A Review of Woven Tracheal Stents: Materials, Structures, and Application
Abstract
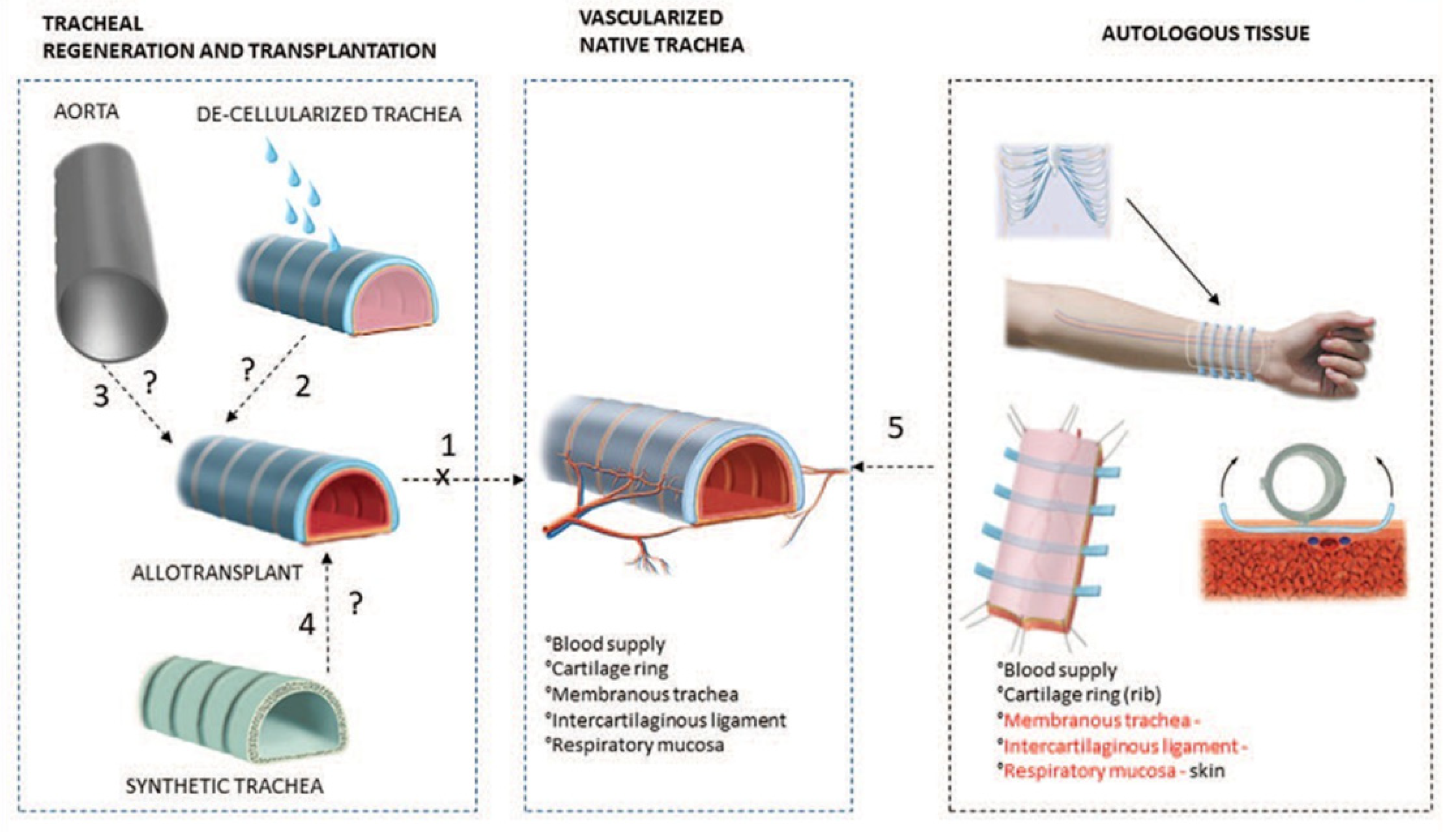
:1. Introduction
2. Materials Used in Woven Tracheal Stent
- Excellent biocompatibility.
- Excellent mechanical properties.
- The degradation rate of the material matched that of the new tissue.
- Immune rejection is within acceptable range.
2.1. Non-Degradable Material
2.2. Biodegradable Synthetic Materials
2.2.1. PDO
2.2.2. PPDO
2.2.3. PLA
2.3. Biodegradable Natural Materials
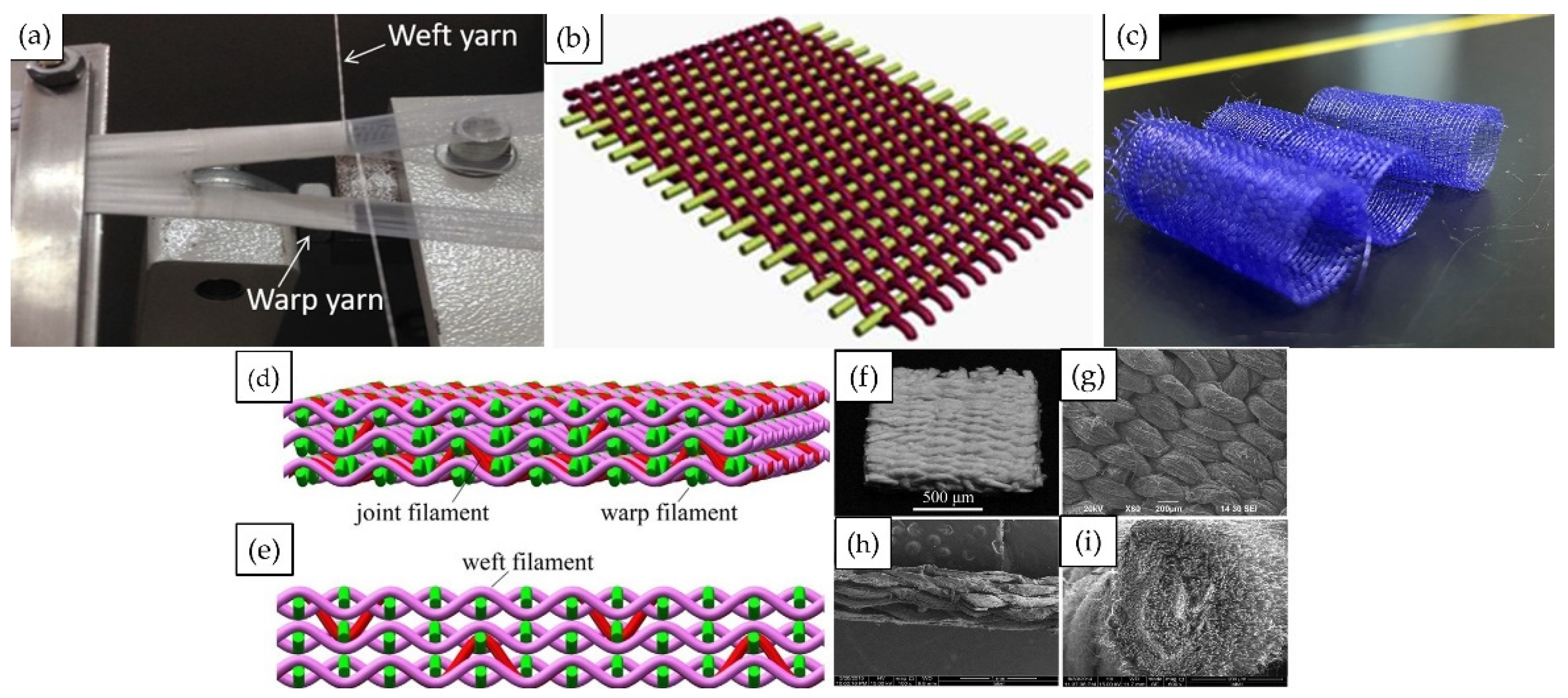
3. Structure of Woven Tracheal Stent
3.1. 2D Weaving and 3D Weaving
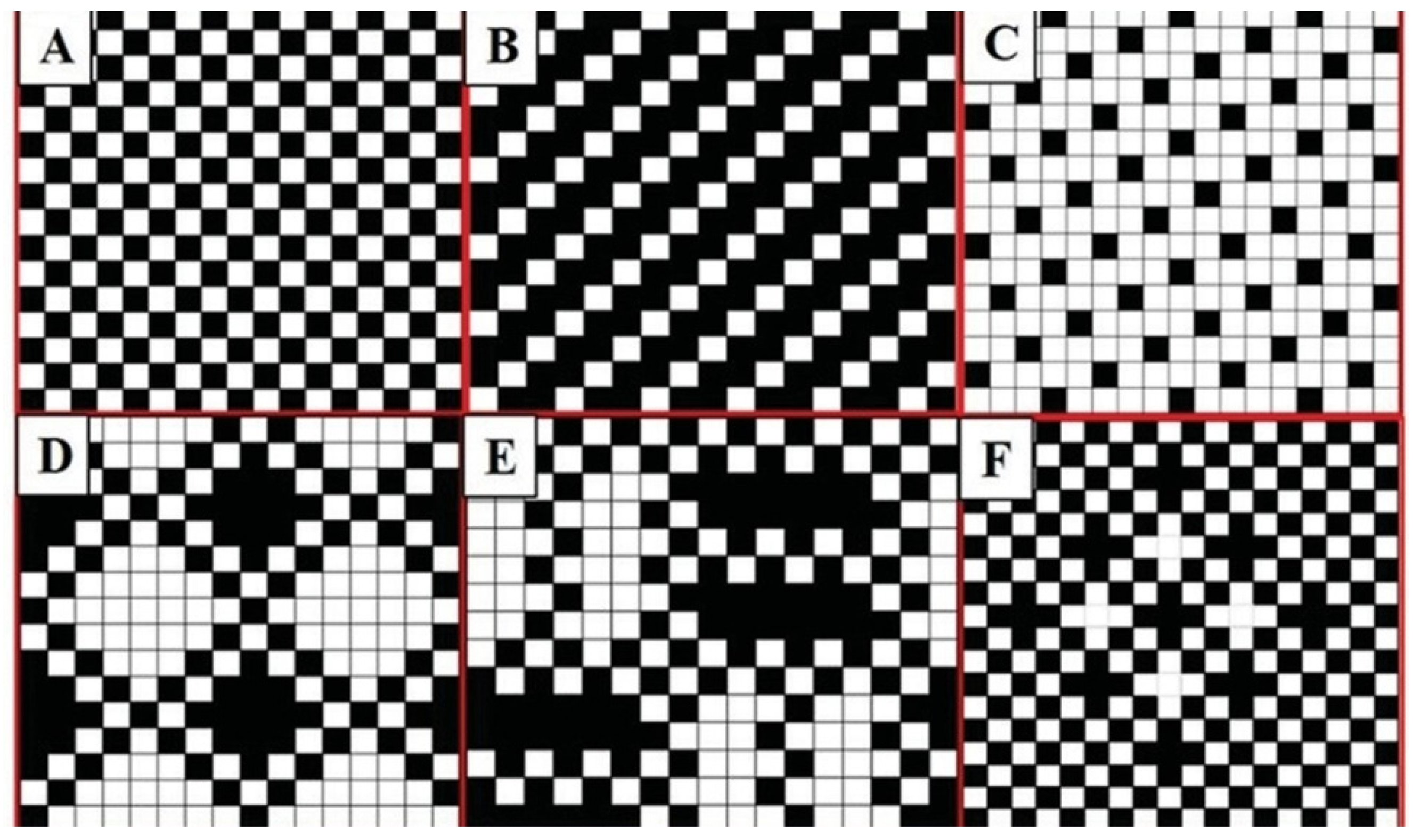
3.2. Woven Patterns
3.3. Weaving Process Parameters
4. Challenges and Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, C.B.; Son, K.H.; Yu, Y.S.; Kim, T.H.; Lee, J.I.; Lee, J.W. Development of a flexible 3D printed scaffold with a cell-adhesive surface for artificial trachea. Biomed. Mater. 2019, 14, 055001. [Google Scholar] [CrossRef]
- Choi, J.S.; Lee, M.S.; Kim, J.; Eom, M.R.; Jeong, E.J.; Lee, M.; Park, S.A.; Jeong, J.H.; Kwon, S.K. Hyaluronic acid coating on hydrophobic tracheal scaffold enhances mesenchymal stem cell adhesion and tracheal regeneration. Tissue Eng. Regen. Med. 2021, 18, 225–233. [Google Scholar] [CrossRef]
- Yu, Y.; Ahn, C.; Son, K.; Lee, J. Motility improvement of biomimetic trachea scaffold via hybrid 3D-bioprinting technology. Polymers 2021, 13, 971. [Google Scholar] [CrossRef]
- Soriano, L.; Khalid, T.; Whelan, D.; O’Huallachain, N.; Redmond, K.C.; O’Brien, F.J.; O’Leary, C.; Cryan, S.-A. Development and clinical translation of tubular constructs for tracheal tissue engineering: A review. Eur. Respir. Rev. 2021, 30, 210154. [Google Scholar] [CrossRef]
- Grillo, H.C. Tracheal replacement: A critical review. Ann. Thorac. Surg. 2002, 73, 1995–2004. [Google Scholar] [CrossRef]
- Etienne, H.; Fabre, D.; Caro, A.G.; Kolb, F.; Mussot, S.; Mercier, O.; Mitilian, D.; Stephan, F.; Fadel, E.; Dartevelle, P. Tracheal replacement. Eur. Respir. J. 2018, 51, 1702211. [Google Scholar] [CrossRef]
- Vranckx, J.J.; Delaere, P. The current status and outlook of trachea transplantation. Curr. Opin. Organ Transplant. 2020, 25, 601–608. [Google Scholar] [CrossRef]
- Ścierski, W.; Lisowska, G.; Namysłowski, G.; Misiołek, M.; Pilch, J.; Menaszek, E.; Gawlik, R.; Błażewicz, M. Reconstruction of ovine trachea with a biomimetic composite biomaterial. BioMed Res. Int. 2018, 2018, 2610637. [Google Scholar] [CrossRef]
- Li, D.; Yin, Z.; Liu, Y.; Feng, S.; Liu, Y.; Lu, F.; Xu, Y.; Min, P.; Hou, M.; Li, K.; et al. Regeneration of trachea graft with cartilage support, vascularization, and epithelization. Acta Biomater. 2019, 89, 206–216. [Google Scholar] [CrossRef]
- Wong, M.; Tan, B.-K.; Lim, C.-H. Trachea reconstruction with single-stage composite flaps in a rabbit model. J. Reconstr. Microsurg. 2020, 36, 001–008. [Google Scholar] [CrossRef]
- Mercier, O.; Kolb, F.; Dartevelle, P.G. Autologous tracheal replacement: Surgical technique and outcomes. Thorac. Surg. Clin. 2018, 28, 347–355. [Google Scholar] [CrossRef]
- Sun, F.; Lu, Y.; Wang, Z.; Zhang, B.; Shen, Z.; Yuan, L.; Wu, C.; Wu, Q.; Yang, W.; Zhang, G.; et al. Directly construct microvascularization of tissue engineering trachea in orthotopic transplantation. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112201. [Google Scholar] [CrossRef]
- Delaere, P.; Lerut, T.; Van Raemdonck, D. Tracheal transplantation: State of the art and key role of blood supply in its success. Thorac. Surg. Clin. 2018, 28, 337–345. [Google Scholar] [CrossRef]
- Ershadi, R.; Rahim, M.; Jahany, S.; Rakei, S. Transplantation of the decellularized tracheal allograft in animal model (Rabbit). Asian J. Surg. 2018, 41, 328–332. [Google Scholar] [CrossRef]
- Villalba-Caloca, J.; Sotres-Vega, A.; Giraldo-Gómez, D.M.; Gaxiola-Gaxiola, M.O.; Piña-Barba, M.C.; García-Montes, J.A.; Martínez-Fonseca, S.; Alonso-Gómez, M.; Santibáñez-Salgado, J.A. In vivo performance of decellularized tracheal grafts in the reconstruction of long length tracheal defects: Experimental study. Int. J. Artif. Organs 2021, 44, 718–726. [Google Scholar] [CrossRef]
- Ohno, M.; Fuchimoto, Y.; Higuchi, M.; Yamaoka, T.; Komura, M.; Umezawa, A.; Hsu, H.-C.; Enosawa, S.; Kuroda, T. Long-term observation of airway reconstruction using decellularized tracheal allografts in micro-miniature pigs at growing stage. Regen. Ther. 2020, 15, 64–69. [Google Scholar] [CrossRef]
- Tsukada, H.; Ernst, A.; Gangadharan, S.; Ashiku, S.; Garland, R.; Litmanovich, D.; DeCamp, M. Tracheal Replacement with a silicone-stented, fresh aortic allograft in sheep. Ann. Thorac. Surg. 2010, 89, 253–258. [Google Scholar] [CrossRef]
- Jaillard, S.; Holder-Espinasse, M.; Hubert, T.; Copin, M.-C.; Duterque-Coquillaud, M.; Wurtz, A.; Marquette, C.-H. Tracheal replacement by allogenic aorta in the pig. Chest 2006, 130, 1397–1404. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.H.; Cho, D.W. Comparison of tracheal reconstruction with allograft, fresh xenograft and artificial trachea scaffold in a rabbit model. J. Artif. Organs 2018, 21, 325–331. [Google Scholar] [CrossRef]
- Ren, J.H.; Xu, Y.Y.; Guo, Z.Y.; Ting, R.; Ren, J.B.; Kan, W.; Luo, Y.Q.; Zhu, M.Y.; Qiang, T. Reconstruction of the Trachea and carina: Surgical reconstruction, autologous tissue transplantation, allograft transplantation, and bioengineering. Thorac. Cancer 2022, 13, 284–295. [Google Scholar] [CrossRef]
- Hung, S.-H.; Su, C.-H.; Lin, S.-E.; Tseng, H. Preliminary experiences in trachea scaffold tissue engineering with segmental organ decellularization. Laryngoscope 2016, 126, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Choi, J.S.; Eom, M.R.; Jo, H.H.; Kwon, I.K.; Kwon, S.K.; Park, S.A. Dexamethasone loaded bilayered 3D tubular scaffold reduces restenosis at the anastomotic site of tracheal replacement: In vitro and in vivo assessments. Nanoscale 2020, 12, 4846–4858. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Fan, Z.; Wang, L.; Li, Y.; Sun, W.; Tang, H.; Zhang, L.; Wu, L.; Zheng, H.; Chen, C. 3D printed biomimetic PCL scaffold as framework interspersed with collagen for long segment tracheal replacement. Front. Cell Dev. Biol. 2021, 9, 629796. [Google Scholar] [CrossRef]
- Chan, D.S.; Gabra, N.; Baig, A.; Manoukian, J.J.; Daniel, S.J. Bridging the gap: Using 3D printed polycaprolactone implants to reconstruct circumferential tracheal defects in rabbits. Laryngoscope 2020, 130, E767–E772. [Google Scholar] [CrossRef] [PubMed]
- Kaye, R.; Cao, A.; Goldstein, T.; Grande, D.A.; Zeltsman, D.; Smith, L.P. Biomechanical properties of the ex vivo porcine trachea: A benchmark for three-dimensional bioprinted airway replacements. Am. J. Otolaryngol. 2022, 43, 103217. [Google Scholar] [CrossRef]
- Townsend, J.M.; Ott, L.M.; Salash, J.R.; Fung, K.M.; Easley, J.T.; Seim, H.B., 3rd; Johnson, J.K.; Weatherly, R.A.; Detamore, M.S. Reinforced electrospun polycaprolactone nanofibers for tracheal repair in an in vivo ovine model. Tissue Eng. Part A 2018, 24, 1301–1308. [Google Scholar] [CrossRef]
- O’Leary, C.; Soriano, L.; Fagan-Murphy, A.; Ivankovic, I.; Cavanagh, B.; O’Brien, F.J.; Cryan, S.-A. The fabrication and in vitro evaluation of retinoic acid-loaded electrospun composite biomaterials for tracheal tissue regeneration. Front. Bioeng. Biotechnol. 2020, 8, 190. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, K.; Liu, Y.; Zhang, C.; Wang, B. Application of textile technology in tissue engineering: A review. Acta Biomater. 2021, 128, 60–76. [Google Scholar] [CrossRef]
- Akbari, M.; Tamayol, A.; Bagherifard, S.; Serex, L.; Mostafalu, P.; Faramarzi, N.; Mohammadi, M.H.; Khademhosseini, A. Tex-tile technologies and tissue engineering: A path toward organ weaving. Adv. Healthc. Mater. 2016, 5, 751–766. [Google Scholar] [CrossRef] [Green Version]
- Liao, I.C.; Moutos, F.T.; Estes, B.T.; Zhao, X.H.; Guilak, F. Composite three-dimensional woven scaffolds with interpene-trating network hydrogels to create functional synthetic articular cartilage. Adv. Funct. Mater. 2013, 23, 5833–5839. [Google Scholar] [CrossRef]
- Wu, S.; Duan, B.; Liu, P.; Zhang, C.; Qin, X.-H.; Butcher, J. Fabrication of aligned nanofiber polymer yarn networks for anisotropic soft tissue scaffolds. ACS Appl. Mater. Interfaces 2016, 8, 16950–16960. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.; Yin, F.; Burg, K.J.L. Evaluation of permeability and fluid wicking in woven fiber bone scaffolds. J. Biomed. Mater. Res. Part B 2019, 107, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Choules, B.D.; Rust, J.P.; King, M.W. The Development of an in vitro test method for predicting the abrasion resistance of textile and metal components of endovascular stent grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Cai, J.; Yao, Y.; Chen, Y.; Khan, A.U.R.; Wu, J.; Mo, X. A woven scaffold with continuous mineral gradients for tendon-to-bone tissue engineering. Compos. Part B Eng. 2021, 212, 108679. [Google Scholar] [CrossRef]
- Ali, M.A.; Aly, N.M.; Mabrouk, M.; El-Sayed, S.A.; Beherei, H.H. A novel synthetic approach to produce cellulose-based woven scaffolds impregnated with bioactive glass for bone regeneration. Int. J. Biol. Macromol. 2021, 181, 905–918. [Google Scholar] [CrossRef]
- Persson, M.; Lehenkari, P.P.; Berglin, L.; Turunen, S.; Finnila, M.A.J.; Risteli, J.; Skrifvars, M.; Tuukkanen, J. Osteogenic differentiation of human mesenchymal stem cells in a 3d woven scaffold. Sci. Rep. 2018, 8, 10457. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, Z.; Chen, K.; Li, Y.; Wang, X.; Li, G. A heparin-functionalized woven stent graft for endovascular exclusion. Colloids Surf. B Biointerfaces 2019, 180, 118–126. [Google Scholar] [CrossRef]
- Richard, A.S.; Verma, R.S. Antioxidant α-mangostin coated woven polycaprolactone nanofibrous yarn scaffold for cardiac tissue repair. ACS Appl. Nano Mater. 2022, 5, 5075–5086. [Google Scholar] [CrossRef]
- Han, F.; Liu, S.; Liu, X.; Pei, Y.; Bai, S.; Zhao, H.; Lu, Q.; Ma, F.; Kaplan, D.L.; Zhu, H. Woven silk fabric-reinforced silk nano-fibrous scaffolds for regenerating load-bearing soft tissues. Acta Biomater. 2014, 10, 921–930. [Google Scholar] [CrossRef]
- Joseph, J.; Krishnan, A.G.; Cherian, A.M.; Rajagopalan, B.K.; Jose, R.; Varma, P.; Maniyal, V.; Balakrishnan, S.; Nair, S.V.; Menon, D. Transforming nanofibers into woven nanotextiles for vascular application. ACS Appl. Mater. Interfaces 2018, 10, 19449–19458. [Google Scholar] [CrossRef]
- Gopalakrishnan-Prema, V.; Mohanan, A.; Shivaram, S.B.; Madhusudanan, P.; Raju, G.; Menon, D.; Shankarappa, S.A. Electrical stimulation of co-woven nerve conduit for peripheral neurite differentiation. Biomed. Mater. 2020, 15, 065015. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, W.W. T-tube tracheal stent. Arch. Otolaryngol. 1965, 82, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, J.; Strahan, R.W.; Goode, R.L. Reinforced T-tube tracheal stent. Arch. Otolaryngol. 1969, 90, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.F.; Chen, Y.; Zhong, C.H.; Chen, X.B.; Li, S.Y. Long-term efficacy and safety of the dumon stent for benign tracheal stenosis: A meta-analysis. J. Thorac. Dis. 2021, 13, 82. [Google Scholar] [CrossRef]
- Sabino, M.A.; González, S.; Márquez, L.; Feijoo, J.L. Study of the hydrolytic degradation of polydioxanone Ppdx. Polym. Degrad. Stab. 2000, 69, 209–216. [Google Scholar] [CrossRef]
- Food and Drug Administration. 510(K) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?start_search=1&Center=&Panel=&ProductCode=&KNum-ber=&Applicant=&DeviceName=%20Polydioxanone&Type=&ThirdPartyReviewed=&ClinicalTrials=&Decision=&DecisionDate-From=&DecisionDateTo=06%2F23%2F2022&IVDProducts=&Redact510K=&CombinationProducts=&ZNumber=&PAGENUM=500 (accessed on 11 July 2022).
- Giammanco, G.; Martínez de Ilarduya, A.; Alla, A.; Muñoz-Guerra, S. Hydrolyzable aromatic copolyesters of P-dioxanone. Biomacromolecules 2010, 11, 2512–2520. [Google Scholar] [CrossRef]
- Gil-Castell, O.; Badia, J.D.; Bou, J.; Ribes-Greus, A. Performance of polyester-based electrospun scaffolds under in vitro hydrolytic conditions: From short-term to long-term applications. Nanomaterials 2019, 9, 786. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Feng, S.; Wang, X.; Qi, J.; Lei, D.; Li, Y.; Bai, W. Tuning the mechanical properties and degradation properties of pol-ydioxanone isothermal annealing. Turk. J. Chem 2020, 44, 1430–1444. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhu, X.; Li, Z.; Li, Z.; Qiu, H.; Wu, G. Poly(ether ester) and related block copolymers via organocatalytic ring-opening polymerization. J. Polym. Sci. 2022. [Google Scholar] [CrossRef]
- Kajihara, K. Recent advances in sol–gel synthesis of monolithic silica and silica-based glasses. J. Asian Ceram. Soc. 2013, 1, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Zhang, J.J.; Qiu, Z.C.; Zeng, Q.; Chang, J.J.; Yang, K.K.; Wang, Y.Z. Properties of poly(P-dioxanone-urethane) copolymers with controllable structures. Soft Mater. 2009, 7, 277–295. [Google Scholar] [CrossRef]
- Pawar, R.; Tekale, S.; Shisodia, S.; Totre, J.; Domb, A. Biomedical applications of poly(lactic acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of lactic acid based polymers and their correlation with composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of biomedical scaffolds using biodegradable polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef]
- Park, K.; Xanthos, M. A study on the degradation of polylactic acid in the presence of phosphonium ionic liquids. Polym. Degrad. Stab. 2009, 94, 834–844. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of Pla, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Murariu, M.; Dubois, P. PLA composites: From production to properties. Adv. Drug Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Yin, Z.; Luo, X.; Liu, W.; Zhang, W.; Zhang, Z.; Cao, Y.; Liu, Y.; Zhou, G. Prolonged in vitro precultivation alle-viates post-implantation inflammation and promotes stable subcutaneous cartilage formation in a goat model. Biomed. Mater. 2016, 12, 015006. [Google Scholar] [CrossRef]
- Sindeeva, O.A.; Prikhozhdenko, E.S.; Schurov, I.; Sedykh, N.; Goriainov, S.; Karamyan, A.; Mordovina, E.A.; Inozemtseva, O.A.; Kudryavtseva, V.; Shchesnyak, L.E.; et al. Patterned drug-eluting coatings for tracheal stents based on PLA, PLGA, and PCL for the granulation formation reduction: In vivo studies. Pharmaceutics 2021, 13, 1437. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive scaffolds for bone tissue engineering: Current state and future outlook. J. Funct. Biomater. 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Romanova, O.A.; Tenchurin, T.H.; Demina, T.; Sytina, E.V.; Shepelev, A.D.; Rudyak, S.G.; Klein, O.I.; Krasheninnikov, S.V.; Safronova, E.I.; Kamyshinsky, R.; et al. Non-woven bilayered biodegradable chitosan-gelatin-polylactide scaffold for bioengineering of tracheal epithelium. Cell Prolif. 2019, 52, e12598. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Trček, J. Bacterial cellulose: Production, modification and perspectives in biomedical applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; King, M.W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Health Mater. 2020, 9, 1901358. [Google Scholar] [CrossRef]
- Mouritz, A.P.; Bannister, M.K.; Falzon, P.J.; Leong, K.H. Review of applications for advanced three-dimensional fibre textile composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1445–1461. [Google Scholar] [CrossRef]
- Singh, S.; Vashisth, P.; Shrivastav, A.; Bhatnagar, N. Synthesis and characterization of a novel open cellular Mg-based scaffold for tissue engineering application. J. Mech. Behav. Biomed. Mater. 2019, 94, 54–62. [Google Scholar] [CrossRef]
- Zhu, H.; HuiMin, H. Design and investigations of three-dimensional multi-holesinpattern structure. J. Donghua Univ. (Nat. Sci.) 2010, 36, 633–638. [Google Scholar]
- Wu, Y.; Wang, L.; Guo, B.; Ma, P.X. Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 2017, 11, 5646–5659. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Han, Q.; Sang, F.; Wang, Q.; Chen, L.; Cui, S.; Ding, B. A biomimetic multilayer nanofiber fabric fabricated by electrospinning and textile technology from polylactic acid and tussah silk fibroin as a scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 599–610. [Google Scholar] [CrossRef]
- Jiao, Y.; Li, C.; Liu, L.; Wang, F.; Liu, X.; Mao, J.; Wang, L. Construction and application of textile-based tissue engineering scaffolds: A review. Biomater. Sci. 2020, 8, 3574–3600. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.Y.; Li, Y.L.; Wang, W.Z.; Shu, F.L. Performance analysis of woven artificial Trachea. Tech. Text. 2011, 29, 12–14. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=CYYF201106002&uniplatform=NZKPT&v=abSHRTr2JyKKJFYsF3NAvjswn0msHsnPSOIOcRTyOWMlMYO5k1b_vKh134nLHVK5 (accessed on 11 July 2022). (In Chinese).
- Fu, B.S.; Li, Y.L.; Ruan, Z.; Ma, Y. Development and mechanical properties of a novel woven artificial tracheal stent. J. Donghua Univ. (Engl. Ed.) 2014, 31, 643–645. [Google Scholar]
- Zhao, F.; Sun, J.; Xue, W.; Wang, F.; King, M.W.; Yu, C.; Jiao, Y.; Sun, K.; Wang, L. Development of a polycaprolactone/poly(p-dioxanone) bioresorbable stent with mechanically self-reinforced structure for congenital heart disease treatment. Bioact. Mater. 2021, 6, 2969–2982. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, J.; Wang, J.; Pei, Y.-H.; Qiu, X.-J.; Wang, Y.-L. Paclitaxel drug-eluting tracheal stent could reduce granulation tissue formation in a canine model. Chin. Med. J. 2016, 129, 2708–2713. [Google Scholar] [CrossRef]
- Griffin, M.F.; Palgrave, R.G.; Seifalian, A.M.; Butler, P.E.; Kalaskar, D.M. Enhancing tissue integration and angiogenesis of a novel nanocomposite polymer using plasma surface polymerisation, an in vitro and in vivo study. Biomater. Sci. 2016, 4, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Zhong, Y.; Shan, Y.; Liu, X.; Xiao, Y.; Shi, H. Selection of the optimum 3D-printed pore and the surface modification techniques for tissue engineering tracheal scaffold in vivo reconstruction. J. Biomed. Mater. Res. Part A 2019, 107, 360–370. [Google Scholar] [CrossRef]
- Matsui, H.; Hiroma, T.; Hasegawa, H.; Ogiso, Y. Decreased granulomatous reaction by polyurethane-coated stent in the trachea. Pediatr. Int. 2014, 56, 817–821. [Google Scholar] [CrossRef]
- Jin, Z.; Chen, Z.; Wu, K.; Shen, Y.; Guo, S. Investigation of migration-preventing tracheal stent with high dose of 5-fluorouracil or paclitaxel for local drug delivery. ACS Appl. Bio Mater. 2018, 1, 1328–1336. [Google Scholar] [CrossRef]
- Law, J.X.; Liau, L.L.; Aminuddin, B.S.; Ruszymah, B.H.I. Tissue-engineered trachea: A review. Int. J. Pediatr. Otorhinolaryngol. 2016, 91, 55–63. [Google Scholar] [CrossRef]
- Jacobs, I.N.; Redden, R.A.; Goldberg, R.; Hast, M.; Salowe, R.; Mauck, R.L.; Doolin, E.J. Pediatric laryngotracheal reconstruction with tissue-engineered cartilage in a rabbit model. Laryngoscope 2016, 126, S5–S21. [Google Scholar] [CrossRef]


| Year | Material | Application Areas | Research Contents | Reference |
|---|---|---|---|---|
| 2013 | Alginate/polyacrylamide/poly(ε-caprolacto/ne) | Articular cartilage | Formation of a dual-network “tough-gel” to establish load-bearing and tribological properties similar to native cartilage | [30] |
| 2018 | Hydrogel/polyacrylonitrile/N, N-Dimethylformamide/polycaprolactone/polyurethane | Heart valves | Integration of woven fiber networks into bioactive hydrogels to produce stents with anisotropic biomechanics and valve ECM like microenvironment | [31] |
| 2018 | Poly-l-lactide/poly-l-lactide-co-E-caprolactone | Bone | The permeability and porosity of the stent were evaluated by adjusting the material combination, weave configuration, and fiber Geometry | [32] |
| 2014 | Polyester/nitinol | Endovascular | Abrasion resistance testing of intravascular fabrics and metal stent grafts. | [33] |
| 2021 | Hydroxyapatite/polylactic acid/poly(L-lactide-co- ε-caprolactone/silk fibroin | Tendon-to-bone | The combination of electrostatic spinning and weaving technology enables gradient release of calcium ions, stents are structurally anisotropic and promote osteogenic differentiation and osteoblast proliferation in rats | [34] |
| 2021 | Bioactive glass/silver nanoparticles | Bone | Woven structures are used to stimulate the growth of cells by virtue of their balanced yarns intersections on the structures’ surfaces. Stents loaded with g bioactive glass containing Ag 0.5% demonstrated remarkable biomineralization. | [35] |
| 2018 | Poly (lactic acid)/hydroxyapatite | Bone | The 3D architecture of woven stent supports the differentiation of the hMSCs into osteoblast cells and enhances the production of mineralized bone matrix | [36] |
| 2019 | Silk fibroin/heparin | Heparin | The filamentous silk fibroin/heparin stent uses woven bifurcation technology and steam/air treatment to achieve anticoagulant properties and improve permeability. | [37] |
| 2022 | α-Mangostin/Polycaprolactone | Cardiac | Woven nanofiber yarn stents exhibit customizable flexible structures, excellent mechanical strength, proper cell adhesion, and degradation properties in vitro. | [38] |
| 2014 | Silk | skin | Significantly higher final tensile strength, elongation at break, and suture retention strength of silk woven stents prepared by enhanced lyophilization with degumming | [39] |
| 2018 | Poly(caprolactone)- Collagen/Poly-L-lactic Acid/ | Vascular | The patterned woven structure promotes protein adsorption, as well as cell attachment and spreading | [40] |
| 2020 | poly-(L-lactic acid)/polypyrrole/copper/platinum | nerve conduits | The design of the braided stent can be used to incorporate conductive materials into polymer yarns to develop electrically stimulable nerve conduits | [41] |
| Type of Material | Fibrous Material | Advantages | Disadvantages | |
|---|---|---|---|---|
| Non-biodegradable | Silicone Nitinol |
|
| |
| Biodegradable | Synthetic | PLA PDO PCL PPDO |
|
|
| natural | Gelatin Silk fibroin |
|
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Ma, Y.; Huang, H.; Ruan, Z.; Li, Y. A Review of Woven Tracheal Stents: Materials, Structures, and Application. J. Funct. Biomater. 2022, 13, 96. https://doi.org/10.3390/jfb13030096
Xu C, Ma Y, Huang H, Ruan Z, Li Y. A Review of Woven Tracheal Stents: Materials, Structures, and Application. Journal of Functional Biomaterials. 2022; 13(3):96. https://doi.org/10.3390/jfb13030096
Chicago/Turabian StyleXu, Chen, Yanxue Ma, Haihua Huang, Zheng Ruan, and Yuling Li. 2022. "A Review of Woven Tracheal Stents: Materials, Structures, and Application" Journal of Functional Biomaterials 13, no. 3: 96. https://doi.org/10.3390/jfb13030096
APA StyleXu, C., Ma, Y., Huang, H., Ruan, Z., & Li, Y. (2022). A Review of Woven Tracheal Stents: Materials, Structures, and Application. Journal of Functional Biomaterials, 13(3), 96. https://doi.org/10.3390/jfb13030096






