Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Storage of Compounds
2.2. Cell Culture and Treatment Conditions
2.3. Cell Viability Assay
2.4. Synthesis of 22 kDa L-PEI and Rhodamine–PEI Conjugate
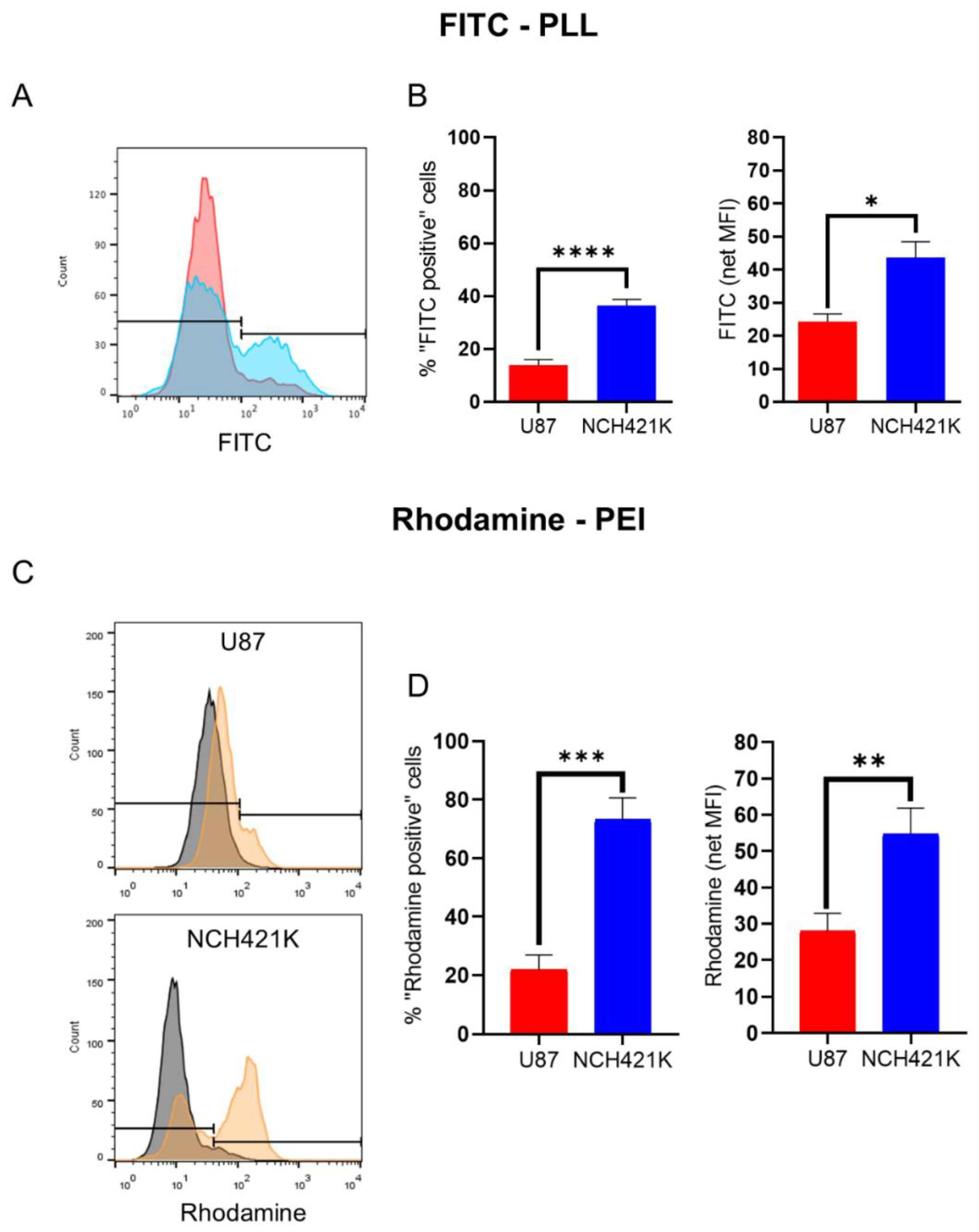
2.5. Cationic Polymer Affinity Assay
2.5.1. U87 and NCH421K Cells
2.5.2. CHO-K1 and pgsA-745 Cells
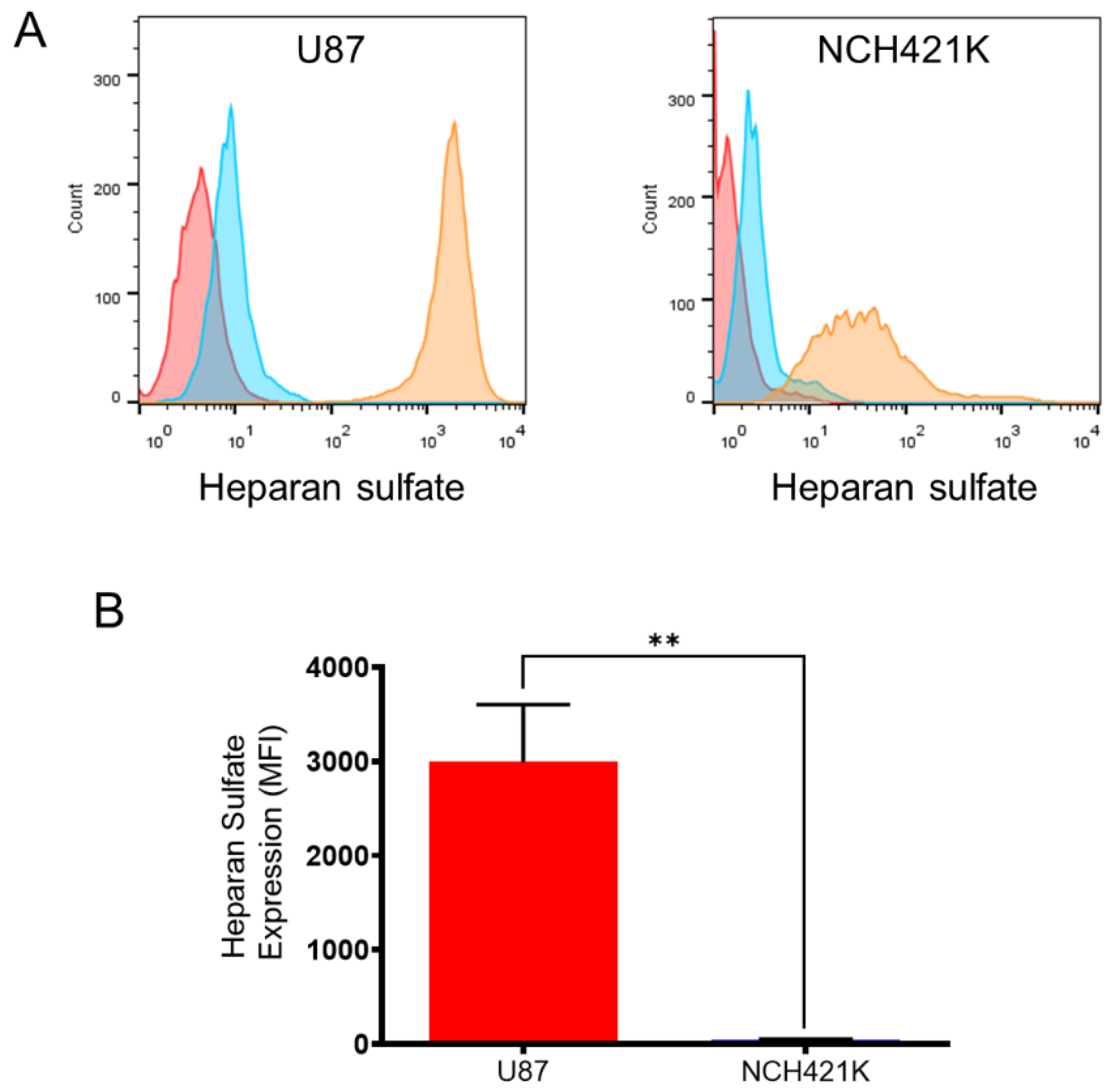
2.6. Heparan Sulfate Expression
3. Results and Discussion
3.1. GSC Specific Toxicity Is Not a General Characteristic of Polycations
3.2. GSC Polycation Affinity Is Not Linked to Heparan Sulfate Expression
3.3. GSC Polycation Toxicity Is Size-Dependent
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B-PEI | Branched PEI |
| CSC | Cancer stem cell |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| FBS | Fetal bovine serum |
| GSC | Glioblastoma stem cell |
| L-PEI | Linear PEI |
| MFI | Median/Geometric mean fluorescence intensity |
| PDC | Polymer–drug conjugate |
| PEI | Polyethylenimine |
| PLL | Poly-L-lysine |
| RLU | Relative luminescence unit |
| RPMI | Roswell Park Memorial Institute |
References
- Moore, C.; Gao, W.; Fatehi, P. Cationic Lignin Polymers as Flocculant for Municipal Wastewater. Polymers 2021, 13, 3871. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A Versatile Vector for Gene and Oligonucleotide Transfer into Cells in Culture and in Vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic Polymers and Their Therapeutic Potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Chen, W.C.W.; Heo, Y.; Wang, Y. Polycations and Their Biomedical Applications. Prog. Polym. Sci. 2016, 60, 18–50. [Google Scholar] [CrossRef]
- Moreau, E.; Domurado, M.; Chapon, P.; Vert, M.; Domurado, D. Biocompatibility of Polycations: In Vitro Agglutination and Lysis of Red Blood Cells and in Vivo Toxicity. J. Drug Target. 2002, 10, 161–173. [Google Scholar] [CrossRef]
- Richter, F.; Leer, K.; Martin, L.; Mapfumo, P.; Solomun, J.I.; Kuchenbrod, M.T.; Hoeppener, S.; Brendel, J.C.; Traeger, A. The Impact of Anionic Polymers on Gene Delivery: How Composition and Assembly Help Evading the Toxicity-Efficiency Dilemma. J. Nanobiotechnol. 2021, 19, 292. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Wang, B.; Yin, D.X.; Zhang, J.; Wu, W.X.; Yu, Q.Y.; Yu, X.Q. Linear TACN-Based Cationic Polymers as Non-Viral Gene Vectors. RSC Adv. 2014, 4, 59164–59174. [Google Scholar] [CrossRef]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging Innate Biological Properties of Nano-Drug Delivery Systems: A Focus on PAMAM Dendrimers and Their Clinical Potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. The Influence of Surface Modification on the Cytotoxicity of PAMAM Dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Chekkat, N.; Dahm, G.; Chardon, E.; Wantz, M.; Sitz, J.; Decossas, M.; Lambert, O.; Frisch, B.; Rubbiani, R.; Gasser, G.; et al. N-Heterocyclic Carbene-Polyethylenimine Platinum Complexes with Potent in Vitro and in Vivo Antitumor Efficacy. Bioconjug. Chem. 2016, 27, 1942–1948. [Google Scholar] [CrossRef]
- Wantz, M.; Bouché, M.; Dahm, G.; Chekkat, N.; Fournel, S.; Bellemin-Laponnaz, S. N-Heterocyclic Carbene-Polyethyleneimine (PEI) Platinum Complexes Inducing Human Cancer Cell Death: Polymer Carrier Impact. Int. J. Mol. Sci. 2018, 19, 3472. [Google Scholar] [CrossRef] [PubMed]
- Cherng, J.Y.; Van De Wetering, P.; Talsma, H.; Crommelin, D.J.A.; Hennink, W.E. Effect of Size and Serum Proteins on Transfection Efficiency of Poly ((2-Dimethylamino)Ethyl Methacrylate)-Plasmid Nanoparticles. Pharm. Res. 1996, 13, 1038–1042. [Google Scholar] [CrossRef]
- Ekladious, I.; Colson, Y.L.; Grinstaff, M.W. Polymer–Drug Conjugate Therapeutics: Advances, Insights and Prospects. Nat. Rev. Drug Discov. 2019, 18, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Fahira, A.I.; Abdulah, R.; Barliana, M.I.; Gatera, V.A.; Amalia, R. Polyethyleneimine (PEI) as a Polymer-Based Co-Delivery System for Breast Cancer Therapy. Breast Cancer Targets Ther. 2022, 14, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Dufès, C.; Keith, W.N.; Bilsland, A.; Proutski, I.; Uchegbu, I.F.; Schätzlein, A.G. Synthetic Anticancer Gene Medicine Exploits Intrinsic Antitumor Activity of Cationic Vector to Cure Established Tumors. Cancer Res. 2005, 65, 8079–8084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Suzuki, K.; Mikami, T.; Okawa, Y.; Tokoro, A.; Suzuki, S.; Suzuki, M. Antitumor Effect of Hexa-N-Acetylchitohexaose and Chitohexaose. Carbohydr. Res. 1986, 151, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in Characterisation and Biological Activities of Chitosan and Chitosan Oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- He, W.; Liang, P.; Guo, G.; Huang, Z.; Niu, Y.; Dong, L.; Wang, C.; Zhang, J. Re-Polarizing Myeloid-Derived Suppressor Cells (MDSCs) with Cationic Polymers for Cancer Immunotherapy. Sci. Rep. 2016, 6, 24506. [Google Scholar] [CrossRef]
- Chen, B.; Le, W.; Wang, Y.; Li, Z.; Wang, D.; Ren, L.; Lin, L.; Cui, S.; Hu, J.J.; Hu, Y.; et al. Targeting Negative Surface Charges of Cancer Cells by Multifunctional Nanoprobes. Theranostics 2016, 6, 1887–1898. [Google Scholar] [CrossRef]
- Motomura, M.; Ichihara, H.; Matsumoto, Y. Nano-Chemotherapy Using Cationic Liposome That Strategically Targets the Cell Membrane Potential of Pancreatic Cancer Cells with Negative Charge. Bioorganic Med. Chem. Lett. 2018, 28, 1161–1165. [Google Scholar] [CrossRef]
- Li, Z.; Ruan, J.; Zhuang, X. Effective Capture of Circulating Tumor Cells from an S180-Bearing Mouse Model Using Electrically Charged Magnetic Nanoparticles. J. Nanobiotechnol. 2019, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Beyerle, A.; Irmler, M.; Beckers, J.; Kissel, T.; Stoeger, T. Toxicity Pathway Focused Gene Expression Profiling of PEI-Based Polymers for Pulmonary Applications. Mol. Pharm. 2010, 7, 727–737. [Google Scholar] [CrossRef]
- Fischer, D.; Li, Y.; Ahlemeyer, B.; Krieglstein, J.; Kissel, T. In Vitro Cytotoxicity Testing of Polycations: Influence of Polymer Structure on Cell Viability and Hemolysis. Biomaterials 2003, 24, 1121–1131. [Google Scholar] [CrossRef]
- Monnery, B.D.; Wright, M.; Cavill, R.; Hoogenboom, R.; Shaunak, S.; Steinke, J.H.G.; Thanou, M. Cytotoxicity of Polycations: Relationship of Molecular Weight and the Hydrolytic Theory of the Mechanism of Toxicity. Int. J. Pharm. 2017, 521, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Merisaari, J.; Le Joncour, V.; Peurla, M.; Karaman, D.Ş.; Casals, E.; Laakkonen, P.; Westermarck, J.; Rosenholm, J.M. Circumventing Drug Treatment? Intrinsic Lethal Effects of Polyethyleneimine (PEI)-Functionalized Nanoparticles on Glioblastoma Cells Cultured in Stem Cell Conditions. Cancers 2021, 13, 2631. [Google Scholar] [CrossRef]
- Knauer, N.; Arkhipova, V.; Li, G.; Hewera, M.; Pashkina, E.; Nguyen, P.-H.; Meschaninova, M.; Kozlov, V.; Zhang, W.; Croner, R.; et al. In Vitro Validation of the Therapeutic Potential of Dendrimer-Based Nanoformulations against Tumor Stem Cells. Int. J. Mol. Sci. 2022, 23, 5691. [Google Scholar] [CrossRef] [PubMed]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular Matrix-Mediated Regulation of Cancer Stem Cells and Chemoresistance. Int. J. Biochem. Cell Biol. 2019, 109, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef]
- Najafi, M.; Mortezaee, K.; Majidpoor, J. Cancer Stem Cell (CSC) Resistance Drivers. Life Sci. 2019, 234, 116781. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mack, S.C.; Mulkearns-Hubert, E.E.; Valentim, C.L.L.; Rich, J.N. Cancer Stem Cells in Glioblastoma. Genes Dev. 2015, 29, 1203–1217. [Google Scholar] [CrossRef]
- McCartin, C.; Dussouillez, C.; Bernhard, C.; Mathieu, E.; Blumberger, J.; Dontenwill, M.; Herold-Mende, C.; Idbaih, A.; Lavalle, P.; Bellemin-Laponnaz, S.; et al. Polyethylenimine, an Autophagy-Inducing Platinum-Carbene-Based Drug Carrier with Potent Toxicity towards Glioblastoma Cancer Stem Cells. Cancers 2022, 14, 5057. [Google Scholar] [CrossRef]
- Smith, A.G.; Macleod, K.F. Autophagy, Cancer Stem Cells and Drug Resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef]
- Esko, J.D.; Elgavish, A.; Prasthofer, T.; Taylor, W.H.; Weinke, J.L. Sulfate Transport-Deficient Mutants of Chinese Hamster Ovary Cells. Sulfation of Glycosaminoglycans Dependent on Cysteine. J. Biol. Chem. 1986, 261, 15725–15733. [Google Scholar] [CrossRef]
- Brissault, B.; Kichler, A.; Guis, C.; Leborgne, C.; Danos, O.; Cheradame, H. Synthesis of Linear Polyethylenimine Derivatives for DNA Transfection. Bioconjug. Chem. 2003, 14, 581–587. [Google Scholar] [CrossRef]
- Günay, K.A.; Ceccato, T.L.; Silver, J.S.; Bannister, K.L.; Bednarski, O.J.; Leinwand, L.A.; Anseth, K.S. PEG–Anthracene Hydrogels as an On-Demand Stiffening Matrix to Study Mechanobiology. Angew. Chem. Int. Ed. 2019, 58, 9912–9916. [Google Scholar] [CrossRef]
- Campos, B.; Wan, F.; Farhadi, M.; Ernst, A.; Zeppernick, F.; Tagscherer, K.E.; Ahmadi, R.; Lohr, J.; Dictus, C.; Gdynia, G.; et al. Differentiation Therapy Exerts Antitumor Effects on Stem-like Glioma Cells. Clin. Cancer Res. 2010, 16, 2715–2728. [Google Scholar] [CrossRef]
- Podergajs, N.; Brekka, N.; Radlwimmer, B.; Herold-Mende, C.; Talasila, K.M.; Tiemann, K.; Rajcevic, U.; Lah, T.T.; Bjerkvig, R.; Miletic, H. Expansive Growth of Two Glioblastoma Stem-like Cell Lines Is Mediated by BFGF and Not by EGF. Radiol. Oncol. 2013, 47, 330–337. [Google Scholar] [CrossRef]
- Francoia, J.-P.; Vial, L. Everything You Always Wanted to Know about Poly-l-Lysine Dendrigrafts (But Were Afraid to Ask). Chem. A Eur. J. 2018, 24, 2806–2814. [Google Scholar] [CrossRef]
- McCartin, C.; Mathieu, E.; Dontenwill, M.; Herold-Mende, C.; Idbaih, A.; Bonfiglio, A.; Mauro, M.; Fournel, S.; Kichler, A. An N-Heterocyclic Carbene Iridium(III) Complex as a Potent Anti-Cancer Stem Cell Therapeutic. Chem. Biol. Interact. 2022, 367, 110167. [Google Scholar] [CrossRef]
- Santos Franco, S.; Raveh-Amit, H.; Kobolák, J.; Alqahtani, M.H.; Mobasheri, A.; Dinnyes, A. The Crossroads between Cancer Stem Cells and Aging. BMC Cancer 2015, 15 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Weiswald, L.-B.; Bellet, D.; Dangles-Marie, V. Spherical Cancer Models in Tumor Biology. Neoplasia 2015, 17, 1–15. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Bishop, J.R.; Schuksz, M.; Esko, J.D. Heparan Sulphate Proteoglycans Fine-Tune Mammalian Physiology. Nature 2007, 446, 1030–1037. [Google Scholar] [CrossRef]
- Mislick, K.A.; Baldeschwieler, J.D. Evidence for the Role of Proteoglycans in Cation-Mediated Gene Transfer. Proc. Natl. Acad. Sci. USA 1996, 93, 12349–12354. [Google Scholar] [CrossRef]
- Poon, G.M.K.; Gariépy, J. Cell-Surface Proteoglycans as Molecular Portals for Cationic Peptide and Polymer Entry into Cells. Biochem. Soc. Trans. 2007, 35, 788–793. [Google Scholar] [CrossRef]
- Kopatz, I.; Remy, J.-S.; Behr, J.-P. A Model for Non-Viral Gene Delivery: Through Syndecan Adhesion Molecules and Powered by Actin. J. Gene Med. 2004, 6, 769–776. [Google Scholar] [CrossRef]
- Xiong, A.; Kundu, S.; Forsberg-Nilsson, K. Heparan Sulfate in the Regulation of Neural Differentiation and Glioma Development. FEBS J. 2014, 281, 4993–5008. [Google Scholar] [CrossRef]
- Steck, P.A.; Moser, R.P.; Bruner, J.M.; Liang, L.; Freidman, A.N.; Hwang, T.L.; Yung, W.K. Altered Expression and Distribution of Heparan Sulfate Proteoglycans in Human Gliomas. Cancer Res. 1989, 49, 2096–2103. [Google Scholar]
- Bertolotto, A.; Magrassi, M.L.; Orsi, L.; Sitia, C.; Schiffer, D. Glycosaminoglycan Changes in Human Gliomas. A Biochemical Study. J. Neurooncol. 1986, 4, 43–48. [Google Scholar] [CrossRef]
- Holley, R.J.; Meade, K.A.; Merry, C.L.R. Using Embryonic Stem Cells to Understand How Glycosaminoglycans Regulate Differentiation. Biochem. Soc. Trans. 2014, 42, 689–695. [Google Scholar] [CrossRef]
- Sasaki, N.; Hirano, T.; Kobayashi, K.; Toyoda, M.; Miyakawa, Y.; Okita, H.; Kiyokawa, N.; Akutsu, H.; Umezawa, A.; Nishihara, S. Chemical Inhibition of Sulfation Accelerates Neural Differentiation of Mouse Embryonic Stem Cells and Human Induced Pluripotent Stem Cells. Biochem. Biophys. Res. Commun. 2010, 401, 480–486. [Google Scholar] [CrossRef]
- Vitale, D.; Kumar Katakam, S.; Greve, B.; Jang, B.; Oh, E.S.; Alaniz, L.; Götte, M. Proteoglycans and Glycosaminoglycans as Regulators of Cancer Stem Cell Function and Therapeutic Resistance. FEBS J. 2019, 286, 2870–2882. [Google Scholar] [CrossRef]
- Esko, J.D.; Stewart, T.E.; Taylor, W.H. Animal Cell Mutants Defective in Glycosaminoglycan Biosynthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 3197–3201. [Google Scholar] [CrossRef]
- Kunath, K.; von Harpe, A.; Fischer, D.; Petersen, H.; Bickel, U.; Voigt, K.; Kissel, T. Low-Molecular-Weight Polyethylenimine as a Non-Viral Vector for DNA Delivery: Comparison of Physicochemical Properties, Transfection Efficiency and in Vivo Distribution with High-Molecular-Weight Polyethylenimine. J. Control. Release 2003, 89, 113–125. [Google Scholar] [CrossRef]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A Novel Non-Viral Vector for DNA Delivery Based on Low Molecular Weight, Branched Polyethylenimine: Effect of Molecular Weight on Transfection Efficiency and Cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef]
- Choksakulnimitr, S.; Masuda, S.; Tokuda, H.; Takakura, Y.; Hashida, M. In Vitro Cytotoxicity of Macromolecules in Different Cell Culture Systems. J. Control. Release 1995, 34, 233–241. [Google Scholar] [CrossRef]
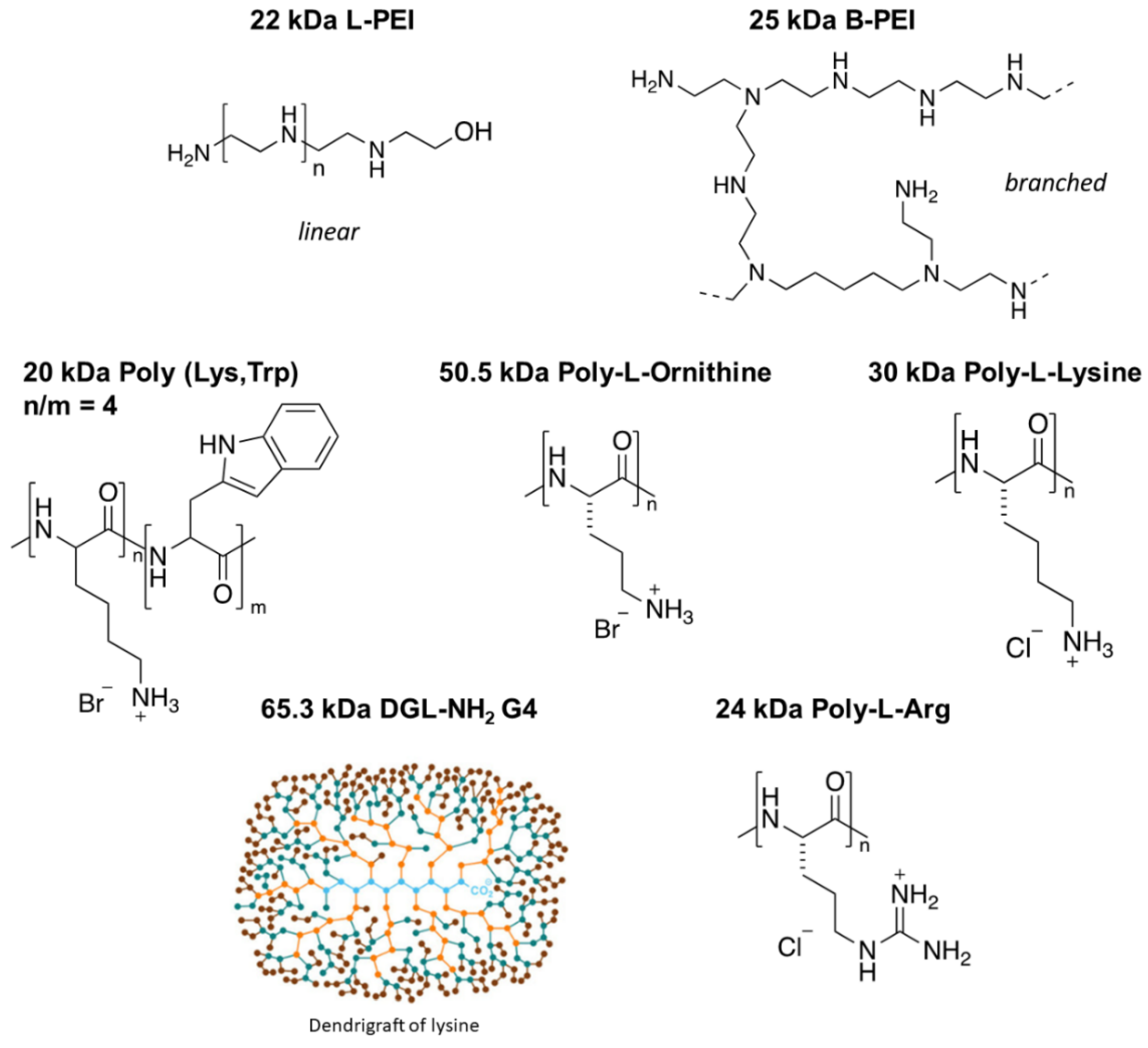

| POLYMERS | IC50 ± SEM (nM Polymer) | ||
|---|---|---|---|
| [Approx. Number of Monomers/Molecule] | U87 | NCH421K | |
| 22 kDa L-PEI [523] | 1547 ± 206 | **** | 194 ± 23.5 |
| 25 kDa B-PEI [595] | 508.8 ± 12.3 | *** | 146.2 ± 12.9 |
| 30 kDa PLL [182] | 583.4 ± 17.1 | ** | 280.5 ± 18.2 |
| 20 kDa P-(Lys,Trp) [164] | 514.9 ± 9.7 | ** | 257.1 ± 17.3 |
| 50.5 kDa PL-Orn [259] | 344.2 ± 26.7 | * | 133.3 ± 5.1 |
| 65.3 kDa DGL-NH2G4 [365] | 558.8 ± 16.5 | * | 292.2 ± 35.9 |
| 24 kDa PL-Arg [120] | 1570 ± 85 | Ns | 620 ± 55 |
| Poly-Arginine [Monomers/Molecule] | IC50 ± SEM (nM Polymer) |
|---|---|
| 1.9 kDa PL-Arg [10] | >8000 |
| 5.8 kDa PL-Arg [30] | 2380 ± 64 |
| 13 kDa PL-Arg [70] | 890 ± 98 **** |
| 24 kDa PL-Arg [120] | 620 ± 64 * |
| 38.5 kDa PL-Arg [200] | 460 ± 75 ns |
| Polyethylenimine | IC50 ± SEM (nM PEI) | ||
|---|---|---|---|
| [Monomers/Molecule] | U87 | NCH421K | |
| 0.73 kDa L-PEI [17] | ND | / | >32,000 |
| 4 kDa L-PEI [60] | 3337.2 ± 178.8 | **** | 1285 ± 188.7 |
| 22 kDa L-PEI [523] | 1547 ± 205.9 **** | **** | 194 ± 23.5 **** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCartin, C.; Blumberger, J.; Dussouillez, C.; Fernandez de Larrinoa, P.; Dontenwill, M.; Herold-Mende, C.; Lavalle, P.; Heurtault, B.; Bellemin-Laponnaz, S.; Fournel, S.; et al. Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells. J. Funct. Biomater. 2023, 14, 17. https://doi.org/10.3390/jfb14010017
McCartin C, Blumberger J, Dussouillez C, Fernandez de Larrinoa P, Dontenwill M, Herold-Mende C, Lavalle P, Heurtault B, Bellemin-Laponnaz S, Fournel S, et al. Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells. Journal of Functional Biomaterials. 2023; 14(1):17. https://doi.org/10.3390/jfb14010017
Chicago/Turabian StyleMcCartin, Conor, Juliette Blumberger, Candice Dussouillez, Patricia Fernandez de Larrinoa, Monique Dontenwill, Christel Herold-Mende, Philippe Lavalle, Béatrice Heurtault, Stéphane Bellemin-Laponnaz, Sylvie Fournel, and et al. 2023. "Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells" Journal of Functional Biomaterials 14, no. 1: 17. https://doi.org/10.3390/jfb14010017
APA StyleMcCartin, C., Blumberger, J., Dussouillez, C., Fernandez de Larrinoa, P., Dontenwill, M., Herold-Mende, C., Lavalle, P., Heurtault, B., Bellemin-Laponnaz, S., Fournel, S., & Kichler, A. (2023). Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells. Journal of Functional Biomaterials, 14(1), 17. https://doi.org/10.3390/jfb14010017







