Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Synthesis of Fe@Fe3O4 Nanoparticles
2.3. The Cytotoxicity Analysis
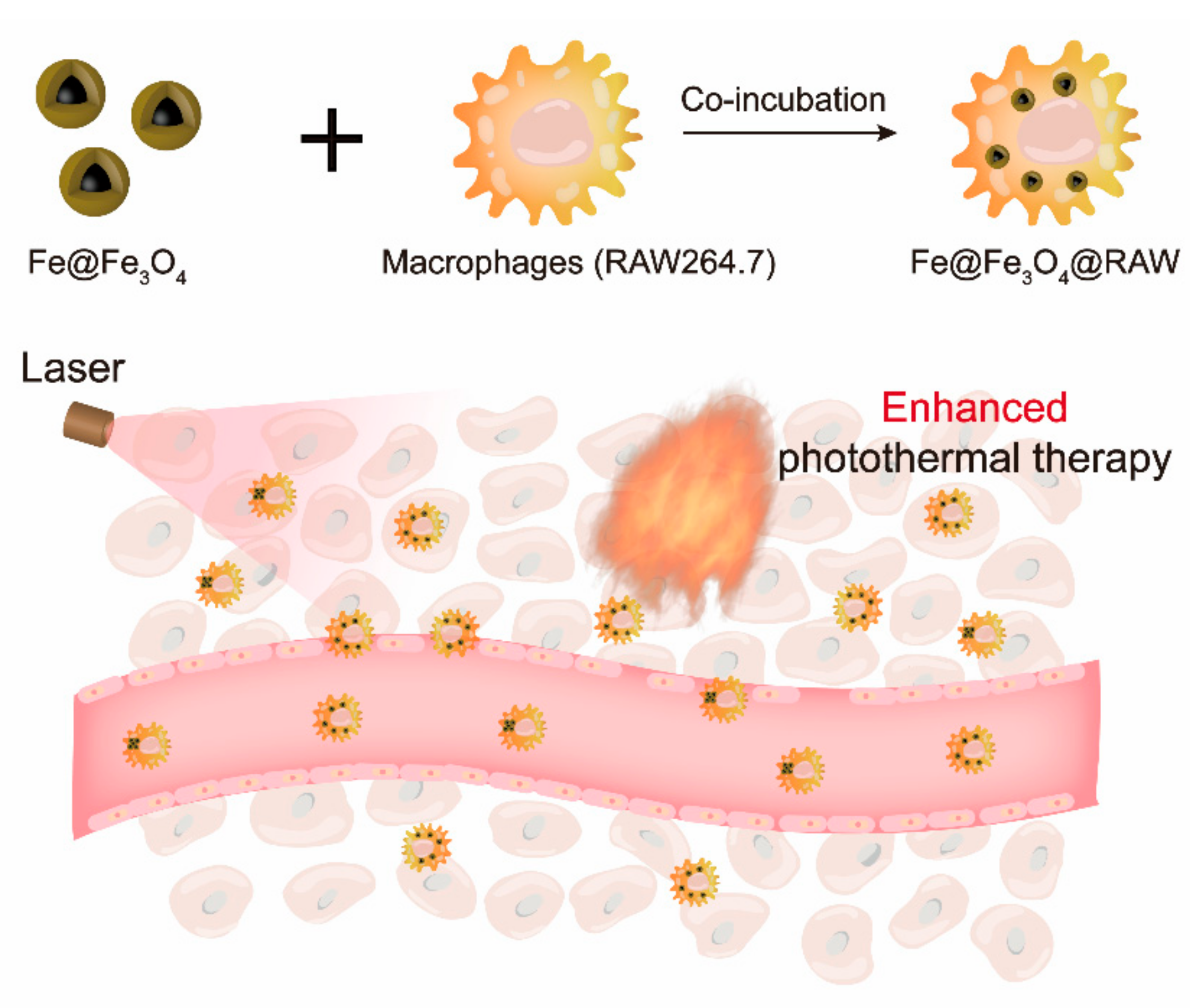
2.4. Construction of Fe@Fe3O4@RAW
2.5. MRI Analysis
2.6. Characterization of Photothermal Properties
2.7. Ethical Statement for the Tumor Model
2.8. Statistical Analyses
3. Results and Discussion
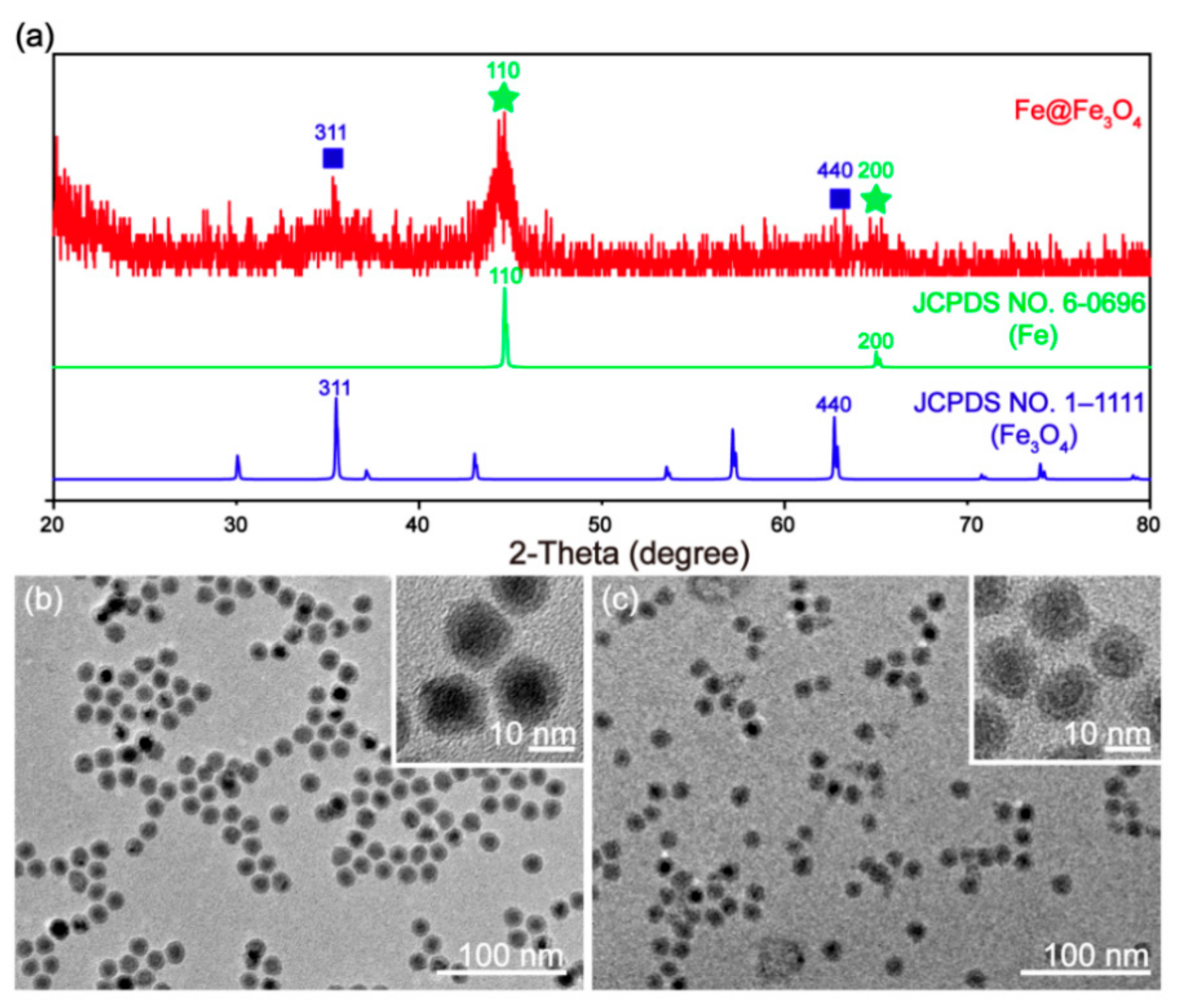
3.1. Synthesis and Characterization of Fe@Fe3O4 Nanoparticles
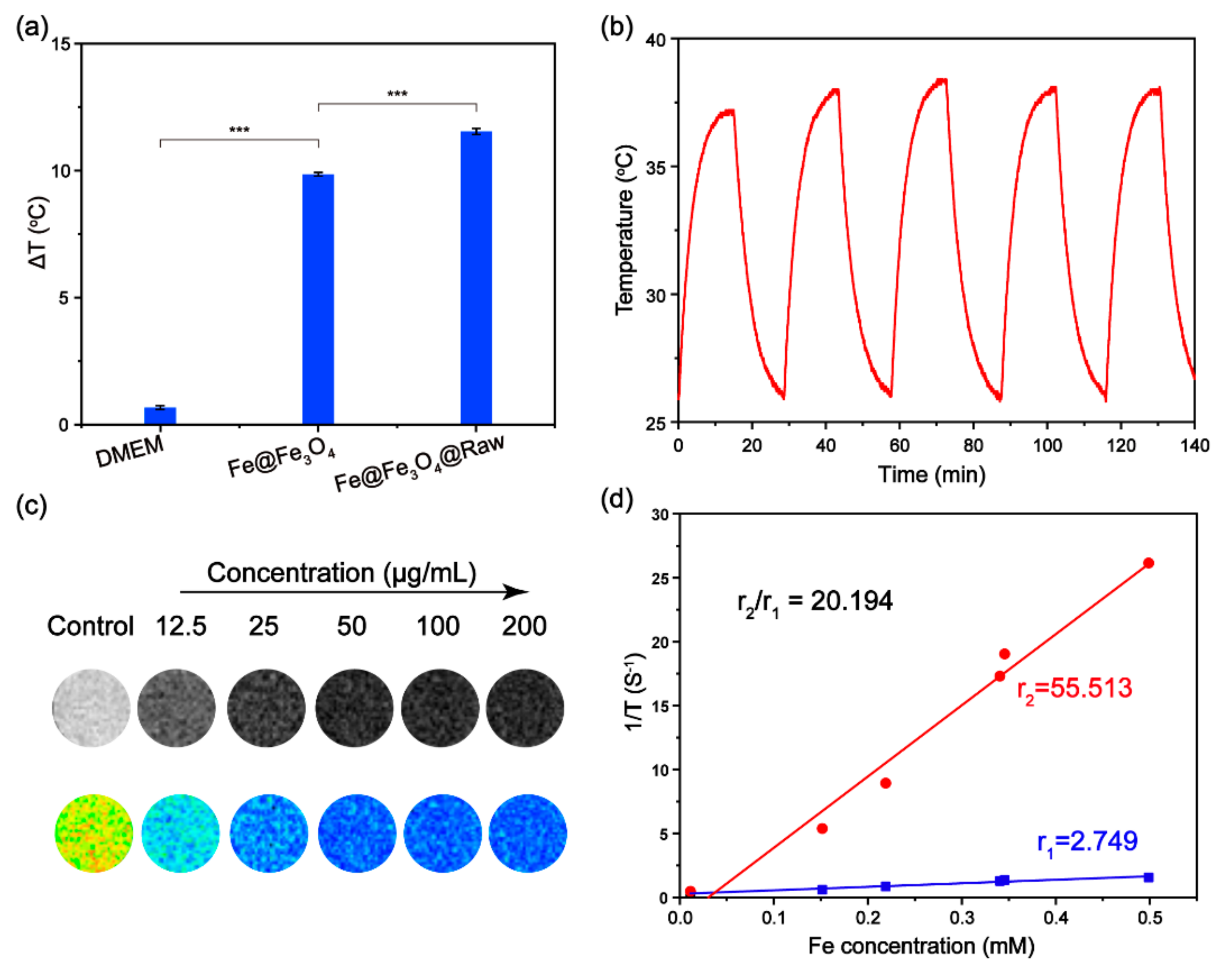
3.2. Photothermal Performance of Fe@Fe3O4 Nanoparticles
3.3. Macrophages Loaded with Fe Nanoparticles
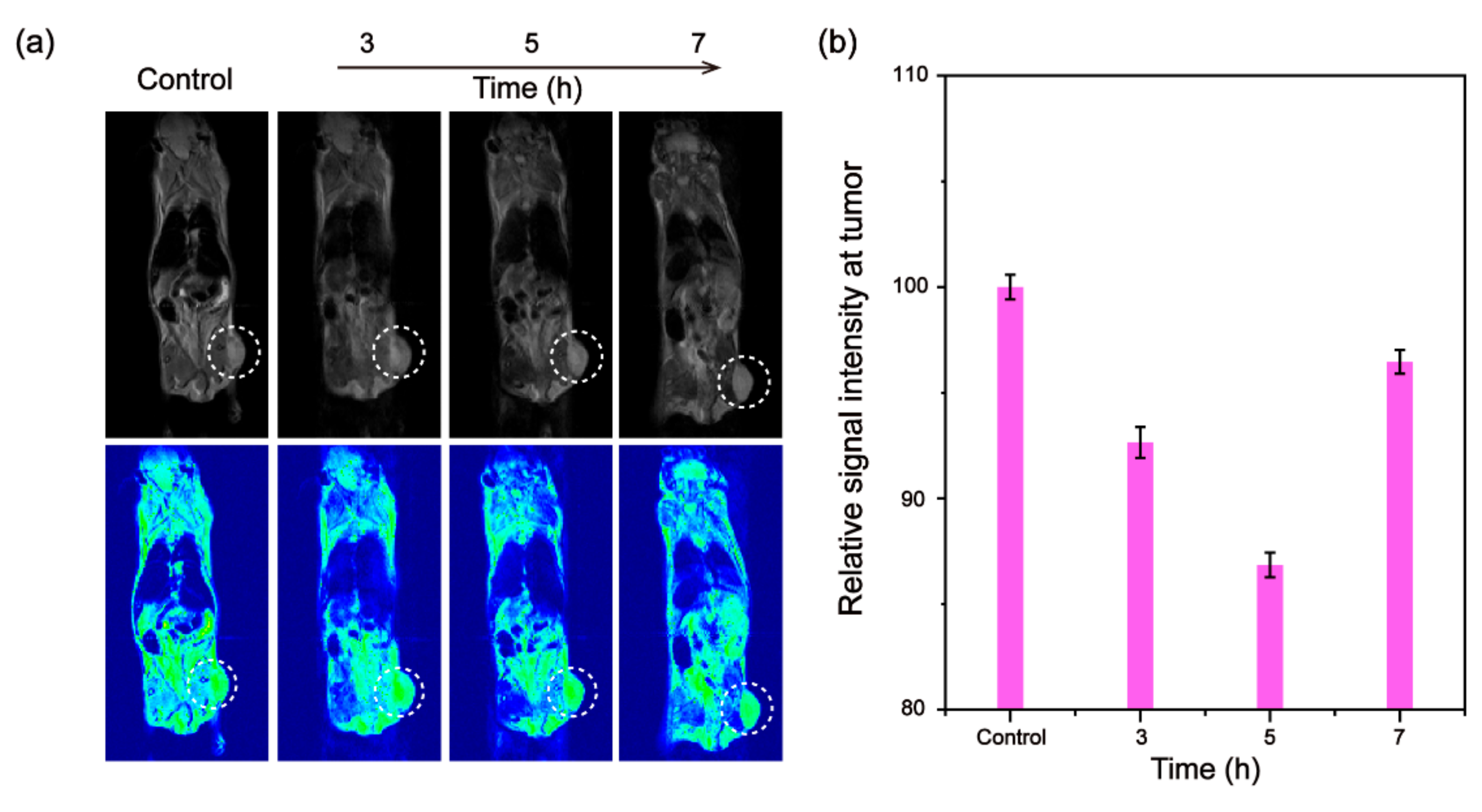
3.4. In Vivo Tumor MRI
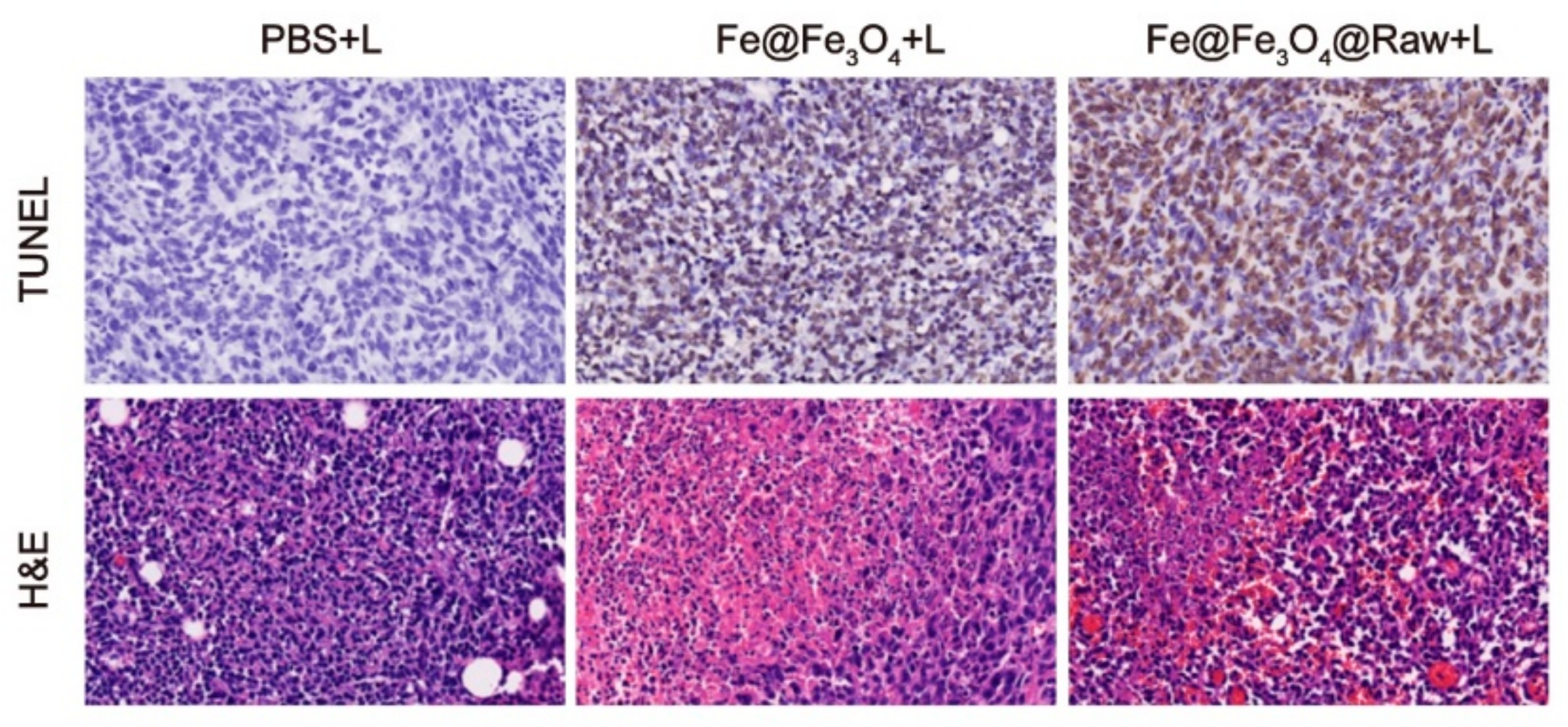
3.5. In Vivo Photothermal Tumor Ablation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, D.; Sun, X.; Sun, S. Monodisperse magnetic nanoparticles for theranostic applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mei, T.; Liu, Y.; Zhang, Y.; Zhang, Z.; Hu, Y.; Wang, Y.; Wu, M.; Yang, C.; Zhong, X.; et al. Dual-targeted and MRI-guided photothermal therapy via iron-based nanoparticles-incorporated neutrophils. Biomater. Sci. 2021, 9, 3968–3978. [Google Scholar] [CrossRef]
- Alphandéry, E. Light-interacting iron-based nanomaterials for localized cancer detection and treatment. Acta Biomater. 2021, 124, 50–71. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Kaczmarek-Szczepańskac, B.; Sahu, N.K. Magneto-thermal response of Fe3O4@CTAB nanoparticles for cancer hyperthermia applications. Mater. Today Commun. 2021, 28, 102583. [Google Scholar] [CrossRef]
- Nicolás-Boluda, A.; Vaquero, J.; Laurent, G.; Renault, G.; Bazzi, R.; Donnadieu, E.; Roux, S.; Fouassier, L.; Gazeau, F. Photothermal depletion of cancer-associated fibroblasts normalizes tumor stiffness in desmoplastic cholangiocarcinoma. ACS Nano 2020, 14, 5738–5753. [Google Scholar] [CrossRef]
- Qin, J.; Liu, Q.; Zhang, J.; Chen, J.; Chen, S.; Zhao, Y.; Du, J. Rationally separating the corona and membrane functions of polymer vesicles for enhanced T2 MRI and drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 14043–14052. [Google Scholar] [CrossRef]
- Zhou, P.; Zhao, H.; Wang, Q.; Zhou, Z.; Wang, J.; Deng, G.; Wang, X.; Liu, Q.; Yang, H.; Yang, S. Photoacoustic-enabled self-guidance in magnetic-hyperthermia Fe@Fe3O4 nanoparticles for theranostics in vivo. Adv. Healthc. Mater. 2018, 7, 1701201. [Google Scholar] [CrossRef]
- Atabaev, T.S. PEG-coated superparamagnetic dysprosium-doped Fe3O4 nanoparticles for potential MRI imaging. BioNanoScience 2018, 8, 299–303. [Google Scholar] [CrossRef]
- Nicolas-Boluda, A.; Laurent, G.; Bazzi, R.; Roux, S.; Donnadieu, E.; Gazeau, F. Two step promotion of a hot tumor immune environment by gold decorated iron oxide nanoflowers and light-triggered mild hyperthermia. Nanoscale 2021, 13, 18483–18497. [Google Scholar] [CrossRef]
- Zhu, Y.; Fang, Y.; Kaskel, S. Folate-conjugated Fe3O4@SiO2 hollow mesoporous spheres for targeted anticancer drug delivery. J. Phys. Chem. C 2010, 114, 16382–16388. [Google Scholar] [CrossRef]
- Cheng, K.; Peng, S.; Xu, C.; Sun, S. Porous hollow Fe3O4 nanoparticles for targeted delivery and controlled release of cisplatin. J. Am. Chem. Soc. 2009, 131, 10637–10644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, H.; Li, K.; Shen, M.; Wen, S.; Luo, Y.; Peng, C.; Zhang, G.; Shi, X. Facile assembly of Fe3O4@Au nanocomposite particles for dual mode magnetic resonance and computed tomography imaging applications. J. Mater. Chem. 2012, 22, 15110–15120. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhang, Y.; Wei, J.; Wang, W.; Dong, C.; Xue, Y.; Liu, M.; Pei, R. Engineered Fe3O4-based nanomaterials for diagnosis and therapy of cancer. New J. Chem. 2021, 45, 7918–7941. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, J. Clearance pathways and tumor targeting of imaging nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1–T2 switchable magnetic resonance imaging contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 2020, 32, 2002054. [Google Scholar]
- Lang, T.; Dong, X.; Huang, Y.; Ran, W.; Yin, Q.; Zhang, P.; Zhang, Z.; Yu, H.; Li, Y. Ly6Chi monocytes delivering pH-sensitive micelle loading paclitaxel improve targeting therapy of metastatic breast cancer. Adv. Funct. Mater. 2017, 27, 1701093. [Google Scholar] [CrossRef]
- Li, S.; Feng, S.; Ding, L.; Liu, Y.; Zhu, Q.; Qian, Z.; Gu, Y. Nanomedicine engulfed by macrophages for targeted tumor therapy. Int. J. Nanomed. 2016, 11, 4107–4124. [Google Scholar]
- Feng, Y.; Liu, Q.; Li, Y.; Han, Y.; Liang, M.; Wang, H.; Yao, Q.; Wang, Y.; Yang, M.; Li, Z.; et al. Cell relay-delivery improves targeting and therapeutic efficacy in tumors. Bioact. Mater. 2021, 6, 1528–1540. [Google Scholar] [CrossRef]
- Vercellino, S.; Kokalari, I.; Liz Cantoral, M.; Petseva, V.; Cursi, L.; Casoli, F.; Castagnola, V.; Boselli, L.; Fenoglio, I. Biological interactions of ferromagnetic iron oxide–carbon nanohybrids with alveolar epithelial cells. Biomater. Sci. 2022, 10, 3514–3526. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.-Y.; Ju, E.J.; Jung, J.; Park, J.; Chung, H.-K.; Lee, J.S.; Lee, J.S.; Park, H.J.; Song, S.Y.; et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials 2012, 33, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, D.; Mei, D.; Zhang, H.; Wang, Z.; He, B.; Dai, W.; Zhang, H.; Wang, X.; Zhang, Q. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J. Control Release 2015, 204, 11–19. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wang, Y.; Lin, J.; Tian, Q.; Xie, Y.; Hu, J.; Yang, S. Macrophages-mediated delivery of small gold nanorods for tumor hypoxia photoacoustic imaging and enhanced photothermal therapy. ACS Appl. Mater. Interfaces 2019, 11, 15251–15261. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Min, H.-K.; Kim, H.Y.; Han, J.; Choi, Y.H.; Kim, C.-S.; Park, J.-O.; Choi, E. Primary macrophage-based microrobots: An effective tumor therapy in vivo by dual-targeting function and near-infrared-triggered drug release. ACS Nano 2021, 15, 8492–8506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, M.; Tang, W.; Wen, R.; Zhou, S.; Lee, C.; Wang, H.; Jiang, W.; Delahunty, I.M.; Zhen, Z.; et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv. Mater. 2018, 30, 1805557. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J.; Hirschberg, H. Macrophages as delivery vehicles for anticancer agents. Ther. Deliv. 2019, 10, 189–201. [Google Scholar]
- Alvarenga, B.M.; Melo, M.N.; Frézard, F.; Demicheli, C.; Gomes, J.M.M.; Borba da Silva, J.B.; Speziali, N.L.; Corrêa Junior, J.D. Nanoparticle phosphate-based composites as vehicles for antimony delivery to macrophages: Possible use in leishmaniasis. J. Mater. Chem. B 2015, 3, 9250–9259. [Google Scholar] [CrossRef]
- Burke, B.; Sumner, S.; Maitland, N.; Lewis, C.E. Macrophages in gene therapy: Cellular delivery vehicles and in vivo targets. J. Leukoc. Biol. 2002, 72, 417–428. [Google Scholar]
- Li, J.; Cheng, Q.; Yue, L.; Gao, C.; Wei, J.; Ding, Y.; Wang, Y.; Zheng, Y.; Wang, R. Macrophage-hitchhiking supramolecular aggregates of CuS nanoparticles for enhanced tumor deposition and photothermal therapy. Nanoscale Horiz. 2021, 6, 907–912. [Google Scholar] [CrossRef]
- Lacroix, L.-M.; Frey Huls, N.; Ho, D.; Sun, X.; Cheng, K.; Sun, S. Stable single-crystalline body centered cubic Fe nanoparticles. Nano Lett. 2011, 11, 1641–1645. [Google Scholar] [CrossRef]
- Liu, D.; Li, J.; Wang, C.; An, L.; Lin, J.; Tian, Q.; Yang, S. Ultrasmall Fe@Fe3O4 nanoparticles as T1–T2 dual-mode MRI contrast agents for targeted tumor imaging. Nanomed. NBM 2021, 32, 102335. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, Y.; Shen, J.; Wei, J.; Yu, C.; Kong, B.; Liu, W.; Yang, H.; Yang, S.; Wang, W. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 2014, 35, 7470–7478. [Google Scholar] [CrossRef]
- Chen, H.; Shao, L.; Ming, T.; Sun, Z.; Zhao, C.; Yang, B.; Wang, J. Understanding the photothermal conversion efficiency of gold nanocrystals. Small 2010, 6, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Cao, M.; Zhang, X.; Lin, J.; Tian, Q.; Yang, S. pH and glutathione synergistically triggered release and self-assembly of Au nanospheres for tumor theranostics. ACS Appl. Mater. Interfaces 2020, 12, 8050–8061. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Busquets, M.A. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Wu, Z.; Li, W.; Wang, Z.; Li, Q.; Kong, F.; Zhang, H.; Zhu, X.; Du, Y.P.; Jin, Y.; et al. Appropriate size of magnetic nanoparticles for various bioapplications in cancer diagnostics and therapy. ACS Appl. Mater. Interfaces 2016, 8, 3092–3106. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, S.; Zheng, R.; Zhu, X.; Jiang, X.; Fu, D.; Yang, W. Magnetic nanoparticle clusters for photothermal therapy with near-infrared irradiation. Biomaterials 2015, 39, 67–74. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Zhu, S.; Qin, K.; Fan, X.; An, L. Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors. J. Funct. Biomater. 2022, 13, 94. https://doi.org/10.3390/jfb13030094
Yu L, Zhu S, Qin K, Fan X, An L. Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors. Journal of Functional Biomaterials. 2022; 13(3):94. https://doi.org/10.3390/jfb13030094
Chicago/Turabian StyleYu, Lei, Shuntao Zhu, Kun Qin, Xueyu Fan, and Lu An. 2022. "Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors" Journal of Functional Biomaterials 13, no. 3: 94. https://doi.org/10.3390/jfb13030094
APA StyleYu, L., Zhu, S., Qin, K., Fan, X., & An, L. (2022). Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors. Journal of Functional Biomaterials, 13(3), 94. https://doi.org/10.3390/jfb13030094





