Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing
Abstract
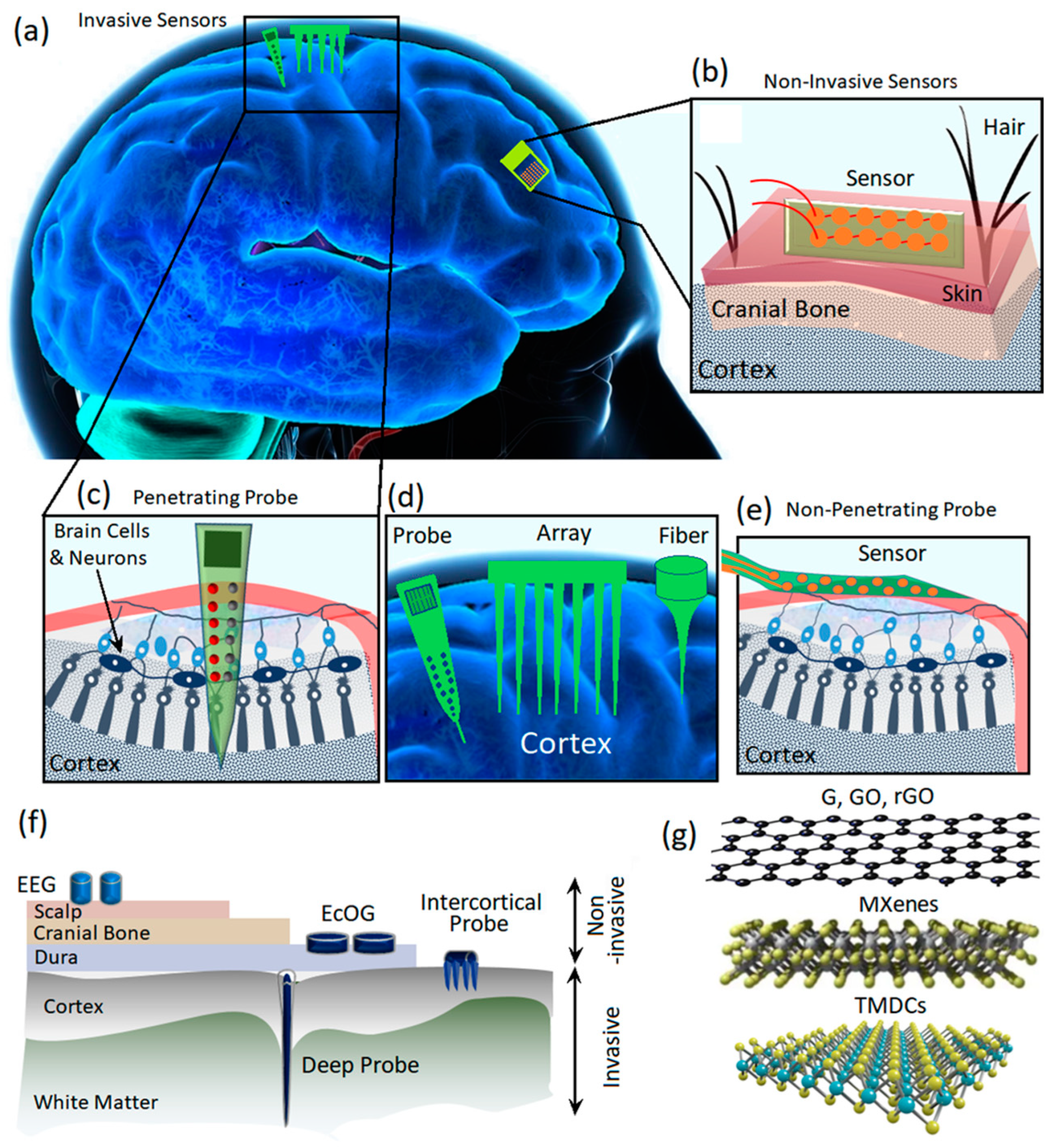
1. Introduction
2. Carbon Based 2D Materials
2.1. Garphene
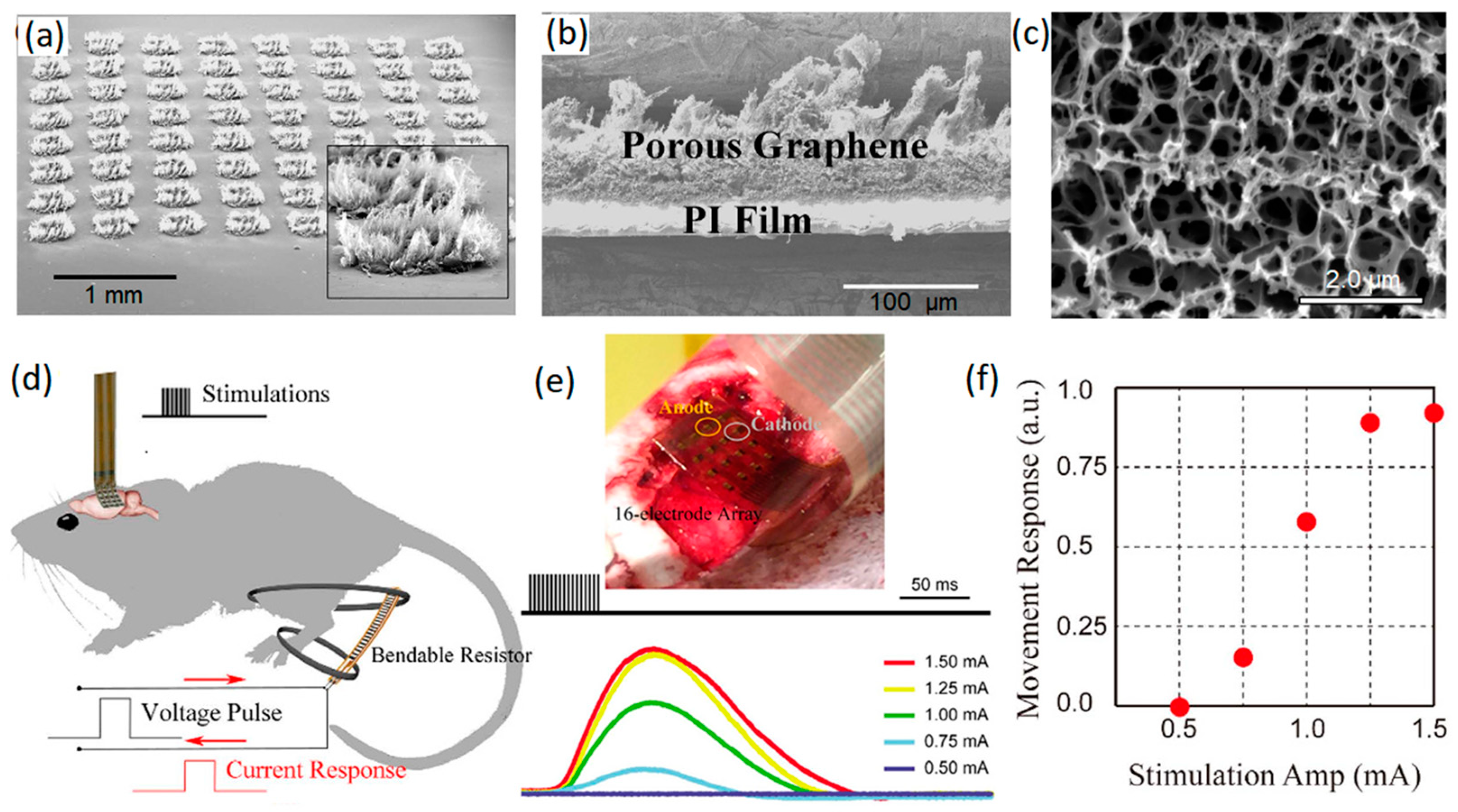
2.1.1. Graphene Microelectrodes
2.1.2. Graphene Field-Effect Transistors (GFETs)
2.2. Other Types of Graphene-Based Materials
3. Two-Dimensional Materials beyond Graphene
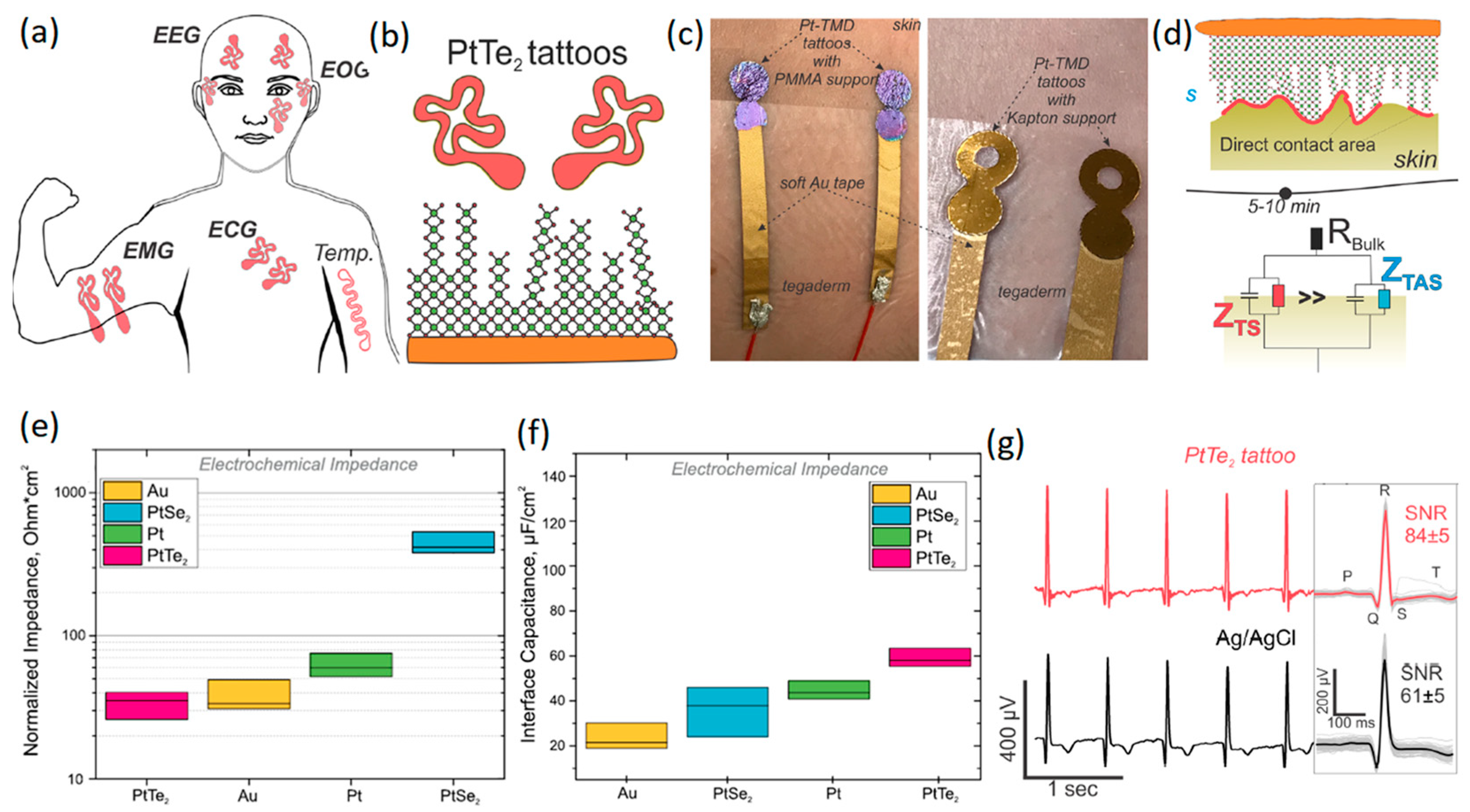
3.1. Transition-Metal Dichalcogenides
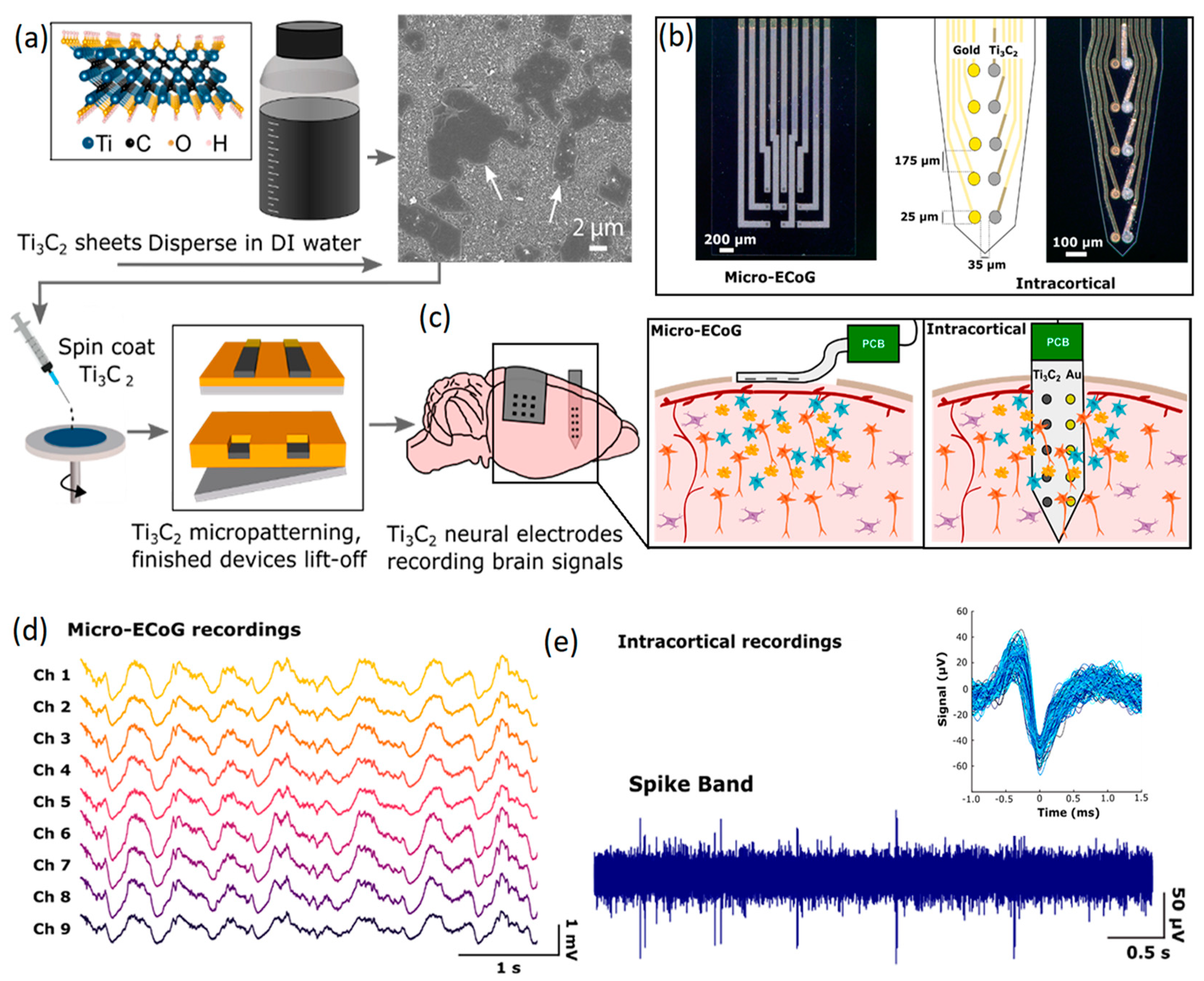
3.2. MXenes
4. Future Opportunities and Challenges
4.1. Impedance and SNR
4.2. Biocompatibility
4.3. Durability
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajeswaran, P.; Orsborn, A.L. Neural interface translates thoughts into type. Nature 2021, 593, 197–198. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, Z.; Liu, X.; Van der Spiegel, J. Electronic neural interfaces. Nat. Electron. 2020, 3, 191–200. [Google Scholar] [CrossRef]
- Sung, C.; Jeon, W.; Nam, K.S.; Kim, Y.J.; Butt, H.; Park, S. Multimaterial and multifunctional neural interfaces: From surface-type and implantable electrodes to fiber-based devices. Mater. Chem. B 2020, 8, 6624–6666. [Google Scholar] [CrossRef] [PubMed]
- Hatsopoulos, N.G.; Donoghue, J.P. The science of neural interface systems. Annu. Rev. Neurosci. 2009, 32, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Spilker, B.; Kamiya, J.; Callaway, E.; Yeager, C.L. Visual evoked responses in subjects trained to control alpha rhythms. Psychophysiology 1969, 5, 683–695. [Google Scholar] [CrossRef]
- Rezeika, A.; Benda, M.; Stawicki, P.; Gembler, F.; Saboor, A.; Volosyak, I. Brain-computer interface spellers: A review. Brain Sci. 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Kastner, S. Promises and limitations of human intracranial electroencephalography. Nat. Neurosci. 2018, 21, 474–483. [Google Scholar] [CrossRef]
- Levinson, D.F. The genetics of depression: A review. Biol. Psychiatry 2006, 60, 84–92. [Google Scholar] [CrossRef]
- Amaral, C.; Mouga, S.; Simões, M.; Pereira, H.C.; Bernardino, I.; Quental, H. A feasibility clinical trial to improve social attention in autistic spectrum disorder (ASD) using a brain computer interface. Front. Neurosci. 2018, 12, 477. [Google Scholar] [CrossRef]
- Hodaie, M.; Wennberg, R.A.; Dostrovsky, J.O.; Lozano, A.M. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia 2002, 43, 603–608. [Google Scholar] [CrossRef]
- Ang, K.K.; Chua, K.S.G.; Phua, K.S.; Wang, C.; Chin, Z.Y.; Kuah, C.W.K. A randomized controlled trial of EEG-Based motor imagery brain-computer interface robotic rehabilitation for stroke. Clin. EEG Neurosci. 2015, 46, 310–320. [Google Scholar] [CrossRef]
- Hochberg, L.R.; Serruya, M.D.; Friehs, G.M.; Mukand, J.A.; Saleh, M.; Caplan, A.H. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 2006, 442, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Baldermann, J.C.; Hardenacke, K.; Hu, X.; Köster, P.; Horn, A.; Freund, H.J. Neuroanatomical characteristics associated with response to deep brain stimulation of the nucleus basalis of meynert for alzheimer’s disease. Neuromodulation 2018, 21, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J. A history of deep brain stimulation: Technological innovation and the role of clinical assessment tools. Soc. Stud. Sci. 2013, 43, 707–728. [Google Scholar] [CrossRef]
- Bell, C.J.; Shenoy, P.; Chalodhorn, R.; Rao, P.N. Control of a humanoid robot by a non-invasive brain-computer interface in humans. J. Neural Eng. 2008, 5, 214. [Google Scholar] [CrossRef]
- Cincotti, F.; Mattia, D.; Aloise, F.; Bufalari, S.; Schalk, G.; Oriolo, G.; Cherubini, A.; Marciani, M.G.; Babiloni, F. Non-Invasive brain-computer interface system: Towards its application an assistive technology. Brain Res. Bull. 2008, 75, 796–803. [Google Scholar] [CrossRef] [PubMed]
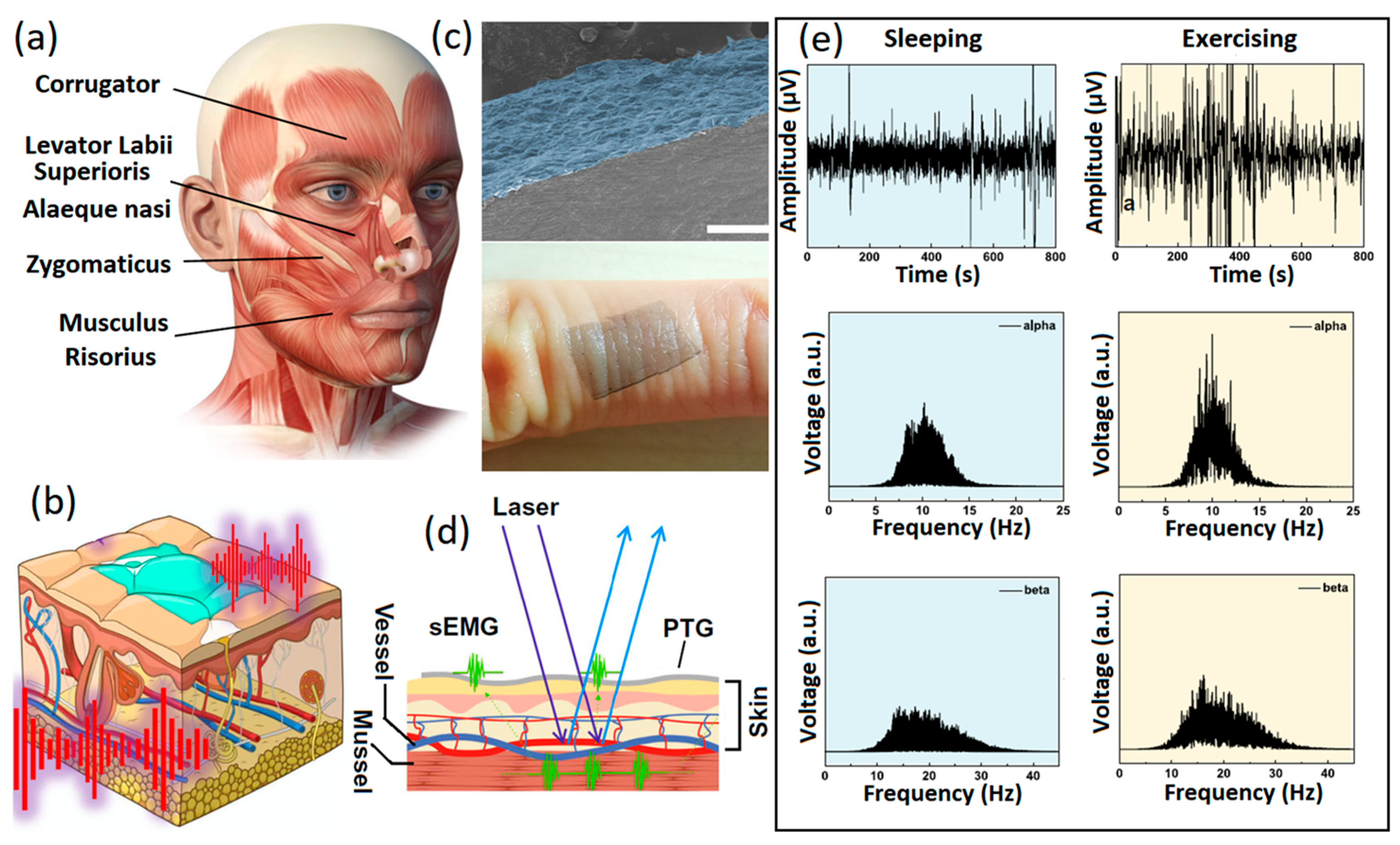
- Li, Z.; Guo, W.; Huang, Y.; Zhu, K.; Yi, H.; Wu, H. On-skin graphene electrodes for large area electrophysiological monitoring and human-machine interfaces. Carbon 2020, 164, 164–170. [Google Scholar] [CrossRef]
- Murastov, G.; Bogatova, E.; Brazovskiy, K.; Amin, I.; Lipovka, A.; Dogadina, E.; Cherepnyov, A.; Ananyeva, A.; Plotnikov, E.; Ryabov, V.; et al. Flexible and water-stable graphene-based electrodes for long-term use in bioelectronics. Biosens. Bioelectron. 2020, 166, 112426–112436. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, S.; Kwon, Y.-T.; Yeo, W.H. Soft wireless bioelectronics and differential electrodermal activity for home sleep monitoring. Sensors 2021, 21, 354. [Google Scholar] [CrossRef]
- Ullah, H.; Wahab, M.A.; Will, G.; Karim, M.R.; Pan, T.; Gao, M.; Lai, D.; Lin, Y.; Miraz, M.H. Recent advances in stretchable and wearable capacitive electrophysiological sensors for long-term health monitoring. Biosensors 2022, 12, 630. [Google Scholar] [CrossRef]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z. 25th anniversary article: The evolution of electronic skin (E-Skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Pan, T.; Xue, M.; Chen, C.; Cui, Y.; Yao, G.; Huang, L.; Liao, F.; Jing, W.; Zhang, H. Thermal release transfer printing for stretchable conformal bioelectronics. Adv. Sci. 2017, 4, 1700251. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xue, M.; Wen, Y.; Yao, G.; Cui, Y.; Liao, F.; Yan, Z.; Huang, L.; Khan, S.A.; Gao, M. A ferroelectric ceramic/polymer composite-based capacitive electrode array for in vivo recordings. Adv. Healthc. Mater. 2017, 6, 1700305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, Y.; Xiao, G.; He, E.; Xie, J.; Dai, Y.; Xing, Y.; Wang, Y.; Xu, S.; Wang, M.; et al. PDMS-parylene hybrid, flexible micro-ECoG electrode array for spatiotemporal mapping of epileptic electrophysiological activity from multicortical brain regions. ACS Appl. Bio Mater. 2021, 4, 8013–8022. [Google Scholar] [CrossRef]
- Penaloza, C.I.; Nishio, S. BMI control of a third arm for multitasking. Sci. Robot. 2018, 3, 1228. [Google Scholar] [CrossRef]
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Karbowski, K. Electroencephalography, the past and future. Eur. Neurol. 2004, 30, 170–175. [Google Scholar] [CrossRef]
- Donoghue, J.P. Bridging the brain to the world: A perspective on neural interface systems. Neuron 2008, 60, 511–521. [Google Scholar] [CrossRef]
- Yan, R.; Park, J.-H.; Choi, Y.; Heo, C.-J.; Yang, S.-M.; Lee, L.P.; Yang, P. Nanowire-based single-cell endoscopy. Nat. Nanotechnol. 2012, 7, 191–196. [Google Scholar] [CrossRef]
- Veronica, A.; Li, Y.; Hsing, I. Minimally invasive & long-lasting neural probes from a materials perspective. Electroanalysis 2019, 31, 586–602. [Google Scholar]
- Donoghue, J.P. Connecting cortex to machines: Recent advances in brain interfaces. Nat. Neurosci. 2002, 5, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Eles, J.R.; Ludwig, K.A.; Seymour, J.P.; Michelson, N.J.; McFadden, W.E.; Vazquez, A.L.; Kozai, D.Y.A. Materials roadmap to functional neural interface design. Adv. Funct. Mater. 2018, 28, 1701269. [Google Scholar] [CrossRef]
- Vomero, M.; Castagnola, E.; Ciarpella, F.; Maggiolini, E.; Goshi, N.; Zucchini, E.; Carli, S.; Fadiga, L.; Kassegne, S.; Ricci, D. Highly stable glassy carbon interfaces for long-term neural stimulation and low-noise recording of brain activity. Sci. Rep. 2017, 7, 40332. [Google Scholar] [CrossRef] [PubMed]
- Im, C.; Seo, J.M. A review of electrodes for the electrical brain signal recording. Biomed. Eng. Lett. 2016, 6, 104–112. [Google Scholar] [CrossRef]
- Sheng, X.; Qin, Z.; Xu, H.; Shu, X.; Gu, G.; Zhu, X. Soft ionic hydrogel electrodes for electroencephalography signal recording. Sci. China Technol. Sci. 2021, 64, 273–282. [Google Scholar] [CrossRef]
- Lopez-Gordo, M.A.; Sanchez-Morillo, D.; Valle, F.P. Dry EEG electrodes. Sensors 2014, 14, 12847–12870. [Google Scholar] [CrossRef] [PubMed]
- Spüler, M. A high-speed brain-computer interface (BCI) using dry EEG electrodes. PLoS ONE 2017, 12, e0172400. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Wu, J.-T.; Xia, Y.H.; He, Q.G.; Jin, H.G. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 2020, 17, 051004. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Ferguson, J.E.; Boldt, C.; Redish, A.D. Creating low-impedance tetrodes by electroplating with additives. Sens. Actuators A 2009, 156, 388–393. [Google Scholar] [CrossRef]
- Oldroyd, P.; Malliaras, G.G. Achieving long-term stability of thin-film electrodes for neurostimulation. Acta Biomater. 2022, 139, 65–81. [Google Scholar] [CrossRef]
- LaRocco, J.; Le, M.D.; Paeng, D.G. A systemic review of available low-cost EEG headsets used for drowsiness detection. Front. Neuroinform. 2020, 14, 553352. [Google Scholar] [CrossRef]
- Boehler, C.; Stieglitz, T.; Asplund, M. Nanostructured platinum grass Enables superior impedance reduction for neural microelectrodes. Biomaterials 2015, 67, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.D.; Cogan, S.F.; Nguyen, T.H.; Rauh, R.D. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible neural electrode array based-on porous graphene for cortical microstimulation and Sensing. Sci. Rep. 2016, 6, 33526. [Google Scholar] [CrossRef]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef]
- Vitale, F.; Summerson, S.R.; Aazhang, B.; Kemere, C.; Pasquali, M. Neural stimulation and recording with bidirectional, soft carbon nanotube fiber microelectrodes. ACS Nano 2015, 9, 4465–4474. [Google Scholar] [CrossRef] [PubMed]
- Patolsky, F.; Timko, B.P.; Yu, G.; Fang, Y.; Greytak, A.B.; Zheng, G.; Lieber, C.M. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 2006, 313, 1100–1104. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Akbari, M.; Zhuiykov, S. Ultrathin Two-Dimensional Semiconductors for Novel Electronic Applications, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Boca Raton, FL, USA, 2021; pp. 275–307. [Google Scholar]
- Karbalaei Akbari, M.; Zhuiykov, S.; Verpoort, F. State-of-the-art surface oxide semiconductors of liquid metals: An emerging platform for development of multifunctional two-dimensional materials. J. Mater. Chem. A 2021, 9, 34–73. [Google Scholar] [CrossRef]
- Xu, H.; Karbalaei Akbari, M.; Zhuiykov, S. 2D Semiconductor nanomaterials and heterostructures: Controlled synthesis and functional applications. Nanoscale Res. Lett. 2021, 16, 94. [Google Scholar] [CrossRef]
- Wei, Z.H.; Karbalaei Akbari, M. Ultrasensitive, sustainable, and selective electrochemical hydrazine detection by ALD-developed two-dimensional WO3. Chem. Electron. Chem. 2017, 4, 266–272. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, X.; Kuzum, D. Graphene-based neurotechnologies for advanced neural interfaces. Curr. Opin. Biomed. Eng. 2018, 6, 138–147. [Google Scholar] [CrossRef]
- Bramini, M.; Alberini, G.; Colombo, E.; Chiacchiaretta, M.; DiFrancesco, M.L.; Maya-Vetencourt, J.F.; Maragliano, L.; Benfenati, F.; Cesca, F. Interfacing graphene-based materials with neural cells. Front. Syst. Neurosci. 2018, 12, 12. [Google Scholar] [CrossRef]
- Kostarelos, K.; Vincent, M.; Hebert, C.; Garrido, J.A. Graphene in the design and engineering of next-generation neural interface. Adv. Mater. 2017, 29, 1700909. [Google Scholar] [CrossRef]
- Park, D.W.; Schendel, A.; Mikael, S.; Brodnick, S.; Richner, T.; Ness, J.; Hayat, R.; Atry, F.; Frye, S.; Pashaie, R.; et al. Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications. Nat. Commun. 2014, 5, 5258. [Google Scholar] [CrossRef]
- Faisal, S.N.; Amjadipour, M.; Izzo, K.; Singer, J.; Bendavid, A.; Lin, C.T.; Iacop, F. Non-invasive on-skin sensors for brain machine interfaces with epitaxial graphene. J. Neural Eng. 2021, 18, 066035. [Google Scholar] [CrossRef]
- Ferro, M.D.; Melosh, N.A. Electronic and ionic materials for neurointerfaces. Adv. Funct. Mater. 2018, 28, 1704335. [Google Scholar] [CrossRef]
- Chen, R.; Canales, A.; Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017, 2, 16093. [Google Scholar] [CrossRef]
- Stosiek, C.; Garaschuk, O.; Holthoff, K.; Konnerth, A. In vivo two photon calcium imaging of neuronal networks. Proc. Nat. Acad. Sci. USA 2003, 100, 7319–7324. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Sun, X.R.; Giovannucci, A.; Lynch, L.A.; Wang, S.S.-H. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics 2014, 1, 025008. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Adhikari, A.; Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci. 2017, 18, 222–235. [Google Scholar] [CrossRef]
- Kuzum, D.; Takano, H.; Shim, E.; Reed, J.C.; Juul, H.; Richardson, A.G.; De Vries, J.; Bink, H.; Dichter, M.A.; Lucas, T.H. Transparent and flexible low noise graphene electrodes for simultaneous electrophysiology and neuroimaging. Nat. Commun. 2014, 5, 5259. [Google Scholar] [CrossRef]
- Liu, X.; Lu, Y.; Iseri, E.; Shi, Y.; Kuzum, D. A compact closed-loop optogenetics system based on artifact-free transparent graphene electrodes. Front. Neurosci. 2018, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Makino, H.; Ren, C.; Liu, H.; Kim, A.N.; Kondapaneni, N.; Liu, X.; Kuzum, D.; Komiyama, T. Transformation of cortex-wide emergent properties during motor learning. Neuron 2017, 94, 880–890.e888. [Google Scholar] [CrossRef]
- Yan, Z.; Peng, Z.; Tour, J.M. Chemical vapor deposition of graphene single crystals. Acc. Chem. Res. 2014, 47, 1327–1337. [Google Scholar] [CrossRef]
- Forsyth, R.; Devadoss, A.; Guy, O.J. Graphene field effect transistors for biomedical applications: Current status and future prospects. Diagnostics 2017, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y.; Iseri, E.; Ren, C.; Liu, H.; Komiyama, T.; Kuzum, D. Transparent artifact-free graphene electrodes for compact closed-loop optogenetics systems. In Proceedings of the 2017 IEEE international electron devices meeting, San Francisco, CA, USA, 2–6 December 2017. [Google Scholar]
- Thunemann, M.; Lu, Y.; Liu, X.; Kõlõç, K.; Desjardins, M.; Vandenberghe, M.; Sadegh, S.; Saisan, P.A.; Cheng, Q.; Weldy, K.L. Deep 2-photon imaging and artifact-free optogenetics through transparent graphene microelectrode arrays. Nat. Commun. 2018, 9, 2035. [Google Scholar] [CrossRef]
- D’Arsié, L.; Esconjauregui, S.; Weatherup, R.S.; Wu, X.; Arter, W.E.; Sugime, H.; Cepek, C.; Robertson, J. Stable, efficient P-type doping of graphene by nitric acid. RSC. Adv. 2016, 6, 113185–113192. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef] [PubMed]
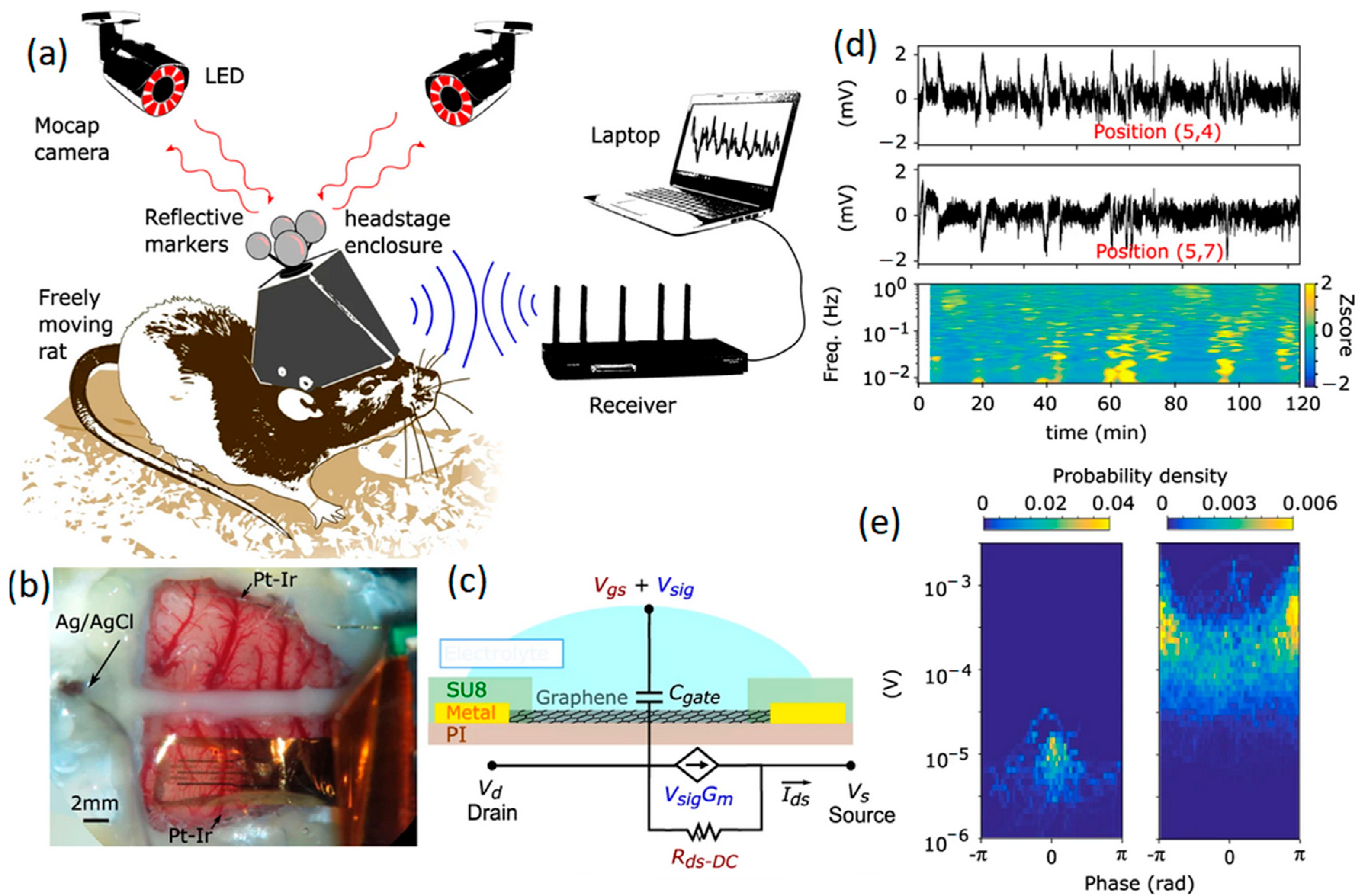
- Garcia-Cortadella, R.; Schafer, N.; Cisneros-Fernandez, J.; Re, L.; Illa, X.; Schwesig, G.; Moya, A.; Santiago, S.; Guirado, G.; Villa, R.; et al. Switchless multiplexing of graphene active sensor arrays for brain mapping. Nano Lett. 2020, 20, 3528–3537. [Google Scholar] [CrossRef] [PubMed]
- Masvidal-Codina, E.; Illa, X.; Dasilva, M.; Calia, A.B.; Dragojevic, T.; Vidal-Rosas, E.E.; Prats-Alfonso, E.; Martinez-Aguilar, J.; De la Cruz, J.M.; Garcia-Cortadella, R.; et al. High-resolution mapping of infraslow cortical brain activity enabled by graphene microtransistors. Nat. Mater. 2019, 18, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortadella, R.; Schwesig, G.; Jeschke, C.; Illa, X.; Gray, A.L.; Savage, S.; Stamatidou, E.; Schiessl, I.; Masvidal-Codina, E.; Kostarelos, K.; et al. Graphene active sensor arrays for long-term and wirelessly mapping of wide frequency band epicortical brain activity. Nat. Commun. 2021, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Blaschke, B.M.; Tort-Colet, N.; Guimerà-Brunet, A.; Weinert, J.; Rousseau, L.; Heimann, A.; Drieschner, S.; Kempski, O.; Villa, R.; Sanchez-Vives, M.V. Mapping brain activity with flexible graphene micro-transistors. 2D Mater. 2017, 4, 025040. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Qu, L. Graphene-based fibers: Recent advances in preparation and application. Adv. Mater. 2020, 32, 1901979. [Google Scholar] [CrossRef]
- Wang, K.; Frewin, C.L.; Esrafilzadeh, D.; Yu, C.; Wang, C.; Pancrazio, J.J.; Romero-Ortega, M.; Jalili, R.; Wallace, G. High performance graphene-fiber based neural recording microelectrodes. Adv. Mater. 2019, 31, 1805867. [Google Scholar] [CrossRef] [PubMed]
- Bonaccini Calia, A.; Masvidal-Codina, E.; Smith, T.M.; Schafer, N.; Rathore, D.; Rodriguez-Lucas, E.; Illa, X.; De la Cruz, J.M.; Del Corro, E.; Prats-Alfonso, E.; et al. Fullbandwidth electrophysiology of seizures and epileptiform activity enabled by flexible graphene microtransistor depth neural probes. Nat. Nanotechnol. 2022, 17, 301–309. [Google Scholar] [CrossRef]
- Das, P.S.; Park, S.H.; Baik, K.Y.; Lee, J.W.; Park, J.Y. Thermally reduced graphene oxide-nylon membrane based epidermal sensor using vacuum filtration for wearable electrophysiological signals and human motion monitoring. Carbon 2020, 158, 386–393. [Google Scholar] [CrossRef]
- Sanderson, K. Electronic skin: From flexibility to a sense of touch. Nature 2021, 591, 685–687. [Google Scholar] [CrossRef]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, S.; Park, S. Electronic skin: Recent progress and future prospects for skin attachable devices for health monitoring, robotics, and prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef]
- Ameri, S.K.; Kim, M.; Kuang, I.A.; Perera, W.K.; Alshiekh, M.; Jeong, H.; Topcu, U.; Akinwande, D.; Lu, N. Imperceptible electrooculography graphene sensor system for human-robot interface. NPJ 2D Mater. Appl. 2018, 2, 19. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, X.; Wang, J.; Ji, S.; Hirtz, T.; Tian, H.; Jian, J.; Cui, T.; Dong, Y.; Xu, X.; et al. Intelligent and multifunctional graphene nanomesh electronic skin with high comfort. Small 2022, 18, 2104810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Yu, T.; Zhang, Y.; Ye, G.; Cui, H.; He, C.; Jiang, W.; Zhai, Y.; Lu, C.; et al. Ultra-conformal skin electrodes with synergistically enhanced conductivity for long-time and low-motion artifact epidermal electrophysiology. Nat. Commun. 2021, 12, 4880. [Google Scholar] [CrossRef] [PubMed]
- Golparvar, A.J.; Yapici, M.K. Electrooculography by wearable graphene textile. IEE Sens. 2018, 18, 8971–8978. [Google Scholar] [CrossRef]
- Ba, H.; Truong-Phuoc, L.; Papaefthimiou, V.; Sutter, C.; Pronkin, S.; Bahouka, A.; Lafue, Y.; Nguyen-Dinh, L.; Giambastiani, G.; Pham-Huu, C. Cotton fabrics coated with few-layer graphene as highly responsive surface heaters and integrated lightweight electronic-textile circuits. ACS Appl. Nano Mater. 2020, 3, 9771–9783. [Google Scholar] [CrossRef]
- Shuvo, I.I.; Shah, A.; Dagdeviren, C. Electronic textile sensor for decoding vital body signals: State-of-the-art review on characterizations and recommendations. Adv. Intell. Syst. 2022, 4, 2100223. [Google Scholar] [CrossRef]
- Afroj, S.; Karim, N.; Wang, Z.; Tan, S.; He, P.; Holwill, M.; Ghazaryan, D.; Fernando, A.; Novoselov, K.S. Engineering graphene flakes for wearable textile sensors via highly scalable and ultrafast yarn dyeing technique. ACS Nano 2019, 13, 3847–3857. [Google Scholar] [CrossRef]
- Apollo, N.V.; Maturana, M.I.; Tong, W.; Nayagam, D.A.; Shivdasani, M.N.; Foroughi, J.; Wallace, G.G.; Prawer, S.; Ibbotson, M.R.; Garrett, D.J. Soft, flexible freestanding neural stimulation and recording electrodes fabricated from reduced graphene oxide. Adv. Funct. Mater. 2015, 25, 3551–3559. [Google Scholar] [CrossRef]
- Sun, F.; Ghosh, H.; Wang, J.; Tan, Z.; Sivoththaman, S. Improving the electrical conductivity of reduced graphene oxide transparent electrodes for photovoltaics. In Proceedings of the 47th IEEE Photovoltaic Specialists Conference (PVSC), Calgary, AB, Canada, 15 June–21 August 2020. [Google Scholar] [CrossRef]
- Liu, T.C.; Chuang, M.C.; Chu, C.Y.; Huang, W.C.; La, H.Y.; Wang, C.T.; Chu, W.L.; Chen, S.Y.; Chen, Y.Y. Implantable graphene-based neural electrode interfaces for electrophysiology and neurochemistry in in vivo hyperacute stroke mode. ACS Appl. Mater. Interfaces 2016, 8, 187–196. [Google Scholar] [CrossRef]
- Ameri, S.K.; Ho, R.; Jang, H.; Tao, L.; Wang, Y.; Wang, L.; Schnyer, D.M.; Akinwande, D.; Lu, N. Graphene electronic tattoo. Sensors. ACS Nano 2017, 11, 7634–7641. [Google Scholar] [CrossRef] [PubMed]
- Shathi, M.A.; Chen, M.; Khoso, N.A.; Rahman, M.T.; Bhattacharjee, B. Graphene coated textile based highly flexible and washable sports bra for human health monitoring. Mater. Des. 2020, 193, 108792. [Google Scholar] [CrossRef]
- Qiu, J.; Yu, T.; Zhang, W.; Zhao, Z.; Zhang, Y.; Ye, G.; Zhao, Y.; Du, X.; Liu, X.; Yang, L.; et al. A bioinspired, durable, and nondisposable transparent graphene skin electrode for electrophysiological signal detection. ACS Mater. Lett. 2020, 2, 999–1007. [Google Scholar] [CrossRef]
- Bakhshaee Babaroud, N.; Palmar, M.; Velea, A.I.; Coletti, C.; Weingärtner, S.; Vos, F.; Serdijn, W.A.; Vollebregt, S.; Giagka, V. Multilayer CVD graphene electrodes using a transfer-free process for the next generation of optically transparent and MRI-compatible neural interfaces. Microsyst. Nanoeng. 2022, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Kong, N.; Ji, X.; Zhang, Y.; Sharma, A.; Ouyang, J.; Qi, B.; Wang, J.; Xie, N.; Kang, C.; et al. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019, 48, 2891. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Zhang, L.; Wei, S.H. A unified understanding of the thickness-dependent bandgap transition in hexagonal two-dimensional semiconductors. J. Phys. Chem. Lett. 2016, 7, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D Transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Villaos, R.A.B.; Crisostomo, C.P.; Huang, Z.Q.; Huang, S.M.; Padama, A.A.B.; Albao, M.A.; Lin, H.; Chuang, F.C. Thickness dependent electronic properties of Pt dichalcogenides. Npj 2D Mater. Appl. 2019, 3, 2. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Pourbaix, M.J.N.; Van Muylder, J.; de Zoubov, N. Electrochemical properties of the platinum metals. Platinum Met. Rev. 1959, 3, 47–53. [Google Scholar]
- Geninatti, T.; Bruno, G.; Barile, B.; Hood, R.L.; Farina, M.; Schmulen, J.; Canavese, G.; Grattoni, A. Impedance characterization degradation, and in vitro biocompatibility for platinum electrodes on BioMEMS. Biomed. Microdevices 2015, 17, 24. [Google Scholar] [CrossRef]
- Guo, G.Y.; Liang, W.Y. The electronic structures of platinum dichalcogenides: PtS2, PtSe2 and PtTe2. J. Phys. C Solid State Phys. 1986, 19, 995–1008. [Google Scholar] [CrossRef]
- Yan, M.; Huang, H.; Zhang, K.; Wang, E.; Yao, W.; Deng, K.; Wan, G.; Zhang, H.; Arita, M.; Yang, H.; et al. Lorentz-violating type-II dirac fermions in transition metal dichalcogenide PtTe2. Nat. Commun. 2017, 8, 257. [Google Scholar] [CrossRef]
- Soled, S.; Wold, A.; Gorochov, O. Crystal growth and characterization of platinum ditelluride. Mater. Res. Bull. 1975, 10, 831–835. [Google Scholar] [CrossRef]
- Yim, C.; Lee, K.; McEvoy, N.; O’Brien, M.; Riazimehr, S.; Berner, N.C.; Cullen, C.P.; Kotakoski, J.; Meyer, J.C.; Lemme, M.C.; et al. High-performance hybrid electronic devices from layered PtSe2 films grown at low temperature. ACS Nano 2016, 10, 9550–9558. [Google Scholar] [CrossRef] [PubMed]
- Okogbue, E.; Han, S.S.; Ko, T.J.; Chung, H.S.; Ma, J.; Shawkat, M.S.; Kim, J.H.; Kim, J.H.; Ji, E.; Oh, K.H.; et al. Multifunctional two-dimensional PtSe2-layer kirigami conductors with 2000% stretchability and metallic-to-semiconducting tunability. Nano Lett. 2019, 19, 7598–7607. [Google Scholar] [CrossRef]
- Han, S.S.; Kim, J.H.; Noh, C.; Kim, J.H.; Ji, E.; Kwon, J.; Yu, S.M.; Ko, T.J.; Okogbue, E.; Oh, K.H.; et al. Horizontal-to-vertical transition of 2D layer orientation in low-temperature chemical vapor deposition-grown PtSe2 and its influences on electrical properties and device applications. ACS Appl. Mater. Interfaces 2019, 11, 13598–13607. [Google Scholar] [CrossRef] [PubMed]
- Okogbue, E.; Ko, T.J.; Han, S.S.; Shawkat, M.S.; Wang, M.; Chung, H.S.; Oh, K.H.; Jung, Y. Wafer-scale 2D PtTe2 layers for high-efficiency mechanically flexible electro-thermal smart window applications. Nanoscale 2020, 12, 10647. [Google Scholar] [CrossRef]
- Wang, M.; Ko, T.J.; Shawkat, M.S.; Han, S.S.; Okogbue, E.; Chung, H.S.; Bae, T.S.; Sattar, S.; Gil, J.; Noh, C.; et al. Wafer-scale growth of 2D PtTe2 with layer orientation tunable high electrical conductivity and superior hydrophobicity. ACS Appl. Mater. Interfaces 2020, 12, 10839–10851. [Google Scholar] [CrossRef]
- Kireev, D.; Okogbue, E.; Jayanth, R.T.; Ko, T.J.; Jung, Y.; Akinwande, D. Multipurpose and reusable ultrathin electronic tattoos based on PtSe2 and PtTe2. ACS Nano 2021, 15, 2800–2811. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, D.; Lin, Z.; Wang, P.; Cao, B.; Ren, H.; Song, F.; Wan, C.; Wang, L.; Zhou, J.; et al. Highly stretchable van der Waals thin films for adaptable and breathable electronic membranes. Science 2022, 375, 852–859. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mats. 2017, 2, 16098. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.Y.; Anasori, B.; Kim, C.K.; Choi, Y.K.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, X.; Xu, W.; Pan, G.; Zhou, D.; Zhu, J.; Wang, H.; Bai, X.; Dong, B.; Song, H. Ratiometric photoluminescence sensing based on Ti3C2 MXene quantum dots as an intracellular PH sensor. Nanoscale 2018, 10, 1111–1118. [Google Scholar] [CrossRef]
- Xuan, J.; Wang, Z.; Chen, Y.; Liang, D.; Cheng, L.; Yang, X.; Liu, Z.; Ma, R.; Sasaki, T.; Geng, F. Organic-base-driven intercalation and delamination for the production of functionalized titanium carbide nanosheets with superior photothermal therapeutic performance. Angew. Chem. Int. Ed. 2016, 55, 14569–14574. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D tantalum carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, M.; Zhang, W.; Zhen, X.; Pei, Z.; Xue, Q.; Zhi, C.; Shi, P. Ultrathin MXene-micropattern-based field-effect transistor for probing neural activity. Adv. Mater. 2016, 28, 3333–3339. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Kota, S.; Lin, Z.; Zhao, M.Q.; Shpigel, N.; Levi, M.D.; Halim, J.; Taberna, P.L.; Barsoum, M.W.; Simon, P.; et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nature Energy. 2017, 2, 17105. [Google Scholar] [CrossRef]
- Zhang, C.J.; Anasori, B.; Seral-Ascaso, A.; Park, S.-H.; McEvoy, N.; Shmeliov, A.; Duesberg, G.S.; Coleman, J.N.; Gogotsi, Y.; Nicolosi, V. Transparent, flexible, and conductive 2D titanium carbide (MXene) films with high volumetric capacitance. Adv. Mater. 2017, 29, 1702678. [Google Scholar] [CrossRef]
- Murphy, B.B.; Mulcahey, P.J.; Driscoll, N.; Richardson, A.G.; Robbins, G.T.; Apollo, N.V.; Maleski, K.; Lucas, T.H.; Gogotsi, Y.; Dillingham, T.; et al. A gel-free Ti3C2Tx-based electrode array for high-density, high-resolution surface electromyography. Adv. Mater. Technol. 2020, 5, 2000325. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.L.; Sun, Y.L.; Bi, C.; Li, Q.F. Development of flexible dry electrode based MXene with low contact impedance for biopotential recording. Measurement 2022, 190, 110782. [Google Scholar] [CrossRef]
- Driscoll, N.; Erickson, B.; Murphy, B.B.; Richardson, A.G.; Robbins, G.; Apollo, N.V.; Mentzelopoulos, G.; Mathis, T.; Hantanasirisakul, K.; Bagga, P.; et al. MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation. Sci. Trans. Med. 2021, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, N.; Richardson, A.G.; Maleski, K.; Anasori, B.; Adewole, O.; Lelyukh, P.; Escobedo, L.; Kacy Cullen, D.; Lucas, T.H.; Gogotsi, Y.; et al. Two-dimensional Ti3C2 MXene for high-resolution neural interfaces. ACS Nano 2018, 12, 10419–10429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Wei, H.; Cao, W.; Qi, Y.; Zhou, S.; Zhang, P.; Li, H.; Li, G.L.; Chai, R. Two-dimensional Ti3C2Tx MXene promotes electrophysiological maturation of neural circuits. J. Nanobiotechnol. 2022, 20, 398. [Google Scholar] [CrossRef] [PubMed]
- Albulbul, A. Evaluating major electrodes types for idle biological signal measurements for modern medical technology. Bioengineering 2016, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Q.; Zhang, Z.; Gan, L.; Zhang, Y.; Wu, J. Materials for dry electrodes for the electroencephalography: Advances challenges, perspectives. Adv. Mater. Technol. 2022, 7, 2100612. [Google Scholar] [CrossRef]
- Szostak, K.M.; Grand, L.; Constandinou, T.G. Neural interfaces for intracortical recording: Requirements, fabrication methods, and characteristics. Front. Neurosci. 2017, 11, 665. [Google Scholar] [CrossRef]
- Bendali, A.; Hess, L.H.; Seifert, M.; Forster, V.; Stephan, A.F.; Garrido, J.A.; Picaud, S. Purified neurons can survive on peptide-free graphene layers. Adv. Healthc. Mater. 2013, 2, 929–933. [Google Scholar] [CrossRef]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J.; Anderson, D.G.; Langer, R. In vivo compatibility of graphene oxide with differing oxidation states. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef]
- Sahni, D.; Jea, A.; Mata, J.A.; Marcano, D.C.; Sivaganesan, A.; Berlin, J.M.; Tatsui, C.E.; Sun, Z.; Luerssen, T.G.; Meng, S. Biocompatibility of pristine graphene for neuronal interface. J. Neurosurg. Pediatr. 2013, 11, 575–583. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Fabbro, A.; Scaini, D.; León, V.N.; Vázquez, E.; Cellot, G.; Privitera, G.; Lombardi, L.; Torrisi, F.; Tomarchio, F.; Bonaccorso, F. GraphenebasedGraphene based interfaces do not alter target nerve cells. ACS Nano 2016, 10, 615–623. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1604. [Google Scholar] [CrossRef]
- Martín, C.; Merino, S.; González-Domínguez, J.M.; Rauti, R.; Ballerini, L.; Prato, M.; Vázquez, E. Graphene improves the biocompatibility of polyacrylamide hydrogels: 3D polymeric scaffolds for neuronal growth. Sci. Rep. 2017, 7, 10942. [Google Scholar] [CrossRef]
- Noh, M.; Kim, S.H.; Kim, J.; Lee, J.R.; Jeong, G.J.; Yoon, J.K.; Kang, S.; Bhang, S.H.; Yoon, H.H.; Lee, J.C. Graphene oxide reinforced hydrogels for osteogenic differentiation of human adiposederived stem cells. RSC Adv. 2017, 7, 20779–20788. [Google Scholar] [CrossRef]
- Hu, X.B.; Liu, Y.L.; Wang, W.J.; Zhang, H.W.; Qin, Y.; Guo, S.; Zhang, X.W.; Fu, L.; Huang, W.H. Biomimetic graphene-based 3D scaffold for long-term cell culture and real-time electrochemical monitoring. Anal. Chem. 2018, 90, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Chen, Y.; Jing, X.; Xiang, L.; Yang, D.; Lin, H.; Liu, Z.; Han, X.; Wu, R. Two-dimensional tantalum carbide (MXenes) composite nanosheets for multiple imaging-guided photothermal tumor ablation. ACS Nano 2017, 11, 12696–12712. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, J.; Lin, H.; Wang, Z.; Li, P.; Chen, Y. 2D Ultrathin MXene-Based Drug-Delivery Nanoplatform for Synergistic Photothermal Ablation and Chemotherapy of Cancer. Adv. Healthc. Mater. 2018, 7, 1701394. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, H.; Xu, G.; Liu, Z.; Wu, R.; Chen, Y. Biocompatible 2D Titanium Carbide (MXenes) Composite Nanosheets for PH-Responsive MRI-Guided Tumor Hyperthermia. Chem. Mater. 2017, 29, 8637–8652. [Google Scholar] [CrossRef]
- Wang, Y.; Yokota, T.; Someya, T. Electrospun nanofiberbased soft electronics. NPG Asia Mater. 2021, 13, 22. [Google Scholar] [CrossRef]
- Hu, Q.; Nag, A.; Xu, Y.; Han, T.; Zhang, L. Use of graphene based fabric sensors for monitoring human activities. Sens. Actuators A Phys. 2021, 332, 113172. [Google Scholar] [CrossRef]

| Neural Interface | Application | SNR | Impedance | Electrode Dimensions | Stability Tests | Ref. |
|---|---|---|---|---|---|---|
| rGO microarrays developed on PDMS (non-invasive) | (e-skin) EEG, EMG, EOG | 16.8 dB | ~500 kΩ at 50 Hz | N/A | 60 times reuse 58 h stability | [20] |
| Laser-induced GO developed on PET (non-invasive) | ECG | ~70 dB | ~60 kΩ at 50 HZ | 4 cm2 | >100 h | [21] |
| Porous graphene array developed by laser pyrolysis (invasive) | Planar electrodes for neural activities | N/A | 5 kΩ at 1 KHz | 250 μm2 | 106 cycles | [45] |
| Epitaxial graphene films developed on silicon substrate (non-invasive) | EEG | N/A | 68 ± 4 kΩ at 10 Hz | 1 cm2 | 120 days | [57] |
| Doped single-layered graphene (invasive) | Planar electrodes for neural activities | 40 | 541 kΩ at 1 KHz | 50 × 50 μm2 | 6 months | [63] |
| Liquid-crystal GO fiber coated with parylene C | Filament deep probing | N/A | 50 kΩ at 1 KHz | N/A | 14 days | [77] |
| CVD graphene-based transparent microarray (invasive) | Optogenetics and neural imaging | N/A | 243.5 kΩ at 1 KHz | 3.1 × 3.1 mm2 | 70 days implanted | [56] |
| Multielectrode array based on graphene microtransistor (invasive) | Neural deep probing | N/A | N/A | N/A | 10 weeks implanted | [78] |
| rGO developed on nylon membrane (non-invasive) | ECG, EMG | N/A | ~15 kΩ at 1000 Hz | N/A | 50 h | [79] |
| Laser-developed graphene on PU nanomesh (non-invasive) | EEG, ECG, EOG | 14.12 dB | N/A | 1.5 cm2 | 1000 cycles | [83] |
| CVD graphene for PMMA tattoo (non-invasive) | ECG, EMG, EEG, EOG | 15.22 dB | ~13 kΩ at 1 KHz | 1.225 cm2 | N/A | [92] |
| rGO-PEDOT/PSS fabricated on nylon-lycra (non-invasive) | ECG | 21.6 dB | ~50 kΩ at 100 Hz | 1 × 1 cm2 | 50 cycles | [93] |
| Electrospun fiber with graphene monolayer (non-invasive) | ECG, EEG, sEMG | ~30 dB | 150 Ω | N/A | 10 times | [94] |
| CVD graphene multilayer electrodes developed on parylene | Neural interfaces compatible with MRI | N/A | 27.4 kΩ at 1 KHz | 430 μm diameter | N/A | [95] |
| Neural Interface | Application | SNR | Impedance | Electrode Dimensions | Stability Tests | Ref. |
|---|---|---|---|---|---|---|
| PtSe2 and PtTe2 based sensors (non-invasive) | EMG, ECG, EOG, EMG | 84 ± 6 dB | 4.94 ± 1.61 kΩ at 10 kHz | N/A | 24 h | [111] |
| Ti3C2Tx (MXene) based sensors (non-invasive) | EMG signals | ~39.23 ± 16.25 dB | ~29.78 ± 8.18 kΩ·cm2 at 1 kHz | 1 cm2 | N/A | [122] |
| Ti3C2 microelectrode array (planar electrodes, non-invasive) | Neural interfacing | N/A | 54.6 ± 28.4 kΩ at 1 kHz | 7 mm2 | N/A | [123] |
| 3D mini pillars composed of MXenes (non-invasive) | EEG, EMG, EOG | N/A | 2.8 ± 0.9 kΩ at 1 kHz | 7 mm2 | N/A | [124] |
| Ti3C2 microelectrode array (intracortical electrodes, invasive) | Neural interfacing | 40 dB | 219 ± 60 kΩ at 1 kHz | 25 μm diameter | 7 days in cell culture | [125] |
| Ti3C2Tx (MXene) | Neural stem cell; Neural spiking, Synaptic transmission | N/A | N/A | 0.785 cm2 | N/A | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbalaei Akbari, M.; Siraj Lopa, N.; Shahriari, M.; Najafzadehkhoee, A.; Galusek, D.; Zhuiykov, S. Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing. J. Funct. Biomater. 2023, 14, 35. https://doi.org/10.3390/jfb14010035
Karbalaei Akbari M, Siraj Lopa N, Shahriari M, Najafzadehkhoee A, Galusek D, Zhuiykov S. Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing. Journal of Functional Biomaterials. 2023; 14(1):35. https://doi.org/10.3390/jfb14010035
Chicago/Turabian StyleKarbalaei Akbari, Mohammad, Nasrin Siraj Lopa, Marina Shahriari, Aliasghar Najafzadehkhoee, Dušan Galusek, and Serge Zhuiykov. 2023. "Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing" Journal of Functional Biomaterials 14, no. 1: 35. https://doi.org/10.3390/jfb14010035
APA StyleKarbalaei Akbari, M., Siraj Lopa, N., Shahriari, M., Najafzadehkhoee, A., Galusek, D., & Zhuiykov, S. (2023). Functional Two-Dimensional Materials for Bioelectronic Neural Interfacing. Journal of Functional Biomaterials, 14(1), 35. https://doi.org/10.3390/jfb14010035











