1. Introduction
The broad application of nanoparticles in biological research techniques and medical practice has brought about revolutionary innovations and played a key role in resolving many problems in these realms [
1,
2,
3]. All applications of nanoparticles in biomedicine can generally be divided into two major groups: therapeutic and diagnostic. Therapeutic approaches include the use of nanoparticles to ensure the targeted delivery of drugs or genetic materials to the target tissues. For instance, magnetic nanoparticles with remarkable superparamagnetic properties could be controlled via an applied external gradient of the magnetic field [
4,
5], and this “action at a distance” facilitates the transport and delivery of drugs to the corresponding tumor sites. Moreover, nanoparticles can ensure the delivery of drug molecules even to subcellular structures, such as cell nuclei or mitochondria [
6]. These capacities distinguish them from others, based on factors including reduced off-target drug toxicity and the need for the administration of repetitive doses. Furthermore, magnetic nanoparticles are used for other treatment applications, such as magnetic hyperthermia [
7]. It is considered one of the promising cancer treatment tools, where heating, as a result of various fluctuations of the particle magnetic moments, causes the death of tumor cells [
8].
Nanoparticles are also considered a prime option for the immunotherapy of various tumors due to their multisided physicochemical and structural characteristics [
9,
10]. Nanomedicines—particularly target immune cells or immune reactions, such as liposomes, polylactic-co-glycolic acid (PLGA) nanoparticles, dendrimers, gold and carbon nanoparticles, micelles, polymeric nanoparticles, quantum dots, and microneedles—have been reported [
10].
Certain types of nanoparticles allow for the imaging of individual, pathologically altered cells and even molecules that are markers of disease. It has already been reported that minor metal oxide nanoparticles, such as iron oxide (Fe
3O
4 [
11]), gadolinium oxide (Gd
2O
3 [
12,
13]), and manganese oxide (Mn
3O
4 [
14])—due to their remarkable physical properties—could be applied as contrast agents for more accurate imaging. Moreover, the fluorescence emission properties of nanoparticles, which are dependent on their sizes, make them indispensable for molecular imaging; this provides opportunities for detecting diseases as early as possible at the molecular level [
15,
16].
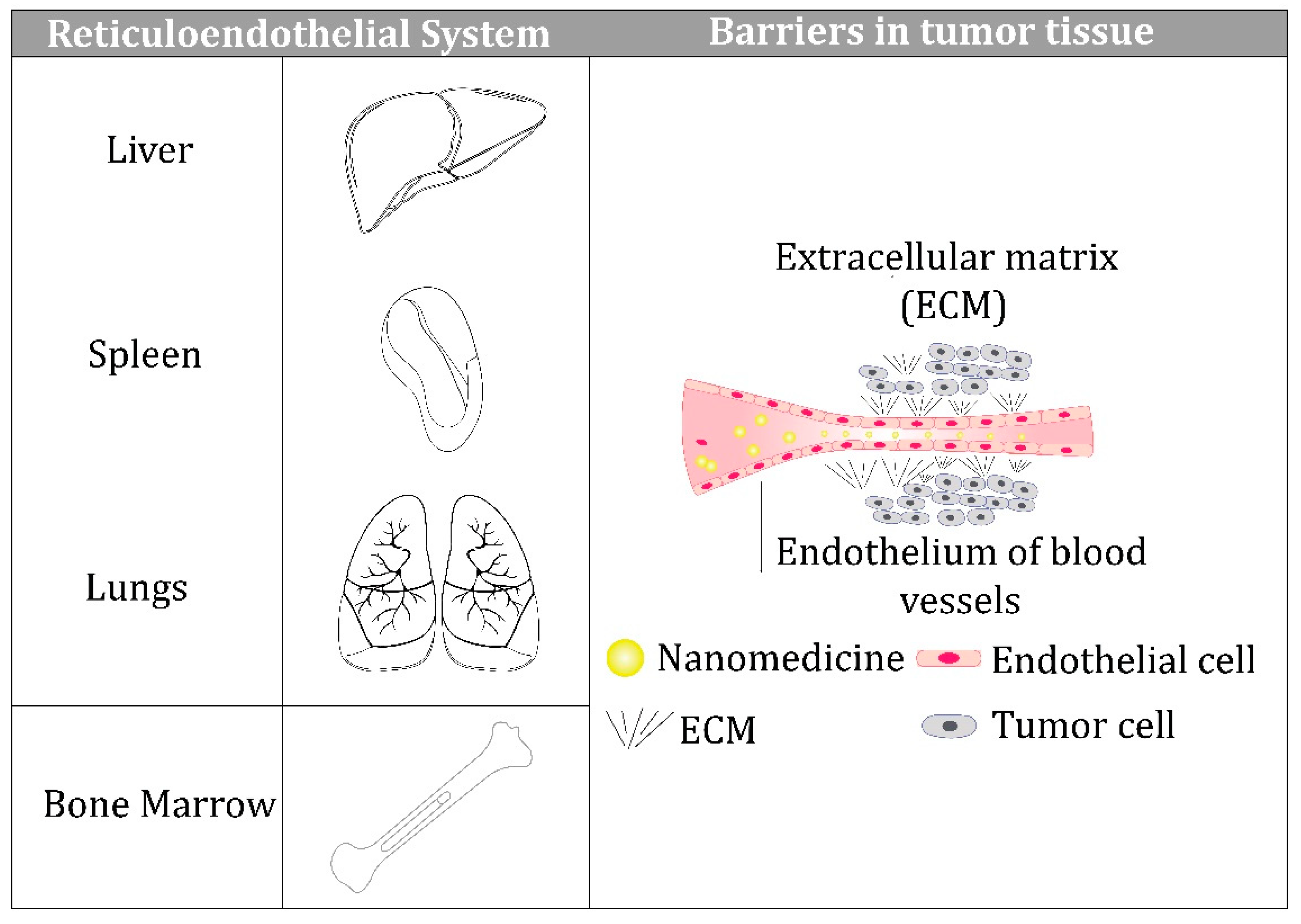
Unfortunately, despite all these advantages, nanoparticles used for therapeutic purposes cannot overcome the various biological barriers that exist on the way from their entry into the blood circulation to the target sites, and this reduces their effectiveness in clinical settings.The barriers that prevent intravenously administered nanoparticles from reaching their target are shown schematically in
Figure 1.
These include the reticuloendothelial system (RES), comprised of the liver, spleen, lungs, and bone marrow [
17,
18]; the endothelium of blood vessels within the target tissues [
19]; and the extracellular matrix (ECM) of the target tissue [
20,
21], which is a highly organized and complex network of structural proteins, proteoglycans, and glycosaminoglycans. Recent studies have demonstrated that nanoparticles can reach a tumor passively through the leaky vasculature surrounding the tumor because of the enhanced permeability and retention (EPR) effect, which opens new perspectives for overcoming tumor chemoresistance [
22]. Furthermore, the physicochemical properties of nanoparticles play decisive roles in overcoming all three barriers, which we discuss below. In addition, we consider the dynamics of protein corona formation and its impact on nanoparticle behavior in a biological environment.
2. Importance of Physicochemical Properties of Nanoparticles
A large number of nanoparticles have demonstrated initial success in preclinical evaluation, but modest therapeutic benefit in clinical settings, partly due to insufficient delivery to the tumor site and penetration of tumor tissue. Therefore, a precise understanding of the relationships betweenthe physicochemical properties of nanoparticles and their interaction with the surrounding microenvironment in the body is extremely important for achieving higher concentrations and better functionality in tumor tissues.
Nanoparticles are characterized by unique properties, such as small spatial dimensions, the possibility of specific surface binding sites for various ligands, and the manifestation of resonance-related absorption under different types of external energy influences, with subsequent relaxation processes [
23]. These features provide a multifunctional platform for the development of various biomedical applications.
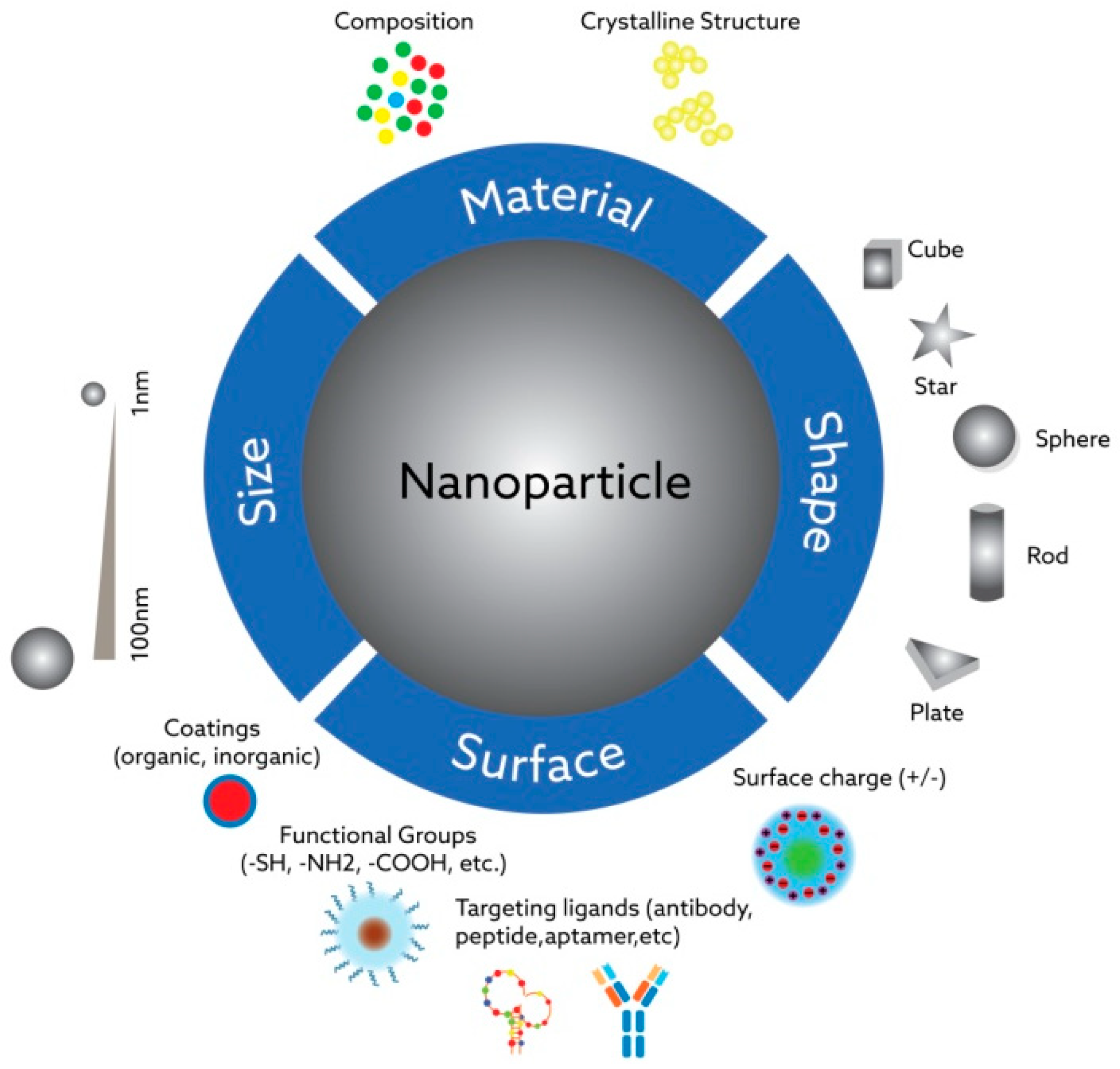
The main powerful feature of nanoparticles is demonstrating fundamentally different physicochemical properties than their micro- and macroscopic analogs—ones that previously have not been seen in certain contexts, for instance, in the human body. The changes after exposure to the biological fluids could be both advantageous and highly deleterious for the expected functionality of nanoparticles in the target site, which should be taken into consideration during the design process. Synthetic nanoparticles can penetrate “the walls” inside cell systems—organelles or nuclei—and interact with natural nano-sized targets in unpredictable ways due to their tunable physicochemical characteristics. The most important characteristics influencing the interaction of nanoparticles with biological systems include their size, shape, hydrophilicity/hydrophobicity properties, surface coating composition, the presence of functional groups on them, and their charge (see
Figure 2).
2.1. Size of Nanoparticles
The small size of nanoparticles is one of the main features allowing them to pass through biological barriers;easily enter cells; and translocate across cells, tissues, and organs [
24,
25]. This significantly influences their therapeutic and diagnostic value [
26]. Certain factors, such as cellular uptake [
27] and elimination [
28], intracellular trafficking [
29], cytotoxicity [
30,
31], tumor penetration [
32], and blood circulation half-time [
33], are influenced by nanoparticle size.
Nanoparticles, as polar molecules, enter the cells by employing an endocytotic pathway, whereas the cell membrane is mostly permeable for small non-polar molecules [
34]. It is generally believed that smaller nanoparticles are taken up by the cells faster than the larger ones [
25]; however, as the particle size decreases, the surface area to volume ratio increases [
35]. This contributes to an elevation in both the rate and extent of cellular uptake but promotes rapid elimination through the kidneys at the same time [
36,
37,
38].
Smaller nanoparticles have exhibited higher toxicity than larger ones. Silver nanoparticles were synthesized into three different sizes (~10 nm, 50 nm, and 100 nm) and were studied in several cell lines, including an MC3T3-E1 clonal murine cell line of immature osteoblasts derived from mice and PC12 rat pheochromocytoma cells.Nanoparticles with a size of 10 nm were found to cause more cell apoptosis than larger nanoparticles. The other group, using the BEAS-2B normal bronchial epithelial cell line and silver nanoparticles (AgNPs) of 10, 40, 50, and 75 nm sizes, showed size-dependent cytotoxicity, where only the 10 nm particles affected the cell viability of human lung cells [
39].
Other in vitro studies have shown maximum cellular uptake in nonphagocytic cells, within the size range of 10–60 nm regardless of nanoparticle core composition or surface charge [
33]. Nanoparticles with sizesdeviatingfrom this range could demonstrate undesired effects. For instance, some nanoparticles with a diameter of less than 10 mm, intended to be used as an imaging tool, may be rapidly eliminated by the kidneys [
37], or a nanoparticle with a diameter greater than 200 nm may activate the complement system and be quickly removed from the blood, accumulating in the liver and spleen [
33]. An in vivo biodistribution study showed that polymeric nanoparticles (RhB-CMCNP and RhB-CHNP), with slight negative charges and a size of 150 nm, tend to accumulate in tumor tissue more effectively [
40].
The cytotoxicity of nanoparticles with different sizes and shapes, produced using the polymerization-induced self-assembly (PISA) methodology, was studied in nude mice bearing HT1080 human fibrosarcoma cells tumor xenografts.It was found that small spherical polystyrene core nanoparticles with a polyethylene glycol (PEG) corona (diameter of 21 nm) have higher tumor accumulation than the larger spherical nanoparticles (diameter of 33 nm) or their rodlike (diameter of 37 nm, contour length of 350–500 nm) or wormlike (diameter of 45 nm, contour length of 1–2 μm) counterparts [
41].
Based on previous studies, the well-accepted size for long-term blood circulation is around 100 nm due to the size-dependent balance between organ filtration and tumor vessel extravasation [
42]. This allows for prolonged circulation lifetimes of the nanoparticles, as well as their better accumulation in tumor tissue through the EPR mechanism, by reducing nonspecific interactions with blood components and other off-targeted cells and urinary excretion. The pores in tumor endothelium are much larger (100 nm to 1 μm, depending on the tumor type)than in the tight endothelial junctions of normal vessels (5–10 nm size), which results in enhanced permeability for relatively larger molecules (in the size range of 20–500 nm) passing through the vascular walls into the interstitium surrounding tumor cells [
43].
Size-switchable nanoparticles were recently introduced to reduce their extravasation through the leaky tumor vasculature. The size change of such nanoparticles is triggered by internal (e.g., low pH in the tumor microenvironment oroverexpressed matrix metalloproteinases [MMPs]) or external (e.g., light, near-infrared [NIR] lasers, temperature, ultrasounds, magnetic fields) stressors and allowsfor prolonged blood circulation, nanocarrier storage at tumor sites, size reduction for penetrating tumor parenchyma, escaping from endo/lysosomes, and swelling or disassembly for drug release [
42].
To achieve good efficacy with less toxicity, the “ideal” size for a nanoparticle as a drug carrier or a diagnostic tool should be determined individually while taking into account its cellular intake in the organism, distribution in the organism, and elimination from the organism.
2.2. Shape of Nanoparticles
Along with the size of the nanoparticles, their shape is also considered a major factor in their functionality [
44]. Nanoparticles could be synthesized in various shapes, such as tubes, fibers, and spheres. Studies report that the shape of nanoparticles plays an important role in cellular uptake, blood circulation, anti-tumor activity, and biodistribution [
45,
46].
There are several reasons for characterizing the shape dependence of bio-nano interactions. One of them is a difference in the curvature structure of different-shaped nanoparticles. For example, rod-shaped nanoparticles have a larger contact area for cell membrane receptors than spherical nanoparticles, which, in turn, can reduce the number of available receptor sites for binding [
47]. Another reason is related to the membrane wrapping time, which is greater for the elongated particles—for instance, cylindrical ones. Accordingly, spherical nanoparticles can be internalized more easily and faster through endocytosis compared to others [
48]. In addition, it has been discovered that spherical nanoparticles are relatively less toxic [
49,
50]. It is shown that, in addition to the size, the shape of iron oxide (Fe
2O
3) nanoparticles is a major factor that contributes to particle cytotoxicity. It has been revealed that the use of rod-shaped Fe
2O
3 nanoparticles results in a higher extent of necrosis and a higher degree of membrane damage and reactive oxygen species (ROS) production.
It is reported that spherical nanoparticles demonstrate the highest uptake among these differently shaped nanoparticles [
51]. Previous work has compared nanoparticles with identical surface area, ligand-receptor interaction strength, and grafting density of the polyethylene glycol and has found that spherical nanoparticles exhibit the fastest internalization rate, followed by cubic, and then rod- and disk-like, nanoparticles.
2.3. Surface Modification of Nanoparticles
Nanoparticle surface properties also have significant effects on their interactions with cellular compartments. Among these properties, nanoparticles’ surface charge and density could affect the degree of cellular uptake and particles’ interaction with biomolecules, as well [
52,
53]. Negatively charged structures bind less efficiently to cell surfaces than neutral or positively charged ones and, thus, they are characterized by smaller rates of endocytic uptake [
54]. This is because electrostatic repulsive forces among the anionic surface and cellular environment repel the nanoparticles from entering. Moreover, nanoparticles’ surface charge also influences their toxicity properties in biological environments. It has been discovered that positively charged nanoparticles show more harmful effects in cell lines than negatively charged particles of a similar shape and size [
55,
56].
The hydrophilic/hydrophobic properties of nanoparticles are other key factors that play important roles in their ability to interact with biomolecules and cells. Several studies reveal that the surface membrane uptake of hydrophobic nanoparticles is favored [
57,
58] because the hydrophobic surface tends to interact with lipid tails (see
Figure 3).
Surface chemical modification is necessary to provide the nanoparticles with a sufficient degree of stability in the physiological medium [
59]. However, due to the high chemical reactivity of their surface, nanoparticles can influence cellular reactions in living systems. Moreover, surface functionalization is also necessary to prevent nanoparticles from aggregating [
60] and reduce non-specific cell uptake [
61]. In addition, the chemical modification of the surface is important for decreasing the toxic effects of nanoparticles and achieving a safe-design strategy that plays a crucial role in their potential applications [
62,
63].
2.4. Protein Corona
Another important feature of the biological environment is that the nanoparticles, while exposed to biological fluids, such as blood, lymph, gastric juice, etc., are covered with a kind of “corona”—a layer of proteins that are constantly adsorbed on their surface [
64]. Undoubtedly, this protein (biological macromolecule) can mask several physicochemical properties (such as the surface charge and solubility) of nanoparticles and make them recognizable to the immune system, which may neutralize them before they reach the target site [
65]. The covering of nanoparticles with these proteins largely determines their biological identities and further fate—their distribution through tissues and organs, their rate of excretion from the body, and their opsonization (phagocytosis involving membrane receptors). This is the key factor causing differences in the performance of nanoparticles within in vitro and in vivo [
66] studies, where nanoparticles indicate good results in the first case and usually are worse or non-effective in living organisms [
67,
68].
However, the composition, density, and mechanism of formation of this macromolecular layer depends not only on the physicochemical characteristics of the nanoparticles, association/dissociation constants, competitive binding processes, and species of proteins in the biological medium but also on where the nanoparticle is located [
69,
70].
The formation of this protein corona depends not only on the composition of the nanoparticle—its size, shape, surface state, and exposure time—but also on the type of media, the nanoparticle–to–protein ratio, and the presence of ions and other molecular species that interfere in the interaction between proteins and nanoparticles [
71].
Careful modulation of the protein corona through the tuning of intrinsic and extrinsic parameters may allow nanoparticles to be delivered optimally in tumor tissue [
72]. A study [
73] has shown that linking Z domain-based recombinant affibody (RA) scaffolds to magnetosomes (BMPs), which were then preadsorbed on anti-HER2 humanized mAb (TZ) and incubated with human plasma, resulted in the more effective targeting capability and drug delivery of nanoparticles in a simulated in vivo environment by incorporating the majority of TZ molecules into the nanoparticle–corona complex. It is also crucial to consider external factors, such as temperature, pH, and shear stress, for developing a more efficient protein corona [
74]. Studies on protein corona composition on nanoparticles suggest that choosing the right materials can influence the types of proteins adsorbed from plasma and improve delivery effectiveness [
72].
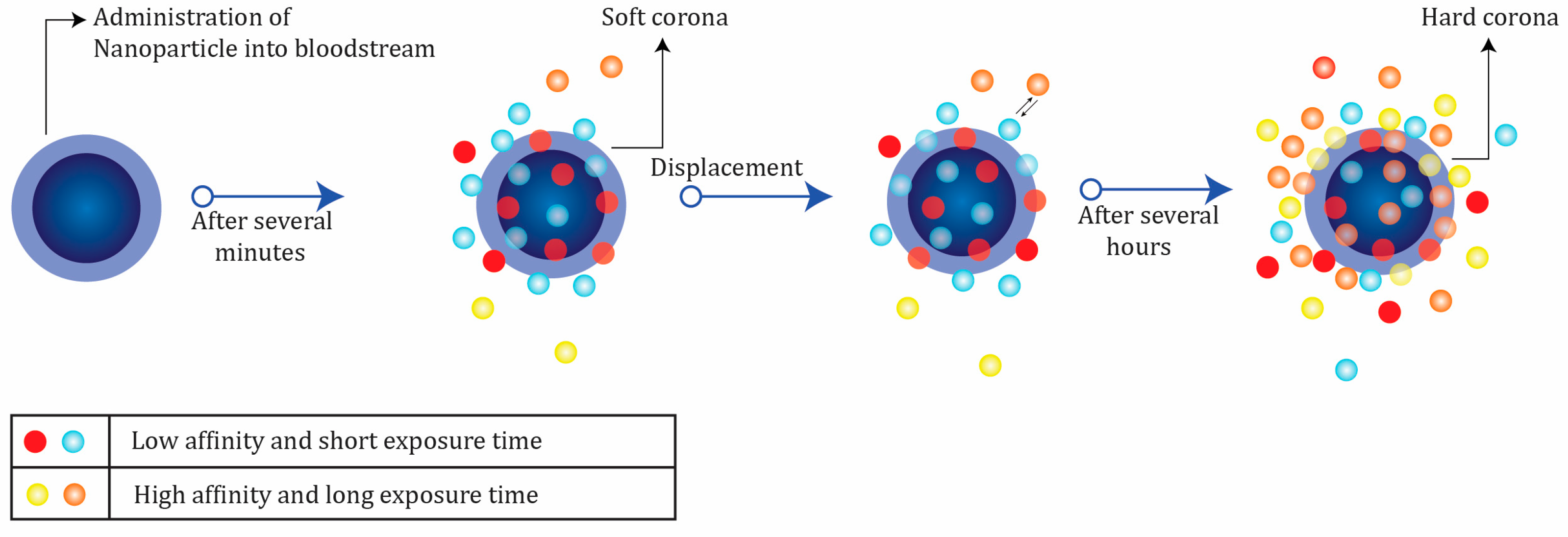
In a dynamic process, protein corona is usually categorized into hard (irreversible) and soft (reversible) forms [
67], depending on their affinity properties and periods of protein exchanges (see
Figure 4).
Proteins in the hard corona are characterized by high affinity on the surface of the nanoparticles and longer exchange times. In contrast, the soft corona represents weakly connected proteins with low affinity on the nanoparticle surface and short exchange periods [
67].
The nanoparticle–protein layer is a formation that “cells see” [
75]; moreover, it plays a significant role in determining the interactions of the nanostructures with the biological environment. In the majority of the nanoparticles, cellular uptake is correlated with the hard corona properties, and they could develop the interactions occurring among nanoparticles and proteins, phospholipids, membranes, and DNA [
76]. In other words, the corona can “control” the process of choosing the biomolecule types and interaction forms. Moreover, the nanoparticle–protein hybrid structure and cell receptor interactions primarily depend on the corona. Some of these interactions emerge due to the presence of nanoparticles and are not inherent to natural biosystems. Therefore, protein coronas that form on the surface of the nanoparticles could stimulate or mitigate an immunological response [
77], which is a crucial aspect of understanding and evaluating their cytotoxic effects [
78]. Consequently, understanding the formation of the protein corona and controlling its properties and interactions with nanoparticle surfaces and other proteins are crucial factors for the application of nanostructures in biomedicine [
79,
80].
3. Conclusions
The current knowledge of nanoparticles as drug carriers or diagnostic tools shows that generalized optimal physicochemical features for more potent nanoparticles cannot be recommended. However, practical design principles based on this knowledge can be identified, which can guide the achievementof reduced premature clearance of nanoparticles from blood circulation, less systemic toxicity, and high tumor penetration.
It is well established that small-sized nanoparticles are easily eliminated from blood circulation through renal filtration without reaching the desired concentration at the target site. Therefore, the consensus is that nanoparticles must be larger than 10 mm. Conversely, it is also well established that as the particle size increases, nanoparticle uptake by the reticuloendothelial system (RES) increases—nanoparticles with a diameter greater than 200 nm may activate the complementary system and be quickly removed from blood circulation, accumulating in the liver and spleen. Therefore, the optimal size of a nanoparticle should be determined on an individual basis while taking into account important factors, such as the amount and rate of cellular uptake, intracellular trafficking, cytotoxicity, tumor penetration, blood circulation half-time, the disease site, and the expected therapeutic goals. This condition is true for the optimal shape and surface modification of nanoparticles.
Further research is required to find optimal combinations of the different physicochemical properties of various nanomaterials in physiological settings, taking into account the target sites in an organism and the goals imposed on them. The design strategy for developing a potent nanoparticle might need to consider the opposing requirements for its physicochemical properties (depending on the intercellular space) where the tunability properties of nanocarriers are advantageous, as their surfaces can be functionalized appropriately to fulfill size requirements. The development of size-switchable intelligent nanoparticles that are activated by the intrinsic tumor micro-environment offers challenging prospects. The potential of endogenous size-tuning triggers (such as curtain enzymes, glucose concentrations, redox reactions, temperatures, and pH differences) should be further explored, whereas some external stressors, such as temperature and light, can damage normal cells and organs.
A better understanding of the importance of the interaction of nanoparticles with the biological environment, after entering blood circulation and targeting tumor tissue, will play a decisive role in designing highly potent nanoparticles. The properties of the nanoparticle surface are one of the main parameters that affect their interaction with cells and body fluids.
The surface load and its density should also be the focus of the design process, as it can affect the degree of the absorption of nanoparticles by the cell, as well as their interaction with biomolecules. The attachment frequency of negatively charged structures with the cell surface is lower than that of neutral or positively charged particles. The neutral nanoparticles are coated with an immune protein corona that retains more IgG or fibrinogen than charged nanoparticles, and an elevated interaction with the immune system is achieved. Another interesting point is that negatively charged nanoparticles are removed from the body faster than positively charged particles, which leads to elevated cytotoxicity.
Another important parameter is the chemical modification of the nanoparticle surface, which allows for a reduction in the toxicity of nanoparticles used for biomedical purposes by controlling and modulating cell penetration and reducing the aggregation of nanoparticles in biological fluids. Further, the presence of large amounts of lipids, sugars, nucleic acids, and especially proteins in biomolecules can significantly affect the stability of nanoparticles in the physiological environment; surface modification plays an important role in solving this problem.
Author Contributions
Conceptualization, V.Y. and A.K.; writing—original draft preparation, V.Y., A.K. and J.H.; writing—review and editing, C.R., A.A., S.B. and M.M.; visualization, A.K.; supervision, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Many thanks to the late Mahammadali Ramazanov, without whom this project would never have been possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Choi, J.; Sun, N. Nanoparticles in Biomedical Applications and Their Safety Concerns. Biomed. Eng.-Theory Appl. 2011, 29, 486. [Google Scholar] [CrossRef]
- McNamara, K.; Tofail, S.A.M. Nanoparticles in biomedical applications. Adv. Phys. X 2017, 2, 54–88. [Google Scholar] [CrossRef]
- Menaa, D.B. The Importance of Nanotechnology in Biomedical Sciences. J. Biotechnol. Biomater. 2011, 1, 1000105. [Google Scholar] [CrossRef]
- Stanicki, D.; Elst, L.V.; Muller, R.N.; Laurent, S. Synthesis and processing of magnetic nanoparticles. Curr. Opin. Chem. Eng. 2015, 8, 7–14. [Google Scholar] [CrossRef]
- Xiao, Y.; Du, J. Superparamagnetic nanoparticles for biomedical applications. J. Mater. Chem. B 2020, 8, 354–367. [Google Scholar] [CrossRef]
- Nag, O.K.; Delehanty, J.B. Active Cellular and Subcellular Targeting of Nanoparticles for Drug Delivery. Pharmaceutics 2019, 11, 543. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- O’Grady, K. Editorial: Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2009, 42, 21–22. [Google Scholar] [CrossRef]
- Surendran, S.P.; Moon, M.J.; Park, R.; Jeong, Y.Y. Bioactive Nanoparticles for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3877. [Google Scholar] [CrossRef]
- Goldberg, M.S. Immunoengineering: How nanotechnology can enhance cancer immunotherapy. Cell 2015, 161, 201–204. [Google Scholar] [CrossRef]
- Yue-Jian, C.; Juan, T.; Fei, X.; Jia-Bi, Z.; Ning, G.; Yi-Hua, Z.; Ye, D.; Liang, G. Synthesis, self-assembly, and characterization of PEG-coated iron oxide nanoparticles as potential MRI contrast agent. Drug Dev. Ind. Pharm. 2010, 36, 1235–1244. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Ahren, M.; Selegard, L.; Klasson, A.; Soderlind, F.; Abrikossova, N.; Skoglund, C.; Bengtsson, T.; Engstrom, M.; Kall, P.O.; Uvdal, K. Synthesis and characterization of PEGylated Gd2O3 nanoparticles for MRI contrast enhancement. Langmuir 2010, 26, 5753–5762. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Xiong, Z.; Shen, M.; Conti, P.S.; Shi, X.; Chen, K. Polyethyleneimine-Coated Manganese Oxide Nanoparticles for Targeted Tumor PET/MR Imaging. ACS Appl. Mater. Interfaces 2018, 10, 34954–34964. [Google Scholar] [CrossRef]
- Boschi, F.; De Sanctis, F. Overview of the optical properties of fluorescent nanoparticles for optical imaging. Eur. J. Histochem. 2017, 61, 2830. [Google Scholar] [CrossRef]
- Nune, S.K.; Gunda, P.; Thallapally, P.K.; Lin, Y.Y.; Laird Forrest, M.; Berkland, C.J. Nanoparticles for biomedical imaging. Expert Opin. Drug Deliv. 2009, 6, 1175–1194. [Google Scholar] [CrossRef]
- Liu, X.; Li, H.; Chen, Y.; Jin, Q.; Ren, K.; Ji, J. Mixed-charge nanoparticles for long circulation, low reticuloendothelial system clearance, and high tumor accumulation. Adv. Healthc. Mater. 2014, 3, 1439–1447. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Gromnicova, R.; Kaya, M.; Romero, I.A.; Williams, P.; Satchell, S.; Sharrack, B.; Male, D. Transport of Gold Nanoparticles by Vascular Endothelium from Different Human Tissues. PLoS ONE 2016, 11, e0161610. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic understanding of nanoparticles’ interactions with extracellular matrix: The cell and immune system. Part. Fibre Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef]
- Hansen, U.; Allen, J.M.; White, R.; Moscibrocki, C.; Bruckner, P.; Bateman, J.F.; Fitzgerald, J. WARP interacts with collagen VI-containing microfibrils in the pericellular matrix of human chondrocytes. PLoS ONE 2012, 7, e52793. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B. Size and Shape Effect on Biomedical Applications of Nanomaterials. In Biomedical Engineering-Technical Applications in Medicine; IntechOpen: London, UK, 2012; pp. 20–40. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Aillon, K.L.; Xie, Y.; El-Gendy, N.; Berkland, C.J.; Forrest, M.L. Effects of nanomaterial physicochemical properties on in vivo toxicity. Adv. Drug Deliv. Rev. 2009, 61, 457–466. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Li, X.; Hu, X.; Han, Y.; Luo, Y.; Wang, Z.; Li, Q.; Aldalbahi, A.; Wang, L.; et al. Size-Dependent Regulation of Intracellular Trafficking of Polystyrene Nanoparticle-Based Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2017, 9, 18619–18625. [Google Scholar] [CrossRef]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. Adv. Exp. Med. Biol. 2014, 811, 73–91. [Google Scholar] [CrossRef]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanoparticle Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA nanoparticles can disassemble at the kidney glomerular basement membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Wallinder, I.O.; Fadeel, B.; Karlsson, H.L. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: The role of cellular uptake, agglomeration and Ag release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Kaga, S.; Truong, N.P.; Esser, L.; Senyschyn, D.; Sanyal, A.; Sanyal, R.; Quinn, J.F.; Davis, T.P.; Kaminskas, L.M.; Whittaker, M.R. Influence of Size and Shape on the Biodistribution of Nanoparticles Prepared by Polymerization-Induced Self-Assembly. Biomacromolecules 2017, 18, 3963–3970. [Google Scholar] [CrossRef]
- Wu, W.; Luo, L.; Wang, Y.; Wu, Q.; Dai, H.B.; Li, J.S.; Durkan, C.; Wang, N.; Wang, G.X. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics 2018, 8, 3038–3058. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhou, Z.; Song, J.; Chen, X. Anisotropic nanomaterials for shape-dependent physicochemical and biomedical applications. Chem. Soc. Rev. 2019, 48, 5140–5176. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, S.; Qiu, H.; Wang, T.; Zhao, Y.; Han, M.; Dong, Z.; Wang, X. Shape of nanoparticles as a design parameter to improve docetaxel antitumor efficacy. Bioconjug. Chem. 2018, 29, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Zhang, Q.; Huang, X.; Meng, X.; Zhang, Y.; Chen, D.; Tang, F.; Li, L. The shape effect of PEGylated mesoporous silica nanoparticles on cellular uptake pathway in Hela cells. Microporous Mesoporous Mater. 2012, 162, 14–23. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C.W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9, 51–63. [Google Scholar] [CrossRef]
- Forest, V.; Leclerc, L.; Hochepied, J.F.; Trouvé, A.; Sarry, G.; Pourchez, J. Impact of cerium oxide nanoparticles shape on their in vitro cellular toxicity. Toxicol. In Vitro 2017, 38, 136–141. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, J.E.; Kim, B.I.; Pak, P.J.; Choi, E.K.; Lee, H.S.; Chung, N. Rod-shaped iron oxide nanoparticles are more toxic than sphere-shaped nanoparticles to murine macrophage cells. Environ. Toxicol. Chem. 2014, 33, 2759–2766. [Google Scholar] [CrossRef]
- Li, Y.; Kröger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015, 7, 16631–16646. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Lin, I.C.; Whittaker, M.R.; Minchin, R.F.; Monteiro, M.J.; Toth, I. Cellular uptake of densely packed polymer coatings on gold nanoparticles. ACS Nano 2010, 4, 403–413. [Google Scholar] [CrossRef]
- Allen, C.; Qiu, T.A.; Pramanik, S.; Buchman, J.T.; Krause, M.O.P.; Murphy, C.J. Research highlights: Investigating the role of nanoparticle surface charge in nano-bio interactions. Environ. Sci. Nano 2017, 4, 741–746. [Google Scholar] [CrossRef]
- Baek, M.; Kim, M.K.; Cho, H.J.; Lee, J.A.; Yu, J.; Chung, H.E.; Choi, S.J. Factors influencing the cytotoxicity of zinc oxide nanoparticles: Particle size and surface charge. J. Phys. Conf. Ser. 2011, 304, 012044. [Google Scholar] [CrossRef]
- Kai, W.; Xiaojun, X.; Ximing, P.; Zhenqing, H.; Qiqing, Z. Cytotoxic effects and the mechanism of three types of magnetic nanoparticles on human hepatoma BEL-7402 cells. Nanoscale Res. Lett. 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Ding, H.M.; Ma, Y.Q. Interactions between Janus particles and membranes. Nanoscale 2012, 4, 1116–1122. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Gu, N. Computational investigation of interaction between nanoparticles and membranes: Hydrophobic/hydrophilic effect. J. Phys. Chem. B 2008, 112, 16647–16653. [Google Scholar] [CrossRef]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Hilliard, L.R.; Tan, W. Surface modification of silica nanoparticles to reduce aggregation and nonspecific binding. Langmuir 2006, 22, 4357–4362. [Google Scholar] [CrossRef]
- Van Haute, D.; Liu, A.T.; Berlin, J.M. Coating Metal Nanoparticle Surfaces with Small Organic Molecules Can Reduce Nonspecific Cell Uptake. ACS Nano 2018, 12, 117–127. [Google Scholar] [CrossRef]
- Cai, X.; Lee, A.; Ji, Z.; Huang, C.; Chang, C.H.; Wang, X.; Liao, Y.P.; Xia, T.; Li, R. Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation. Part. Fibre Toxicol. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.C.; Seligy, V.L.; Massarsky, A.; Moon, T.W.; Rippstein, P.; Tan, J.; Tayabali, A.F. Comparison of toxicity of uncoated and coated silver nanoparticles. J. Phys. Conf. Ser. 2013, 429, 012025. [Google Scholar] [CrossRef]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.Q.; Jiang, L.; Zhang, C.L.; Chen, Q.M.; Zhang, Z.R.; Lin, Y.F. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef] [PubMed]
- Walkey, C.D.; Chan, W.C.W. Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem. Soc. Rev. 2012, 41, 2780–2799. [Google Scholar] [CrossRef]
- Hadjidemetriou, M.; McAdam, S.; Garner, G.; Thackeray, C.; Knight, D.; Smith, D.; Al-Ahmady, Z.; Mazza, M.; Rogan, J.; Clamp, A.; et al. The Human In Vivo Biomolecule Corona onto PEGylated Liposomes: A Proof-of-Concept Clinical Study. Adv. Mater. 2019, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggård, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Baldelli Bombelli, F.; Dawson, K.A. Physical-Chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef]
- Sotnikov, D.V.; Berlina, A.N.; Ivanov, V.S.; Zherdev, A.V.; Dzantiev, B.B. Adsorption of proteins on gold nanoparticles: One or more layers? Colloids Surf. B Biointerfaces 2019, 173, 557–563. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Rosfa, S.; Wlodarski, A.; Kuharev, J.; Rekik, A.; Knauer, S.K.; Bantz, C.; Nawroth, T.; Bier, C.; et al. Nanoparticle size is a critical physicochemical determinant of the human blood plasma corona: A comprehensive quantitative proteomic analysis. ACS Nano 2011, 5, 7155–7167. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E. Formation of the protein corona: The interface between nanoparticles and the immune system. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2017; pp. 52–60. [Google Scholar]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Protein corona-enabled systemic delivery and targeting of nanoparticles. AAPS J. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Ma, S.; Gu, C.; Xu, J.; He, J.; Li, S.; Zheng, H.; Pang, B.; Wen, Y.; Fang, Q.; Liu, W. Strategy for Avoiding Protein Corona Inhibition of Targeted Drug Delivery by Linking Recombinant Affibody Scaffold to Magnetosomes. Int. J. Nanomed. 2022, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Ethiraj, K.; Mukherjee, A. Understanding the relevance of protein corona in nanoparticle-based therapeutics and diagnostics. RSC Adv. 2020, 10, 27161–27172. [Google Scholar] [CrossRef] [PubMed]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Shannahan, J.H.; Lai, X.; Ke, P.C.; Podila, R.; Brown, J.M.; Witzmann, F.A. Silver Nanoparticle Protein Corona Composition in Cell Culture Media. PLoS ONE 2013, 8, e74001. [Google Scholar] [CrossRef] [PubMed]
- Göppert, T.M.; Müller, R.H. Polysorbate-stabilized solid lipid nanoparticles as colloidal carriers for intravenous targeting of drugs to the brain: Comparison of plasma protein adsorption patterns. J. Drug Target. 2005, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Kittler, S.; Greulich, C.; Gebauer, J.S.; Diendorf, J.; Treuel, L.; Ruiz, L.; Gonzalez-Calbet, J.M.; Vallet-Regi, M.; Zellner, R.; Köller, M.; et al. The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles. J. Mater. Chem. 2010, 20, 512–518. [Google Scholar] [CrossRef]
- Kononenko, V.; Narat, M.; Drobne, D. Nanoparticle interaction with the immune system. Arch. Ind. Hyg. Toxicol. 2015, 66, 97–108. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).