa, K.; Suzuki, K. Effect of Priming Time on Tensile Bond Strength to Bovine Teeth and Morphologic Structure of Interfaces Created by Self-Etching Primers. Int. J. Prosthodont. 2001, 14, 225–230. [Google Scholar]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.J.; De Munck, J.; Van Landuyt, K.L. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Maciel, K.; Carvalho, R.; Ringle, R.; Preston, C.; Russell, C.; Pashley, D.H. The Effects of Acetone, Ethanol, HEMA, and Air on the Stiffness of Human Decalcified Dentin Matrix. J. Dent. Res. 1996, 75, 1851–1858. [Google Scholar] [CrossRef] [PubMed]
- Frankenberger, R.; Pashley, D.H.; Reich, S.M.; Lohbauer, U.; Petschelt, A.; Tay, F.R. Characterisation of Resin–Dentine Interfaces by Compressive Cyclic Loading. Biomaterials 2005, 26, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, M.C.; Osorio, R.; Pisani-Proenca, J.; Aguilera, F.S.; Osorio, E.; Breschi, L.; Toledano, M. Effect of Double Layering and Prolonged Application Time on MTBS of Water/Ethanol-Based Self-Etch Adhesives to Dentin. Oper. Dent. 2009, 34, 571–577. [Google Scholar] [CrossRef]
- Fujiwara, S.; Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Imai, A.; Watanabe, H.; Erickson, R.L.; Latta, M.A.; Nakatsuka, T.; Miyazaki, M. Effect of Double-Layer Application on Bond Quality of Adhesive Systems. J. Mech. Behav. Biomed. Mater. 2018, 77, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Lapinska, B.; Konieczka, M.; Zarzycka, B.; Sokolowski, K.; Grzegorczyk, J.; Lukomska-Szymanska, M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus Mutans. Molecules 2019, 24, 532. [Google Scholar] [CrossRef]
- Hardan, L.; Orsini, G.; Bourgi, R.; Cuevas-Suárez, C.E.; Nicastro, M.; Lazarescu, F.; Filtchev, D.; Cornejo-Ríos, E.; Zamarripa-Calderón, J.E.; Sokolowski, K. Effect of Active Bonding Application after Selective Dentin Etching on the Immediate and Long-Term Bond Strength of Two Universal Adhesives to Dentin. Polymers 2022, 14, 1129. [Google Scholar] [CrossRef]
- Belli, R.; Sartori, N.; Dalmagro Peruchi, L.; Coutinho Guimarães, J.; Cardoso Vieira, L.C.; Narciso Baratieri, L.; Monteiro, S., Jr. Effect of Multiple Coats of Ultra-Mild All-in-One Adhesives on Bond Strength to Dentin Covered with Two Different Smear Layer Thicknesses. J. Adhes. Dent. 2011, 13, 507. [Google Scholar] [PubMed]
- Fontes, S.T.; Cubas, G.B.; Flores, J.B.; Montemezzo, M.L.; Pinto, M.B.; Piva, E. Resin-dentin bond strength of 10 contemporary etch-and-rinse adhesive systems after one year of water storage. Gen. Dent. 2010, 58, 257–261. [Google Scholar]
- Chowdhury, A.F.M.A.; Islam, R.; Alam, A.; Matsumoto, M.; Yamauti, M.; Carvalho, R.M.; Sano, H. Variable Smear Layer and Adhesive Application: The Pursuit of Clinical Relevance in Bond Strength Testing. Int. J. Mol. Sci. 2019, 20, 5381. [Google Scholar] [CrossRef] [PubMed]
- Schärer, B.M.; Peutzfeldt, A. Impact of Adhesive Application Errors on Dentin Bond Strength of Resin Composite. Biomater. Investig. Dent. 2022, 9, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Atai, M.; Khamverdi, Z.; Nejad, S.K. The Effect of Nanofiller Addition to an Experimental Dentin Adhesive on Microtensile Bond Strength to Human Dentin. Front. Dent. 2009, 6, 36–41. [Google Scholar]
- Peumans, M.; De Munck, J.; Van Landuyt, K.L.; Poitevin, A.; Lambrechts, P.; Van Meerbeek, B. A 13-Year Clinical Evaluation of Two Three-Step Etch-and-Rinse Adhesives in Non-Carious Class-V Lesions. Clin. Oral Investig. 2012, 16, 129–137. [Google Scholar] [CrossRef]
- Mandava, D.; Ajitha, P.; Narayanan, L.L. Comparative Evaluation of Tensile Bond Strengths of Total-Etch Adhesives and Self-Etch Adhesives with Single and Multiple Consecutive Applications: An in Vitro Study. J. Conserv. Dent. JCD 2009, 12, 55. [Google Scholar] [CrossRef]
- Hashimoto, M.; Sano, H.; Yoshida, E.; Hori, M.; Kaga, M.; Oguchi, H.; Pashley, D.H. Effects of Multiple Adhesive Coatings on Dentin Bonding. Oper. Dent. 2004, 29, 416–423. [Google Scholar]
- Dal-Bianco, K.; Pellizzaro, A.; Patzlaft, R.; de Oliveira Bauer, J.R.; Loguercio, A.D.; Reis, A. Effects of Moisture Degree and Rubbing Action on the Immediate Resin–Dentin Bond Strength. Dent. Mater. 2006, 22, 1150–1156. [Google Scholar] [CrossRef]
- Bowen, R.L. Adhesive bonding of various materials to hard tooth tissues. III. Bonding to dentin improved by pretreatment and the use of surface-active comonomer. J. Dent. Res. 1965, 44, 903–905. [Google Scholar] [CrossRef]
- Migliau, G. Classification Review of Dental Adhesive Systems: From the IV Generation to the Universal Type. Ann. Di Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pimentel de Oliveira, R.; de Paula, B.L.; Ribeiro, M.E.; Alves, E.; Costi, H.T.; Silva, C. Evaluation of the Bond Strength of Self-Etching Adhesive Systems Containing HEMA and 10-MDP Monomers: Bond Strength of Adhesives Containing HEMA and 10-MDP. Int. J. Dent. 2022, 2022, 5756649. [Google Scholar] [CrossRef] [PubMed]
- Fukegawa, D.; Hayakawa, S.; Yoshida, Y.; Suzuki, K.; Osaka, A.; Van Meerbeek, B. Chemical Interaction of Phosphoric Acid Ester with Hydroxyapatite. J. Dent. Res. 2006, 85, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the Art Etch-and-Rinse Adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef]
- Moura, S.K.; Lemos, L.V.F.M.; Myszkovisk, S.; Provenzano, M.G.A.; Balducci, I.; Myaki, S.I. Bonding Durability of Dental Sealants to Deciduous and Permanent Teeth. Braz. J. Oral Sci. 2014, 13, 198–202. [Google Scholar] [CrossRef]
- Azad, E.; Atai, M.; Zandi, M.; Shokrollahi, P.; Solhi, L. Structure–Properties Relationships in Dental Adhesives: Effect of Initiator, Matrix Monomer Structure, and Nano-Filler Incorporation. Dent. Mater. 2018, 34, 1263–1270. [Google Scholar] [CrossRef]
- Silva E Souza Junior, M.H.; Carneiro, K.G.K.; Lobato, M.F.; Rodrigues Silva E Souza, P.d.A.; de Goes, M.F.; Silva e Souza, M.H.J.; Carneiro, K.G.K.; Lobato, M.F.; Silva e Souza, P.d.A.R.; de Goes, M.F. Adhesive Systems: Important Aspects Related to Their Composition and Clinical Use. J. Appl. Oral Sci. 2010, 18, 207–214. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Hayakawa, S.; Okihara, T.; Yoshida, Y.; Van Meerbeek, B. Chemical Interaction of Glycero-Phosphate Dimethacrylate (GPDM) with Hydroxyapatite and Dentin. Dent. Mater. 2018, 34, 1072–1081. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Yao, C.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Extra Bonding Layer Compensates Universal Adhesive’s Thin Film Thickness. J. Adhes. Dent. 2020, 22, 483–501. [Google Scholar]
- Wang, R.; Shi, Y.; Li, T.; Pan, Y.; Cui, Y.; Xia, W. Adhesive Interfacial Characteristics and the Related Bonding Performance of Four Self-Etching Adhesives with Different Functional Monomers Applied to Dentin. J. Dent. 2017, 62, 72–80. [Google Scholar] [CrossRef]
- Ahmed, M.; Yoshihara, K.; Yao, C.; Okazaki, Y.; Van Landuyt, K.; Peumans, M.; Van Meerbeek, B. Multiparameter Evaluation of Acrylamide HEMA Alternative Monomers in 2-Step Adhesives. Dent. Mater. 2021, 37, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Suárez, C.E.; Ramos, T.S.; Rodrigues, S.B.; Collares, F.M.; Zanchi, C.H.; Lund, R.G.; da Silva, A.F.; Piva, E. Impact of Shelf-Life Simulation on Bonding Performance of Universal Adhesive Systems. Dent. Mater. 2019, 35, e204–e219. [Google Scholar] [CrossRef] [PubMed]
- Iliev, G.; Hardan, L.; Kassis, C.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Mancino, D.; Haikel, Y.; Kharouf, N. Shelf Life and Storage Conditions of Universal Adhesives: A Literature Review. Polymers 2021, 13, 2708. [Google Scholar] [CrossRef]
- Bourgi, R.; Daood, U.; Bijle, M.N.; Fawzy, A.; Ghaleb, M.; Hardan, L. Reinforced Universal Adhesive by Ribose Crosslinker: A Novel Strategy in Adhesive Dentistry. Polymers 2021, 13, 704. [Google Scholar] [CrossRef]
- Saeed, N.A.; Tichy, A.; Kuno, Y.; Hosaka, K.; Tagami, J.; Nakajima, M. Effect of Surface Moisture on Bur-Cut Dentin on Bonding of HEMA-Free and HEMA-Containing Universal Adhesives with or without Methacrylamide Monomer. J. Adhes. Dent. 2021, 23, 327–334. [Google Scholar] [PubMed]
- Teshima, I. Degradation of 10-Methacryloyloxydecyl Dihydrogen Phosphate. J. Dent. Res. 2010, 89, 1281–1286. [Google Scholar] [CrossRef]
- El Zohairy, A.; De Gee, A.; De Jager, N.; Van Ruijven, L.; Feilzer, A. The Influence of Specimen Attachment and Dimension on Microtensile Strength. J. Dent. Res. 2004, 83, 420–424. [Google Scholar] [CrossRef]
- Münchow, E.A.; Bossardi, M.; Priebe, T.C.; Valente, L.L.; Zanchi, C.H.; Ogliari, F.A.; Piva, E. Microtensile versus Microshear Bond Strength between Dental Adhesives and the Dentin Substrate. Int. J. Adhes. Adhes. 2013, 46, 95–99. [Google Scholar] [CrossRef]
- Irmak, Ö.; Yaman, B.C.; Orhan, E.O.; Ozer, F.; Blatz, M.B. Effect of Rubbing Force Magnitude on Bond Strength of Universal Adhesives Applied in Self-Etch Mode. Dent. Mater. J. 2018, 37, 139–145. [Google Scholar] [CrossRef]
- Maciel Pires, P.; Dávila-Sánchez, A.; Faus-Matoses, V.; Nuñez Martí, J.M.; Lo Muzio, L.; Sauro, S. Bonding Performance and Ultramorphology of the Resin-Dentine Interface of Contemporary Universal Adhesives. Clin. Oral Investig. 2022, 26, 4391–4405. [Google Scholar] [CrossRef]
- Langer, A.; Ilie, N. Dentin Infiltration Ability of Different Classes of Adhesive Systems. Clin. Oral Investig. 2013, 17, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.S.; Pugach, M.K.; Hilton, J.F.; Watanabe, L.G.; Marshall, S.J.; Marshall Jr, G.W. The Influence of the Dentin Smear Layer on Adhesion: A Self-Etching Primer vs. a Total-Etch System. Dent. Mater. 2003, 19, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Shinchi, M.; Soma, K.; Nakabayashi, N. The Effect of Phosphoric Acid Concentration on Resin Tag Length and Bond Strength of a Photo-Cured Resin to Acid-Etched Enamel. Dent. Mater. 2000, 16, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kharouf, N.; Ashi, T.; Eid, A.; Maguina, L.; Zghal, J.; Sekayan, N.; Bourgi, R.; Hardan, L.; Sauro, S.; Haikel, Y. Does Adhesive Layer Thickness and Tag Length Influence Short/Long-Term Bond Strength of Universal Adhesive Systems? An in-Vitro Study. Appl. Sci. 2021, 11, 2635. [Google Scholar] [CrossRef]
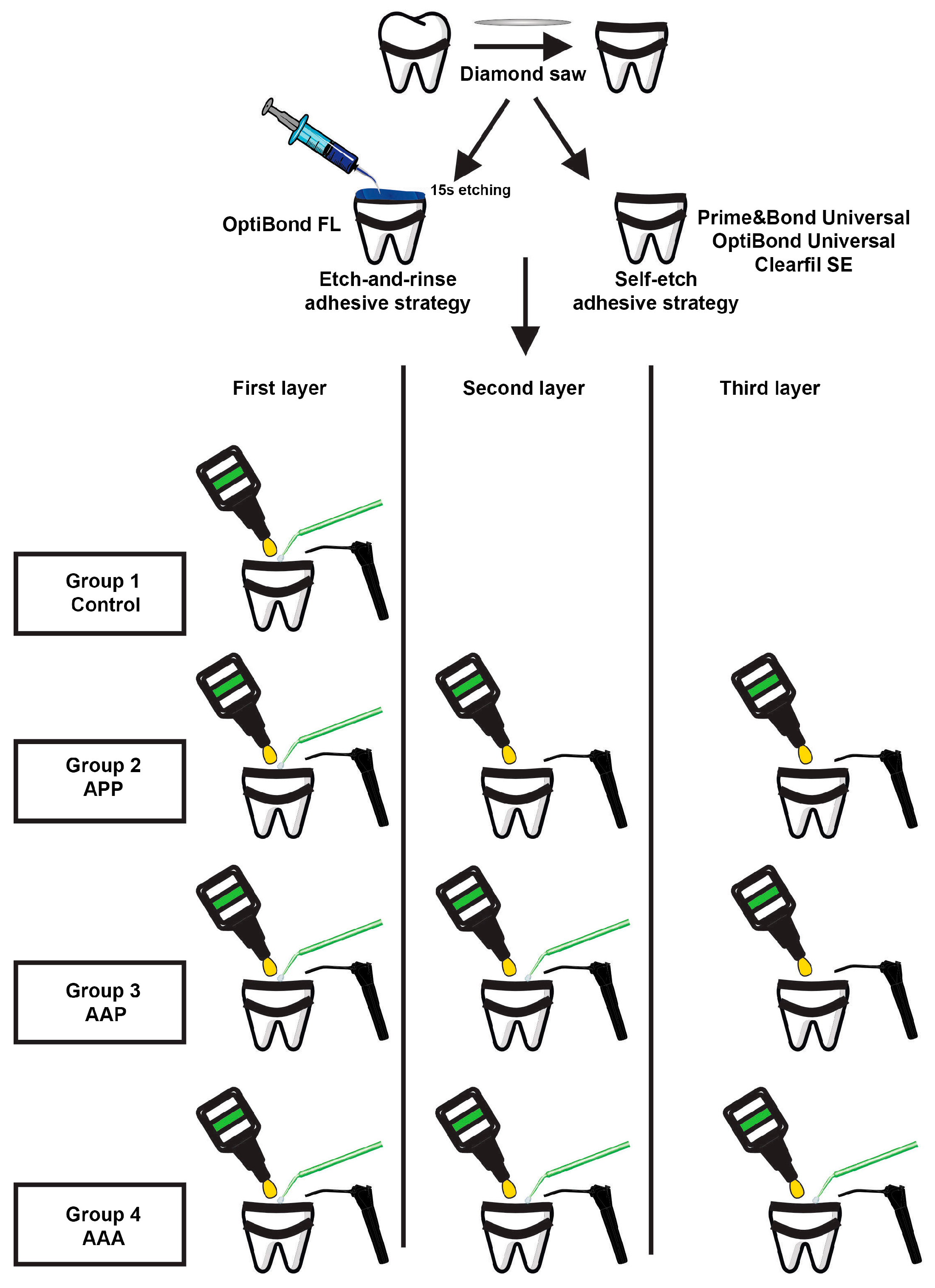
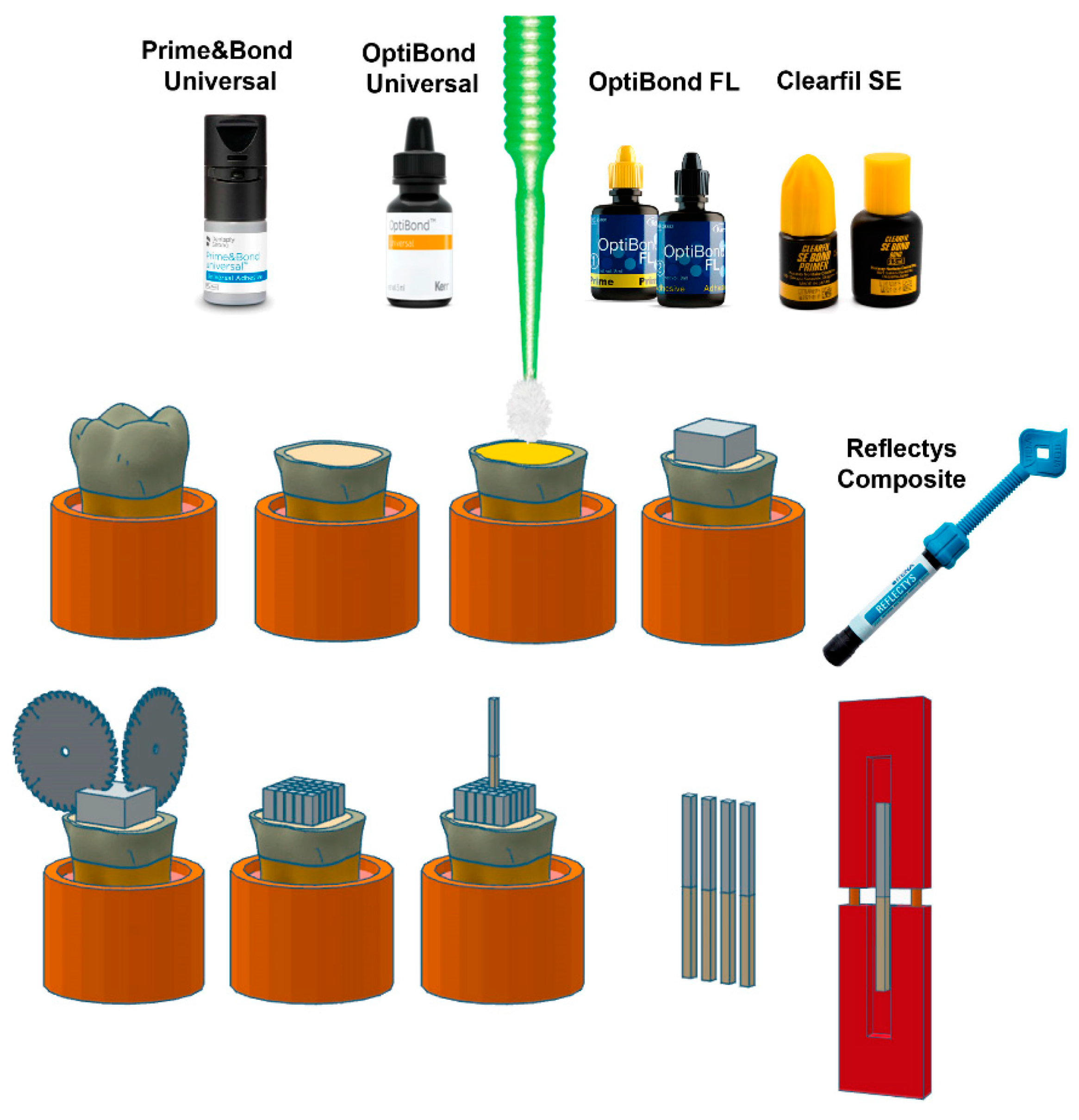
| Material | Classification | Composition * | Manufacturer | Recommendation by the Manufacturer for Adhesive Application |
|---|---|---|---|---|
| PBU | Mild Universal pH = 2.5 | 10-MDP, PENTA, isopropanol, water, photoinitiator, bi- and multifunctional acrylate | Dentsply DeTrey GmbH, Konstanz, Germany | Apply PBU to all cavity surfaces. Avoid pooling. Keep PBU slightly agitated for 20 s. Evaporate solvent with air for at least 5 s. Light cure. |
| OBU | Universal pH = 2.5–3.0 | Acetone, HEMA, GDMA, ethanol, GPDM | Kerr Co, Orange, CA, USA | Using the disposable applicator brush, apply a generous amount of OBU adhesive to the enamel/dentin surface. Scrub the surface with a brushing motion for 20 s. Dry the adhesive with gentle air first and then medium air for at least 5 s with oil-free air. The surface should have a glossy uniform appearance. If not, repeat the bonding and drying steps. Light cure. |
| OBFL | Three-step etch-and- rinse pH primer: 1.9; pH bonding: 6.9 | Etchant: 37.5% H3PO4 Primer: HEMA, GPDM, MMEP, water, ethanol, CQ, and BHT Adhesive: Bis-GMA, HEMA, GDMA, CQ, and filler (fumed SiO2, barium aluminoborosilicat, Na2SiF6), coupling factor A174 | Kerr Co, Orange, CA, USA | Apply OBFL primer using an applicator brush over enamel and dentin surfaces with a light scrubbing motion for 15 s. Gently air dry for approximately 5 s. At this point, the dentin surface should have a slightly shiny appearance. Using a new applicator brush, apply OBFL adhesive to the prepared enamel and dentin surfaces with a light scrubbing motion for 15 s, creating a thin coating. Gently air dry for approximately 5 s. Light cure. |
| CSE | Two-step self-etch pH primer = 1.76 pH bond = 2 | Primer: 10-MDP, HEMA, hydrophilic dimethacrylate, CQ, DEPT, water, ethanol Bond: MDP, HEMA, Bis-GMA, hydrophobic dimethacrylate, CQ, DEPT, silanized colloidal silica | Kuraray Noritake Dental Inc., Tokyo, Japan | Apply primer for 20 s. Dry with mild air flow. Apply bond. Apply air flow gently. Light cure. |
| Technique | CSE | OBFL | OBU | PBU |
|---|---|---|---|---|
| A | X19.02 (3.19) a | X29.66 (5.25) a | X28.3 (5.02) a | X26.16 (8.9) a |
| APP | X16.86 (2.74) a | XY11.87 (4.66) b | XY13.6 (2.25) b | Y7.34 (2.2) b |
| AAP | X17.12 (5.20) a | X15.25 (2.79) b | X12.5 (3.73) b | X10.17 (3.1) b |
| AAA | X19.77 (2.98) a | XY17.16 (6.39) b | X20.6 (8.73) ab | Y11.49 (2.8) b |
| Technique | CSE | OBFL | OBU | PBU |
|---|---|---|---|---|
| A | X17.60 (3.75) a | X13.73 (3.12) a | X16.71 (6.13) a | X20.11 (2.95) a |
| APP | X7 (3.98) b | X10.26 (5.8) b | X6.75 (3.26) b | X7.51 (3.75) b |
| AAP | X10.31 (4.22) b | X8.38 (1.47) b | X8.57 (2.46) b | X6.7 (2.74) b |
| AAA | Y10.43 (2.77) b | X18.03 (5.26) a | Y9.24 (1.76) b | Y9.06 (4.24) b |
| Technique/Adhesive | Aging | |
|---|---|---|
| CSE | 24 h | 6 months |
| A | 19.02 (3.19) X | 17.60 (3.75) X |
| APP | 16.86 (2.74) X | 7 (3.98) Y |
| AAP | 17.12 (5.20) X | 10.31 (4.22) Y |
| AAA | 19.77 (2.98) X | 10.43 (2.77) Y |
| OBFL | 24 h | 6 months |
| A | 29.66 (5.25) X | 13.73 (3.12) Y |
| APP | 11.87 (4.66) X | 10.26 (5.8) X |
| AAP | 15.25 (2.79) X | 8.38 (1.47) Y |
| AAA | 17.16 (6.39) X | 18.03 (5.26) X |
| OBU | 24 h | 6 months |
| A | 28.3 (5.02) X | 16.71 (6.13) Y |
| APP | 13.6 (2.25) X | 6.75 (3.26) Y |
| AAP | 12.5 (3.73) X | 8.57 (2.46) X |
| AAA | 20.6 (8.73) X | 9.24 (1.76) Y |
| PBU | 24 h | 6 months |
| A | 26.16 (8.9) X | 20.11 (2.95) X |
| APP | 7.34 (2.2) X | 7.51 (3.75) X |
| AAP | 10.17 (3.1) X | 6.7 (2.74) X |
| AAA | 11.49 (2.8) X | 9.06 (4.24) X |
| Technique | Material | Aging | Failure Types | |||
|---|---|---|---|---|---|---|
| Adhesive | Mixed | Cohesive Resin | Cohesive Dentin | |||
A | CSE | 24 h | 14 | 16 | 0 | 0 |
| 6 months | 13 | 15 | 1 | 1 | ||
APP | CSE | 24 h | 10 | 14 | 3 | 3 |
| 6 months | 17 | 9 | 2 | 2 | ||
AAP | CSE | 24 h | 13 | 16 | 1 | 0 |
| 6 months | 17 | 11 | 2 | 0 | ||
AAA | CSE | 24 h | 11 | 15 | 2 | 2 |
| 6 months | 16 | 10 | 2 | 2 | ||
| Technique | Material | Aging | Failure Types | |||
| Adhesive | Mixed | Cohesive Resin | Cohesive Dentin | |||
A | OBFL | 24 h | 11 | 19 | 0 | 0 |
| 6 months | 16 | 12 | 1 | 1 | ||
APP | OBFL | 24 h | 15 | 15 | 0 | 0 |
| 6 months | 16 | 14 | 0 | 0 | ||
AAP | OBFL | 24 h | 13 | 15 | 2 | 0 |
| 6 months | 18 | 12 | 0 | 0 | ||
AAA | OBFL | 24 h | 11 | 16 | 2 | 1 |
| 6 months | 14 | 16 | 0 | 0 | ||
| Technique | Material | Aging | Failure Types | |||
|---|---|---|---|---|---|---|
| Adhesive | Mixed | Cohesive Resin | Cohesive Dentin | |||
A | OBU | 24 h | 11 | 17 | 1 | 1 |
| 6 months | 18 | 12 | 0 | 0 | ||
APP | OBU | 24 h | 14 | 16 | 0 | 0 |
| 6 months | 20 | 10 | 0 | 0 | ||
AAP | OBU | 24 h | 15 | 15 | 0 | 0 |
| 6 months | 21 | 9 | 0 | 0 | ||
AAA | OBU | 24 h | 11 | 13 | 3 | 3 |
| 6 months | 18 | 12 | 0 | 0 | ||
| Technique | Material | Aging | Failure Types | |||
|---|---|---|---|---|---|---|
| Adhesive | Mixed | Cohesive Resin | Cohesive Dentin | |||
A | PBU | 24 h | 10 | 20 | 0 | 0 |
| 6 months | 12 | 18 | 0 | 0 | ||
APP | PBU | 24 h | 15 | 15 | 0 | 0 |
| 6 months | 14 | 15 | 0 | 1 | ||
AAP | PBU | 24 h | 14 | 16 | 0 | 0 |
| 6 months | 20 | 9 | 1 | 0 | ||
AAA | PBU | 24 h | 14 | 16 | 0 | 0 |
| 6 months | 15 | 14 | 1 | 0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgi, R.; Kharouf, N.; Cuevas-Suárez, C.E.; Lukomska-Szymańska, M.; Devoto, W.; Kassis, C.; Hasbini, O.; Mancino, D.; Haikel, Y.; Hardan, L. Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin. J. Funct. Biomater. 2023, 14, 522. https://doi.org/10.3390/jfb14100522
Bourgi R, Kharouf N, Cuevas-Suárez CE, Lukomska-Szymańska M, Devoto W, Kassis C, Hasbini O, Mancino D, Haikel Y, Hardan L. Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin. Journal of Functional Biomaterials. 2023; 14(10):522. https://doi.org/10.3390/jfb14100522
Chicago/Turabian StyleBourgi, Rim, Naji Kharouf, Carlos Enrique Cuevas-Suárez, Monika Lukomska-Szymańska, Walter Devoto, Cynthia Kassis, Omar Hasbini, Davide Mancino, Youssef Haikel, and Louis Hardan. 2023. "Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin" Journal of Functional Biomaterials 14, no. 10: 522. https://doi.org/10.3390/jfb14100522
APA StyleBourgi, R., Kharouf, N., Cuevas-Suárez, C. E., Lukomska-Szymańska, M., Devoto, W., Kassis, C., Hasbini, O., Mancino, D., Haikel, Y., & Hardan, L. (2023). Effect of Modified Triple-Layer Application on the Bond Strength of Different Dental Adhesive Systems to Dentin. Journal of Functional Biomaterials, 14(10), 522. https://doi.org/10.3390/jfb14100522













