Cytotoxicity of V-Prep Versus Phosphoric Acid Etchant on Oral Gingival Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Giemsa Staining
2.3. Cell Death Quantification
2.4. Cytotoxicity Assay
2.5. Statistical Analysis
3. Results
3.1. Morphological Changes Gingival Fibroblasts
3.2. Cell Death Quantification
3.3. Cell Viability Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pickett, K.L.; Sadowsky, P.L.; Jacobson, A.; Lacefield, W. Orthodontic In Vivo Bond Strength: Comparison with In Vitro Results. Angle Orthod. 2001, 71, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.; Manso, M.C.; Almeida, R.F.; Martin, C.; Carvalho, Ó.; Henriques, B.; Silva, F.; Pinhão Ferreira, A.; Souza, J.C.M. Bond Strength of Metallic or Ceramic Orthodontic Brackets to Enamel, Acrylic, or Porcelain Surfaces. Materials 2020, 13, 5197. [Google Scholar] [CrossRef] [PubMed]
- Grazioli, G.; Hardan, L.; Bourgi, R.; Nakanishi, L.; Amm, E.; Zarow, M.; Jakubowicz, N.; Proc, P.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M. Residual Adhesive Removal Methods for Rebonding of Debonded Orthodontic Metal Brackets: Systematic Review and Meta-Analysis. Materials 2021, 14, 6120. [Google Scholar] [CrossRef]
- Buonocore, M.G. A Simple Method of Increasing the Adhesion of Acrylic Filling Materials to Enamel Surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Sharma, S.; Tandon, P.; Nagar, A.; Singh, G.P.; Singh, A.; Chugh, V.K. A Comparison of Shear Bond Strength of Orthodontic Brackets Bonded with Four Different Orthodontic Adhesives. J. Orthod. Sci. 2014, 3, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hardan, L.; Bourgi, R.; Kharouf, N.; Mancino, D.; Zarow, M.; Jakubowicz, N.; Haikel, Y.; Cuevas-Suárez, C.E. Bond Strength of Universal Adhesives to Dentin: A Systematic Review and Meta-Analysis. Polymers 2021, 13, 814. [Google Scholar] [CrossRef]
- Flores, A.R.; Sáez, E.G.; Barceló, F. Metallic Bracket to Enamel Bonding with a Photopolymerizable Resin-Reinforced Glass Ionomer. Am. J. Orthod. Dentofac. Orthop. 1999, 116, 514–517. [Google Scholar] [CrossRef]
- Mickenautsch, S.; Yengopal, V.; Banerjee, A. Retention of Orthodontic Brackets Bonded with Resin-Modified GIC versus Composite Resin Adhesives--a Quantitative Systematic Review of Clinical Trials. Clin. Oral Investig. 2012, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Summers, A.; Kao, E.; Gilmore, J.; Gunel, E.; Ngan, P. Comparison of Bond Strength between a Conventional Resin Adhesive and a Resin-Modified Glass Ionomer Adhesive: An in Vitro and in Vivo Study. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 200–206; quiz 254–255. [Google Scholar] [CrossRef]
- Ghoubril, V.; Ghoubril, J.; Khoury, E. A Comparison between RMGIC and Composite with Acid-Etch Preparation or Hypochlorite on the Adhesion of a Premolar Metal Bracket by Testing SBS and ARI: In Vitro Study. Int. Orthod. 2020, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification Review of Dental Adhesive Systems: From the IV Generation to the Universal Type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Blomlöf, J.; Lindskog, S. Periodontal Tissue-Vitality after Different Etching Modalities. J. Clin. Periodontol. 1995, 22, 464–468. [Google Scholar] [PubMed]
- Blomlöf, J.; Jansson, L.; Blomlöf, L.; Lindskog, S. Long-Time Etching at Low PH Jeopardizes Periodontal Healing. J. Clin. Periodontol. 1995, 22, 459–463. [Google Scholar] [CrossRef]
- Akman, A.C.; Demiralp, B.; Güncü, G.N.; Kiremitçi, A.; Sengün, D. Necrosis of Gingiva and Alveolar Bone Caused by Acid Etching and Its Treatment with Subepithelial Connective Tissue Graft. J. Can. Dent. Assoc. 2005, 71, 477–479. [Google Scholar]
- Jurado, C.A.; Fischer, N.G.; Sayed, M.E.; Villalobos-Tinoco, J.; Tsujimoto, A. Rubber Dam Isolation for Bonding Ceramic Veneers: A Five-Year Post-Insertion Clinical Report. Cureus 2021, 13, e20748. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, C.; Li, F.; Chen, J.; Sun, J.; Zou, S.; Sandham, A.; Xu, Q.; Riley, P.; Ye, Q. Enamel Etching for Bonding Fixed Orthodontic Braces. Cochrane Database Syst. Rev. 2013, 2013, CD005516. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kwak, J.; Jo, R.; Jung, D.; Youn, D.; Oh, N.; Jang, J. Effects of Dental Acid Etchants in Oral Epithelial Cells. Oral Biol. Res. 2019, 43, 299–305. [Google Scholar] [CrossRef]
- Terhune, W.F.; Sydiskis, R.J.; Davidson, W.M. In Vitro Cytotoxicity of Orthodontic Bonding Materials. Am. J. Orthod. 1983, 83, 501–506. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, H.-J.; Park, S.-H.; Kim, J.-W.; Cho, K.-M. Iatrogenic chemical burn on facial skin by 37% phosphoric acid etchant. J. Korean Acad. Conserv. Dent. 2009, 34, 38–41. [Google Scholar] [CrossRef]
- Nakajima, A.; Kurihara, H.; Yagita, H.; Okumura, K.; Nakano, H. Mitochondrial Extrusion through the Cytoplasmic Vacuoles during Cell Death. J. Biol. Chem. 2008, 283, 24128–24135. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.S.; Wills, L.P.; Schnellmann, R.G. Measurement of Cell Death in Mammalian Cells. Curr. Protoc. Pharm. 2004, 56, 12.8.1–12.8.24. [Google Scholar] [CrossRef] [PubMed]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Fröb, L.; Rüttermann, S.; Romanos, G.E.; Herrmann, E.; Gerhardt-Szép, S. Cytotoxicity of Self-Etch Versus Etch-and-Rinse Dentin Adhesives: A Screening Study. Materials 2020, 13, 452. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Bernardo, C.F.d.F.; de Souza, F.F.d.F.A.; Michél, M.D.; Ribeiro, C.N.d.M.; Germano, S.; Maluf, D.F. Cytotoxicity of Etch-and-Rinse, Self-Etch, and Universal Dental Adhesive Systems in Fibroblast Cell Line 3T3. Scanning 2017, 2017, 9650420. [Google Scholar] [CrossRef] [PubMed]
- Ozer, F.; Blatz, M.B. Self-Etch and Etch-and-Rinse Adhesive Systems in Clinical Dentistry. Compend. Contin. Educ. Dent. 2013, 34, 12–14, 16, 18; quiz 20, 30. [Google Scholar] [PubMed]
- Cecchin, D.; Farina, A.P.; Vidal, C.M.; Bedran-Russo, A.K. A Novel Enamel and Dentin Etching Protocol Using α-Hydroxy Glycolic Acid: Surface Property, Etching Pattern, and Bond Strength Studies. Oper. Dent. 2018, 43, 101–110. [Google Scholar] [CrossRef] [PubMed]
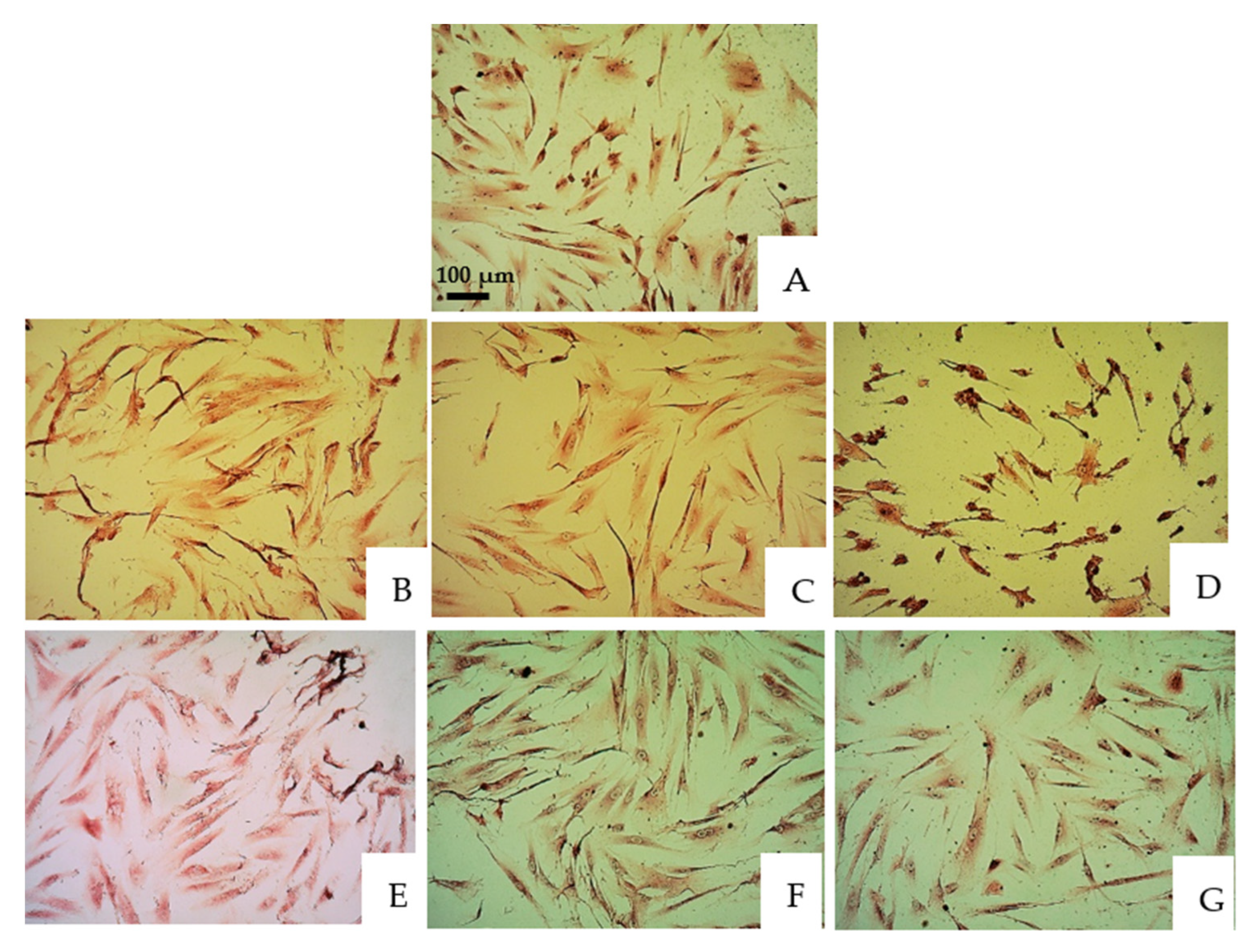
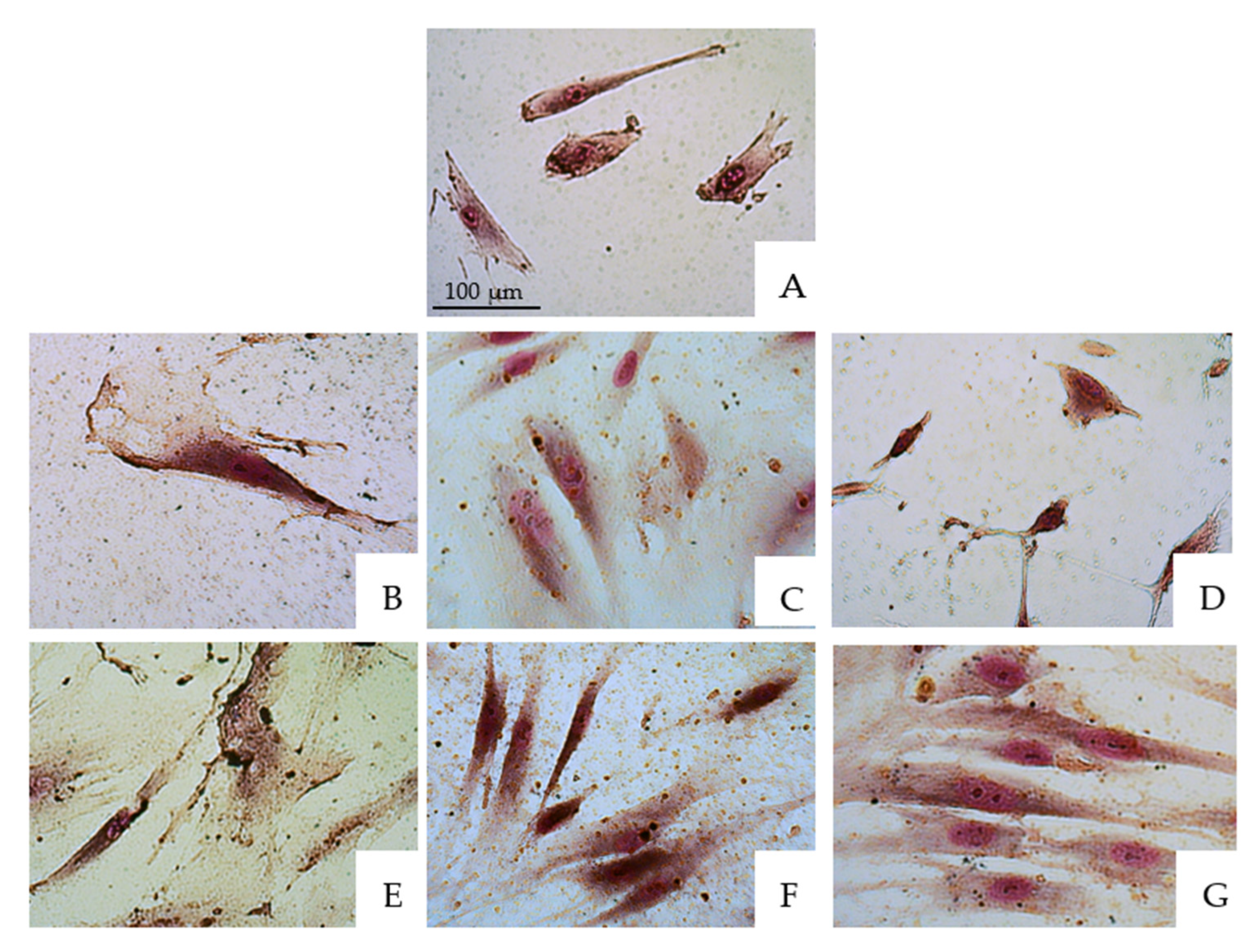
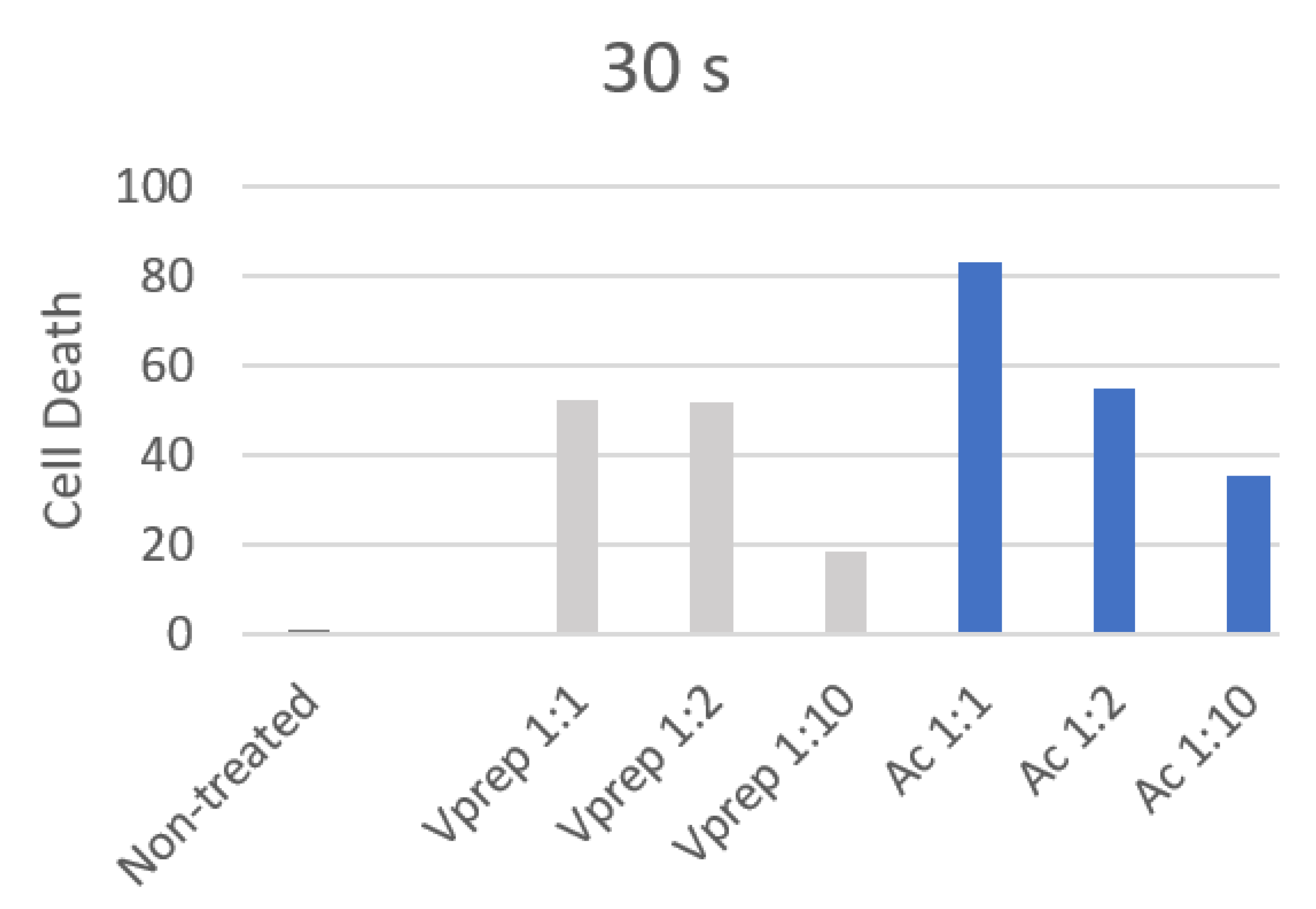
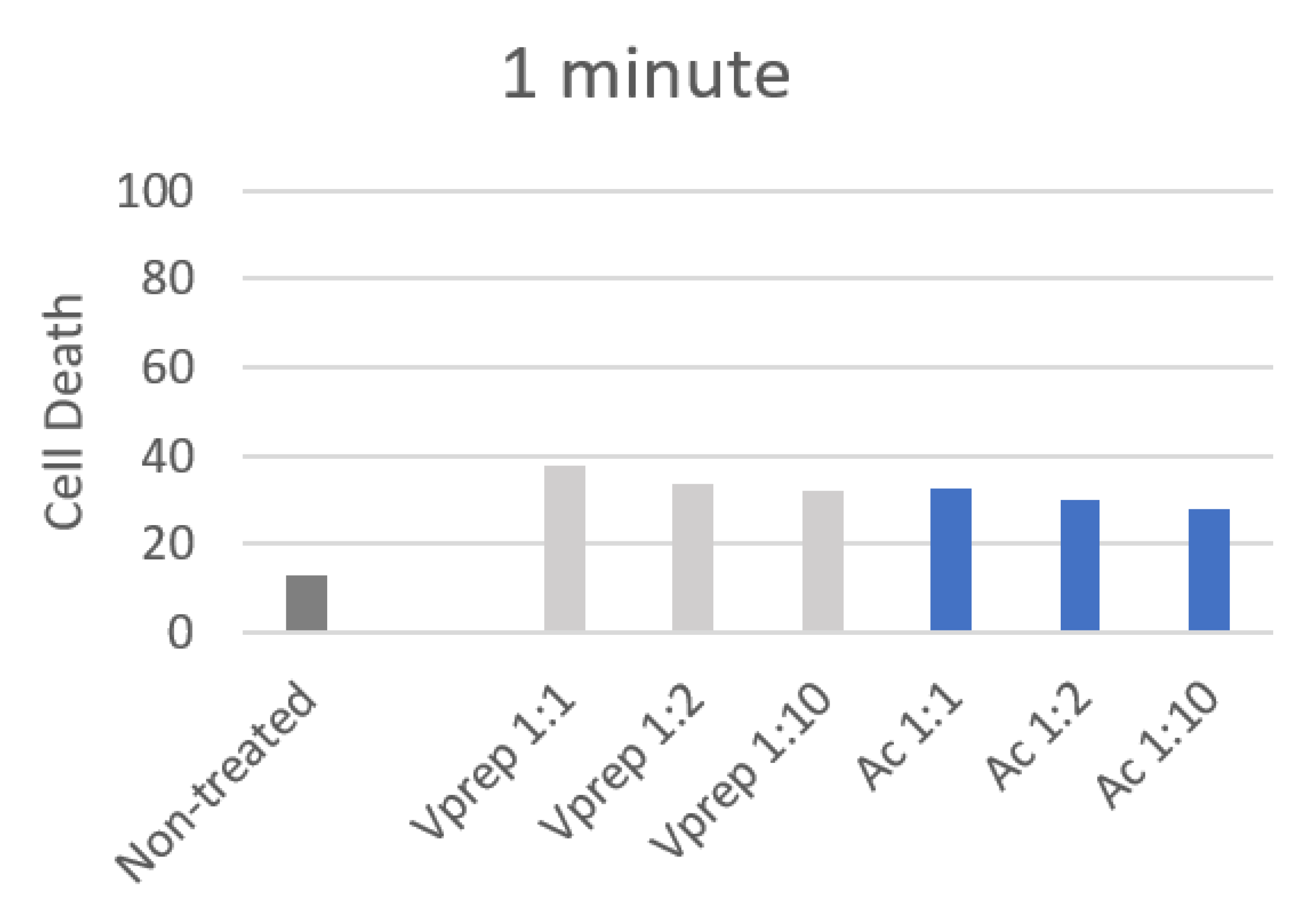
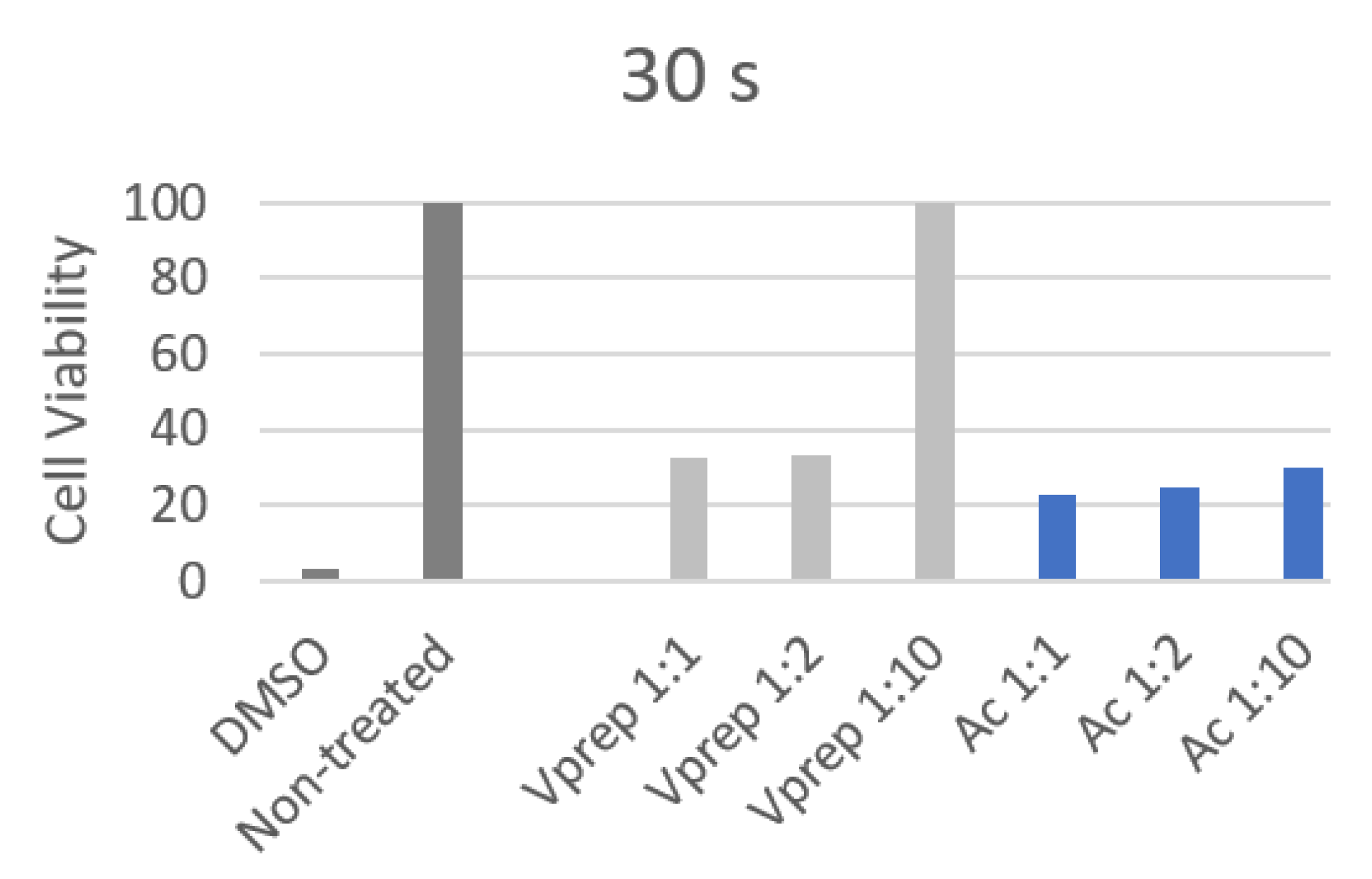
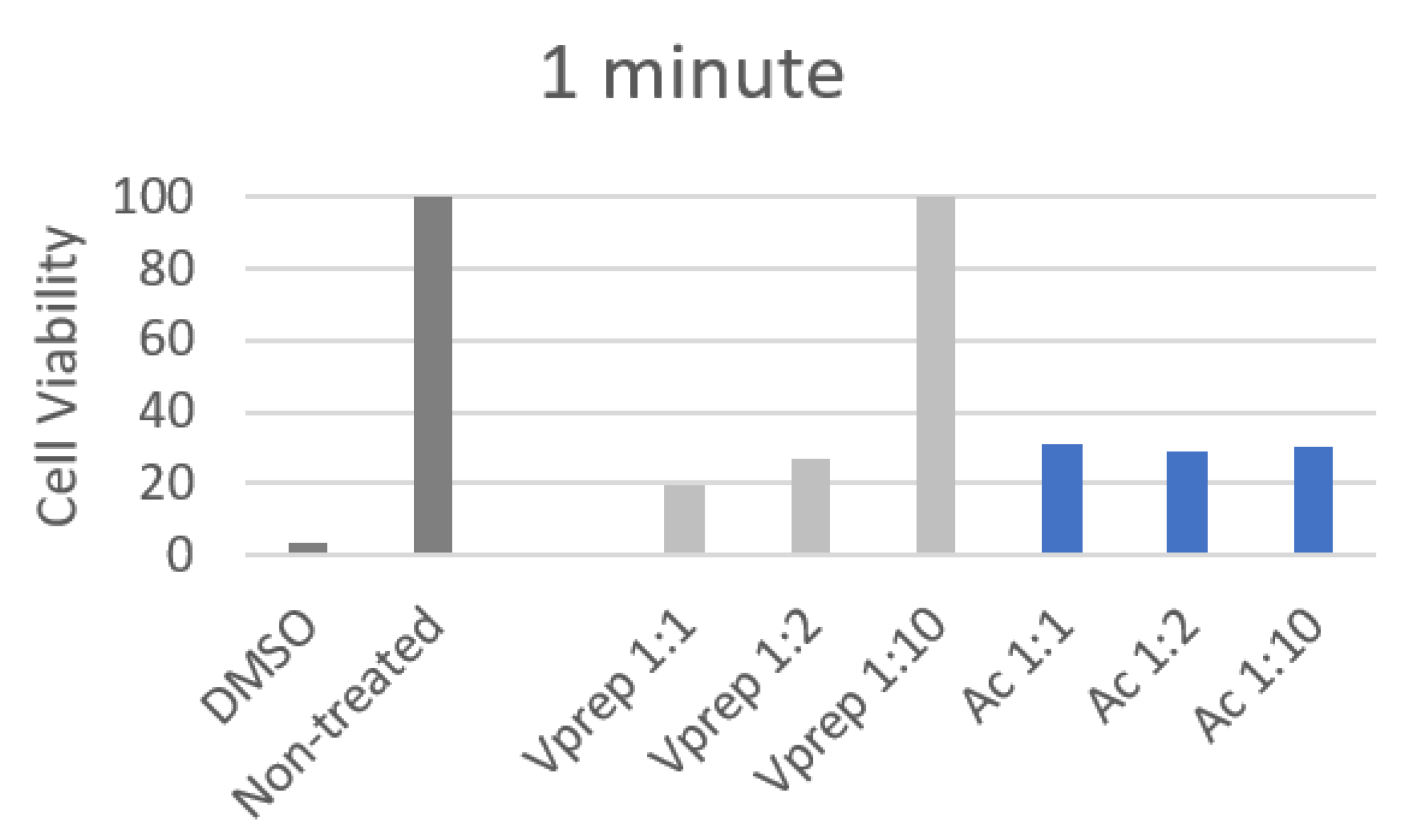
| 30 s | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| A | V-prep 1:1 (undiluted) | Acid etch 1:1 (undiluted) | ||||
| B | V-prep 1:2 | Acid etch 1:2 | ||||
| C | V-prep 1:10 | Acid etch 1:10 | ||||
| D | Non-treated | Non-treated | ||||
| p-Value | Non-Treated | Vprep 1:1 | Vprep 1:2 | Vprep 1:10 | Ac 1:1 | Ac 1:2 | Ac 1:10 |
|---|---|---|---|---|---|---|---|
| Non-treated | 0.000 | 0.000 | 0.097 | 0.000 | 0.000 | 0.000 | |
| Vprep 1:1 | 0.000 | 0.922 | 0.035 | 0.048 | 0.820 | 0.060 | |
| Vprep 1:2 | 0.000 | 0.922 | 0.037 | 0.045 | 0.810 | 0.061 | |
| Vprep 1:10 | 0.097 | 0.035 | 0.037 | 0.002 | 0.032 | 0.047 | |
| Ac 1:1 | 0.000 | 0.048 | 0.045 | 0.002 | 0.055 | 0.011 | |
| Ac 1:2 | 0.000 | 0.820 | 0.810 | 0.032 | 0.055 | 0.058 | |
| Ac 1:10 | 0.000 | 0.060 | 0.061 | 0.047 | 0.011 | 0.058 |
| p-Value | Non-Treated | Vprep 1:1 | Vprep 1:2 | Vprep 1:10 | Ac 1:1 | Ac 1:2 | Ac 1:10 |
|---|---|---|---|---|---|---|---|
| Non-treated | 0.000 | 0.012 | 0.012 | 0.012 | 0.014 | 0.015 | |
| Vprep 1:1 | 0.010 | 0.800 | 0.800 | 0.800 | 0.350 | 0.120 | |
| Vprep 1:2 | 0.012 | 0.800 | 0.880 | 0.880 | 0.770 | 0.120 | |
| Vprep 1:10 | 0.012 | 0.800 | 0.880 | 0.860 | 0.550 | 0.230 | |
| Ac 1:1 | 0.012 | 0.800 | 0.880 | 0.860 | 0.550 | 0.230 | |
| Ac 1:2 | 0.014 | 0.350 | 0.770 | 0.550 | 0.550 | 0.470 | |
| Ac 1:10 | 0.015 | 0.120 | 0.120 | 0.230 | 0.230 | 0.470 |
| p-Value | Non-Treated | Vprep 1:1 | Vprep 1:2 | Vprep 1:10 | Ac 1:1 | Ac 1:2 | Ac 1:10 |
|---|---|---|---|---|---|---|---|
| Non-treated | 0.000 | 0.000 | 0.999 | 0.000 | 0.000 | 0.000 | |
| Vprep 1:1 | 0.000 | 0.880 | 0.000 | 0.030 | 0.035 | 0.034 | |
| Vprep 1:2 | 0.000 | 0.880 | 0.000 | 0.033 | 0.040 | 0.060 | |
| Vprep 1:10 | 0.999 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Ac 1:1 | 0.000 | 0.030 | 0.033 | 0.000 | 0.072 | 0.053 | |
| Ac 1:2 | 0.000 | 0.035 | 0.040 | 0.000 | 0.072 | 0.058 | |
| Ac 1:10 | 0.000 | 0.034 | 0.060 | 0.000 | 0.053 | 0.058 |
| p-Value | Non-Treated | Vprep 1:1 | Vprep 1:2 | Vprep 1:10 | Ac 1:1 | Ac 1:2 | Ac 1:10 |
|---|---|---|---|---|---|---|---|
| Non-treated | 0.000 | 0.000 | 0.999 | 0.000 | 0.000 | 0.000 | |
| Vprep 1:1 | 0.000 | 0.044 | 0.000 | 0.028 | 0.034 | 0.033 | |
| Vprep 1:2 | 0.000 | 0.044 | 0.000 | 0.062 | 0.070 | 0.068 | |
| Vprep 1:10 | 0.999 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| Ac 1:1 | 0.000 | 0.028 | 0.062 | 0.000 | 0.080 | 0.081 | |
| Ac 1:2 | 0.000 | 0.034 | 0.070 | 0.000 | 0.080 | 0.400 | |
| Ac 1:10 | 0.000 | 0.033 | 0.068 | 0.000 | 0.081 | 0.400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoubril, V.; Changotade, S.; Lutomski, D.; Ghoubril, J.; Chakar, C.; Abboud, M.; Hardan, L.; Kharouf, N.; Khoury, E. Cytotoxicity of V-Prep Versus Phosphoric Acid Etchant on Oral Gingival Fibroblasts. J. Funct. Biomater. 2022, 13, 266. https://doi.org/10.3390/jfb13040266
Ghoubril V, Changotade S, Lutomski D, Ghoubril J, Chakar C, Abboud M, Hardan L, Kharouf N, Khoury E. Cytotoxicity of V-Prep Versus Phosphoric Acid Etchant on Oral Gingival Fibroblasts. Journal of Functional Biomaterials. 2022; 13(4):266. https://doi.org/10.3390/jfb13040266
Chicago/Turabian StyleGhoubril, Victor, Sylvie Changotade, Didier Lutomski, Joseph Ghoubril, Carole Chakar, Maher Abboud, Louis Hardan, Naji Kharouf, and Elie Khoury. 2022. "Cytotoxicity of V-Prep Versus Phosphoric Acid Etchant on Oral Gingival Fibroblasts" Journal of Functional Biomaterials 13, no. 4: 266. https://doi.org/10.3390/jfb13040266
APA StyleGhoubril, V., Changotade, S., Lutomski, D., Ghoubril, J., Chakar, C., Abboud, M., Hardan, L., Kharouf, N., & Khoury, E. (2022). Cytotoxicity of V-Prep Versus Phosphoric Acid Etchant on Oral Gingival Fibroblasts. Journal of Functional Biomaterials, 13(4), 266. https://doi.org/10.3390/jfb13040266










