3D Printing of Bioinert Oxide Ceramics for Medical Applications
Abstract
1. Introduction
2. AM Technologies for Ceramics
2.1. VAT Photopolymerization
2.2. Material Extrusion (ME)
2.3. Material Jetting (MJ)
2.4. Binder Jetting (BJ)
2.5. Powder Bed Fusion (PBF)
2.6. Directed Energy Deposition (DED)
2.7. Sheet Lamination
3. Comparison among the Different AM Technologies for Ceramics
4. Bioinert Ceramics
4.1. Zirconia
4.2. Alumina
4.3. Titania
5. Medical Applications of Additive Manufactured Ceramics Materials
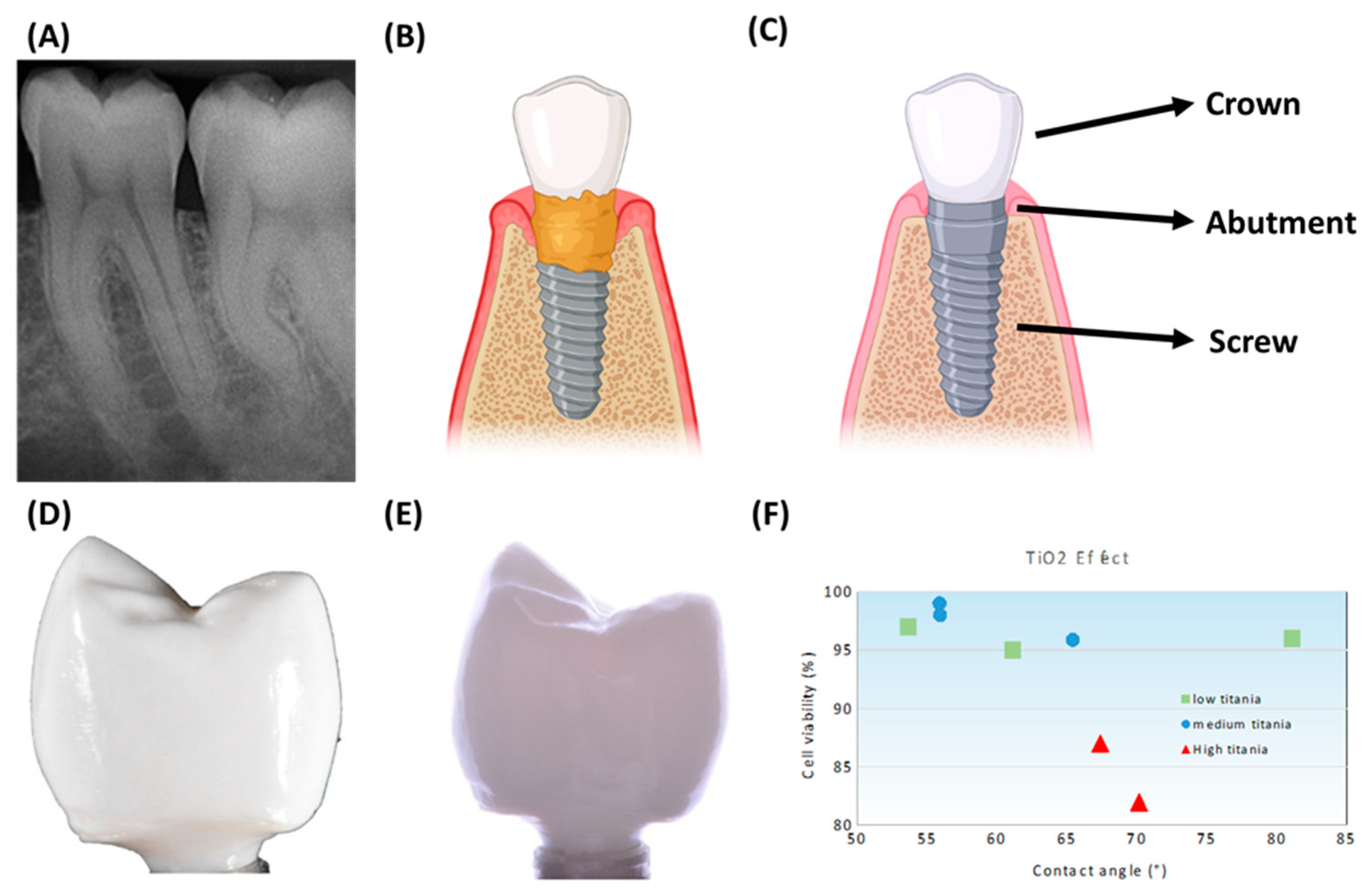
5.1. Dental Implants
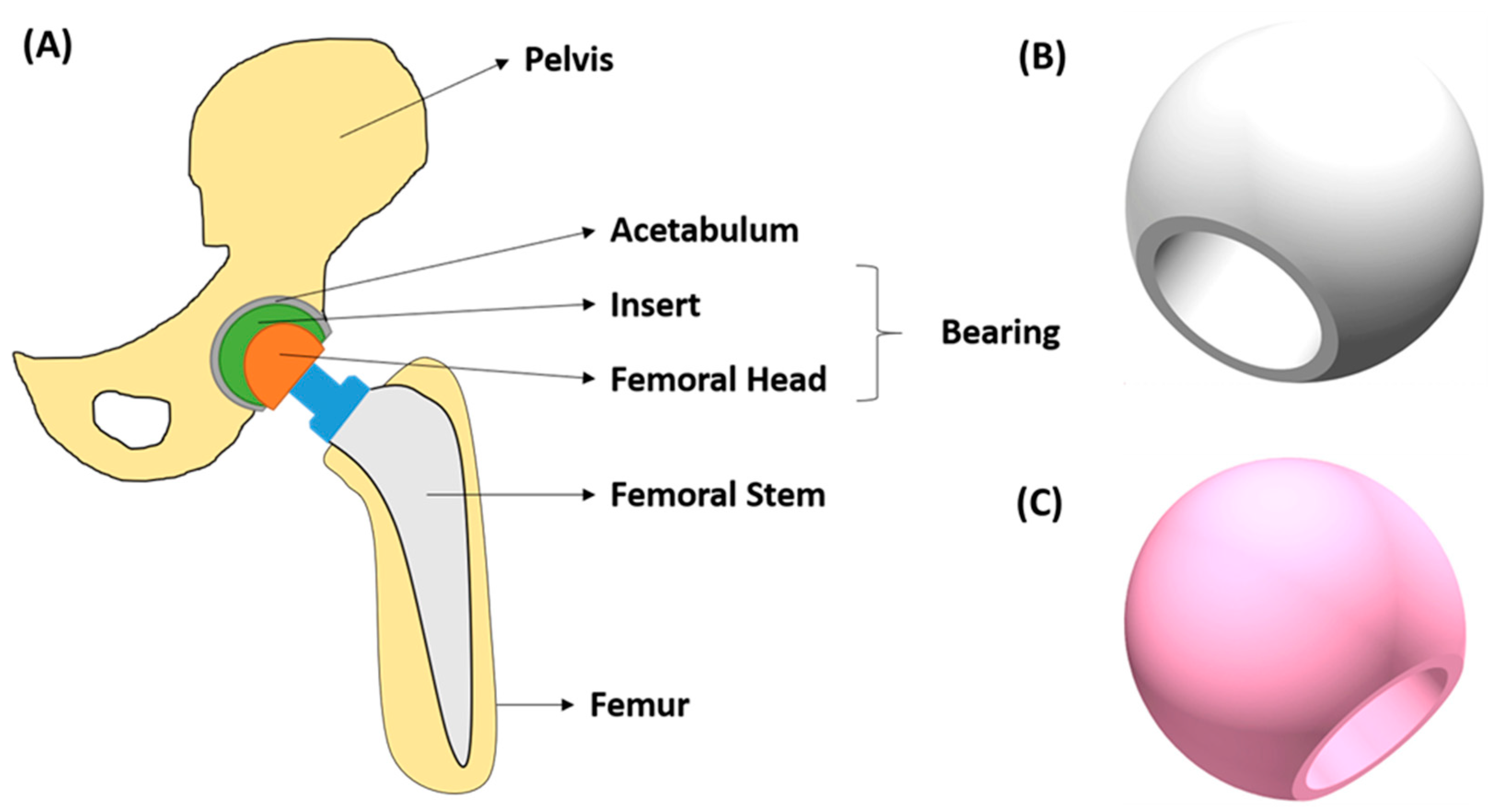
5.2. Hip Implants
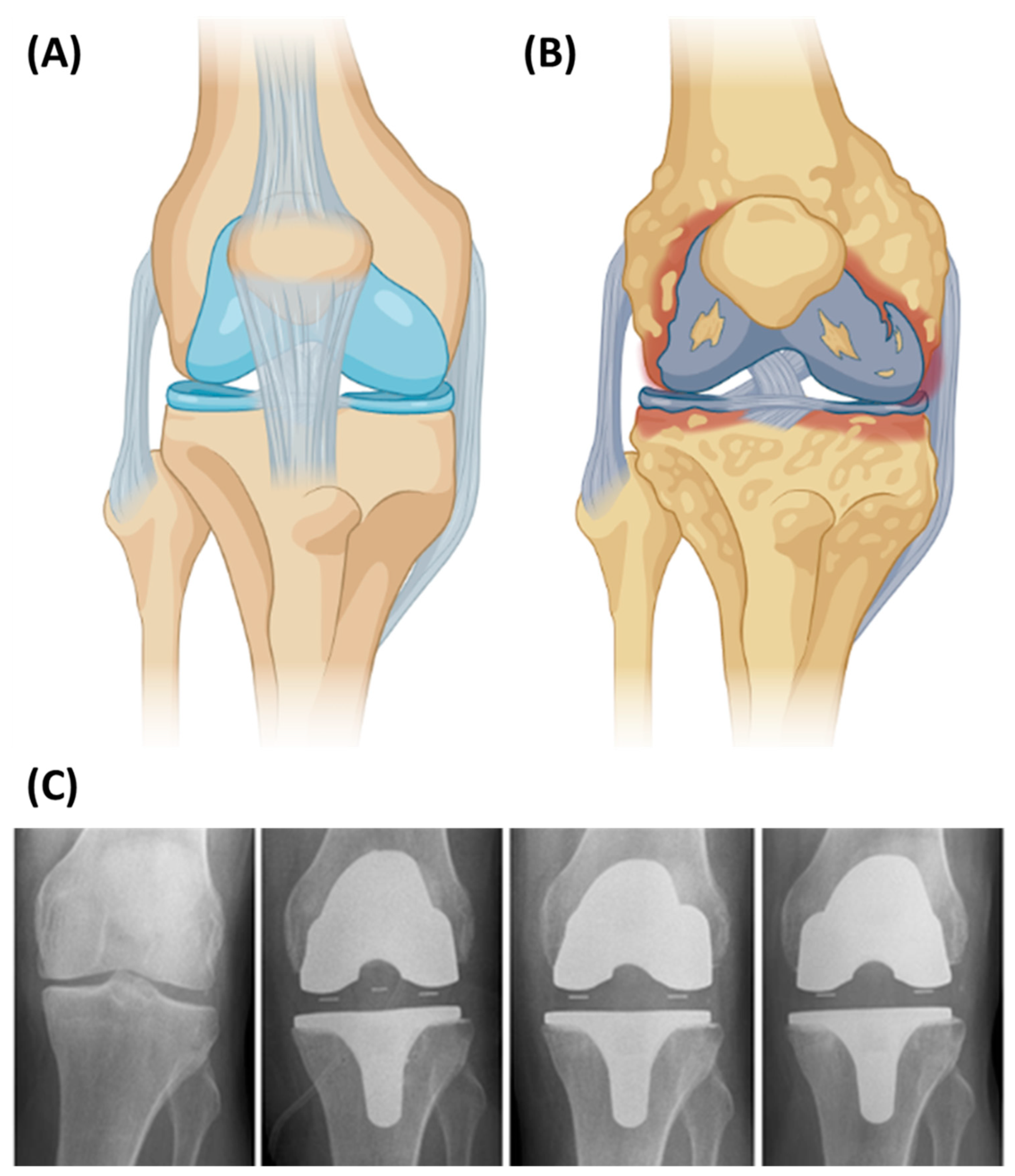
5.3. Knee Implants
5.4. Scaffolds
6. Outlook and Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kishawy, H.A.; Hosseini, A. Ceramics. In Machining Difficult-to-Cut Materials. Basic Principles and Challenges. Materials Forming, Machining and Tribology; Sprnger: Cham, Switzerland, 2019; pp. 179–204. [Google Scholar]
- Anjaneyulu, U.; Ren, P. Bioinert Ceramics for Biomedical Application. Biomedical Sci. and Tech. Series. 2020. Wiley/Scrivener (Open Library). Available online: https://www.researchgate.net/profile/Pei-Gen-Ren/publication/338253271_Bioinert_Ceramics_for_Biomedical_Applications/links/5f478d78a6fdcc14c5cdd9c3/Bioinert-Ceramics-for-Biomedical-Applications.pdf (accessed on 5 September 2022).
- Piconi, C.; Porporati, A.A. Bioinert ceramics: Zirconia and alumina. In Handbook of Bioceramics and Biocomposites; Springer: Cham, Switzerland, 2016; pp. 59–89. [Google Scholar]
- U.S. Food & Drug Administration (FDA). Implants and Prosthetics. Available online: https://www.fda.gov/medical-devices/products-and-medical-procedures/implants-and-prosthetics (accessed on 5 September 2022).
- Ahmed, W.; Elhissi, A.; Jackson, M.; Ahmed, E. 2-Precision machining of medical devices. Des. Manuf. Med. Devices 2012, 59–113. [Google Scholar] [CrossRef]
- Cortizo, M.C.; de Mele, M.F.L.; Cortizo, A.M. Metallic Dental Material Biocompatibility in Osteoblastlike Cells Correlation with Metal Ion Release. Biol. Trace Elem. Res. 2004, 100, 151–168. [Google Scholar] [CrossRef]
- García, T.E.; Rodríguez, C.; Belzunce, F.J.; Suárez, C. Estimation of the mechanical properties of metallic materials by means of the small punch test. J. Alloys Compd. 2014, 582, 708–717. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Tejo-Otero, A.; Fenollosa-Artés, F. Development of am technologies for metals in the sector of medical implants. Metals 2020, 10, 686. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Lei, X.; Zhang, L.; Man, C.; Yao, J. Bio-functional and anti-corrosive 3D printing 316L stainless steel fabricated by selective laser melting. Mater. Des. 2018, 152, 88–101. [Google Scholar] [CrossRef]
- Popov, V.V.; Gary, J.; Kovalevsky, M.A.; Dzhenzhera, G.; Strokin, E.; Kolomiets, A.; Ramon, J.; Muller-kamskii, G. Design and 3D-printing of titanium bone implants: Brief review of approach and clinical cases. Biomed. Eng. Lett. 2018, 8, 337–344. [Google Scholar] [CrossRef]
- Arjunan, A.; Robinson, J.; Baroutaji, A.; Tuñ, A.; Mart, M.; Serrano-aroca, Á. 3D Printed Cobalt-Chromium-Molybdenum Porous Superalloy with Superior Antiviral Activity. Int. J. Mol. Sci. 2021, 22, 12721. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Fenollosa-Artés, F.; Achaerandio, I.; Rey-Vinolas, S.; Buj-Corral, I.; Mateos-Timoneda, M.Á.; Engel, E. Soft-Tissue-Mimicking Using Hydrogels for the Development of Phantoms. Gels 2022, 8, 40. [Google Scholar] [CrossRef]
- Fenollosa-Artes, F.; Jorand, L.; Tejo-Otero, A.; Romero-Sabat, G.; Medel, S. Soft 3D printing of thermoplastic polyurethane: Preliminary study. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2022. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Sanz-Fraile, H.; Ulldemolins, A.; Tejo-Otero, A.; Domínguez-Fernández, A.; Almendros, I.; Otero, J. Characterization of 3D Printed Metal-PLA Composite Scaffolds for Biomedical Applications. Polymers 2022, 14, 2754. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Ritchie, A.C. Biological and mechanical evaluation of mineralized-hydrogel scaffolds for tissue engineering applications. J. Biomater. Appl. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In Vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.C.; Pereira, P.R.; Barbosa, A.M.; Torrado, E.; Alves, N.M. Optimization of silver-containing bioglass nanoparticles envisaging biomedical applications. Mater. Sci. Eng. C 2019, 94, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Papynov, E.K.; Mayorov, V.Y.; Portnyagin, A.S.; Shichalin, O.O.; Kobylyakov, S.P. Application of carbonaceous template for porous structure control of ceramic composites based on synthetic wollastonite obtained via Spark Plasma Sintering. Ceram. Int. 2015, 41, 1171–1176. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Hassan, A. Mechanical properties of wollastonite reinforced thermoplastic composites: A review. Polym. Compos. 2019, 41, 395–429. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Portnyagin, A.S. Reactive Spark Plasma Synthesis of Porous Bioceramic Wollastonite. Russ. J. Inorg. Chem. 2020, 65, 263–270. [Google Scholar] [CrossRef]
- Saxena, V.; Hasan, A.; Pandey, L.M. Effect of Zn/ZnO integration with hydroxyapatite: A review. Mater. Technol. 2018, 33, 79–92. [Google Scholar] [CrossRef]
- Bellucci, D.; Sola, A.; Cannillo, V. Hydroyapatite and tricalcium phosphate composites with bioactive glass as second phase: State of the art and current applications. J. Biomed. Mater. Res. 2016, 104, 1030–1056. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Barba, A.; Diez-Escudero, A.; Maazouz, Y.; Rappe, K.; Espanol, M.; Montufar, E.B.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Öhman-Mägi, C.; et al. Osteoinduction by Foamed and 3D-Printed Calcium Phosphate Scaffolds: Effect of Nanostructure and Pore Architecture. ACS Appl. Mater. Interfaces 2017, 9, 41722–41736. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Ratheesh, V.; Manakari, V.; Parande, G.; Gupta, M.; Wong, R. The potential of Magnesium based Materials in Mandibular reconstruction. Metals 2019, 3, 302. [Google Scholar] [CrossRef]
- ISO/ASTM 52900; Additive Manufacturing—General Principles—Terminology. ISO/ASTM International Standard: Geneva, Switzerland; West Conshohocken, PA, USA, 2015. [CrossRef]
- Chen, Z.; Li, Z.; Li, J.; Liu, C.; Lao, C.; Fu, Y.; Liu, C.; Li, Y.; Wang, P.; He, Y. 3D printing of ceramics: A review. J. Eur. Ceram. Soc. 2019, 39, 661–687. [Google Scholar] [CrossRef]
- Hull, C.W. Apparatus for Production of Three-Dimensional Objects by Stereolithography. U.S. Patent No. 4.575.330A, 11 March 1986. [Google Scholar]
- Xing, B.; Cao, C.; Zhao, W.; Shen, M.; Wang, C.; Zhao, Z. Dense 8 mol% yttria-stabilized zirconia electrolyte by DLP stereolithography. J. Eur. Ceram. Soc. 2020, 40, 1418–1423. [Google Scholar] [CrossRef]
- Formlabs Form 2. Available online: https://formlabs.com/3d-printers/form-2/ (accessed on 5 September 2022).
- Crump, S.S. Apparatus and Method for Creating Three-Dimensional Objects. U.S. Patent No. 5,121,329A, 9 June 1992. [Google Scholar]
- Nakonieczny, D.S.; Kern, F.; Dufner, L.; Antonowicz, M.; Matus, K. Alumina and zirconia-reinforced polyamide pa-12 composites for biomedical additive manufacturing. Materials 2021, 14, 6201. [Google Scholar] [CrossRef]
- BCN3D FDM 3D Printer. Available online: https://www.bcn3d.com/es/ (accessed on 5 September 2022).
- Muniz, N.O.; Vechietti, F.A.; Dos Santos, L.A.L. Influence of several binders on the mechanical properties of alumina parts manufactured by 3D inkjet printing. Mater. Res. Express 2019, 6, 115341. [Google Scholar] [CrossRef]
- XJET XJet Carmel 1400C Ultimate Ceramic AM System. Available online: https://www.xjet3d.com/products/ceramic-systems/ (accessed on 5 September 2022).
- Sachs, E.; Cima, M.; Williams, P.; Brancazio, D.; Cornie, J. Three dimensional printing: Rapid tooling and prototypes directly from a CAD model. J. Manuf. Sci. Eng. Trans. ASME 1992, 114, 481–488. [Google Scholar] [CrossRef]
- Lv, X.; Ye, F.; Cheng, L.; Fan, S.; Liu, Y. Binder jetting of ceramics: Powders, binders, printing parameters, equipment, and post-treatment. Ceram. Int. 2019, 45, 12609–12624. [Google Scholar] [CrossRef]
- Huang, S.; Ye, C.; Zhao, H.; Fan, Z.; Wei, Q. Binder jetting yttria stabilised zirconia ceramic with inorganic colloid as a binder. Adv. Appl. Ceram. 2019, 118, 458–465. [Google Scholar] [CrossRef]
- ComeTRUE ComeTrue® M10 CERAMIC & Binder Jetting 3D Printer. Available online: https://www.cometrue3d.com/en/p/ceramic-3d-printer-en (accessed on 5 September 2022).
- Deckard, C.R.; Beaman, J.J.; Darrah, J.F. Method for Selective Laser Sintering with Layerwise Cross-Scanning. U.S. Patent No. 5,155,324A, 13 October 1992. [Google Scholar]
- Meiners, W.; Wissenbach, K.; Gasser, A. Selective Laser Sitering at Melting Temperature. U.S. Patent 6,215,083B1, 10 April 2001. [Google Scholar]
- Florio, K.; Puccio, D.; Viganò, G.; Pfeiffer, S.; Verga, F.; Grasso, M.; Colosimo, B.M.; Graule, T.; Wegener, K. Process characterization and analysis of ceramic powder bed fusion. Int. J. Adv. Manuf. Technol. 2021, 117, 2105–2116. [Google Scholar] [CrossRef]
- Juste, E.; Petit, F.; Lardot, V.; Cambier, F. Shaping of ceramic parts by selective laser melting of powder bed. J. Mater. Res. 2014, 29, 2086–2094. [Google Scholar] [CrossRef]
- Systems, 3D DTM DTM Sinterstation 2500 Plus. Available online: https://www.treatstock.co.uk/machines/item/303-dtm-sinterstation-2500-plus (accessed on 5 September 2022).
- Hu, Y.; Wang, H.; Cong, W.; Zhao, B. Directed Energy Deposition of Zirconia-Toughened Alumina Ceramic: Novel Microstructure Formation and Mechanical Performance. J. Manuf. Sci. Eng. 2020, 142, 1–10. [Google Scholar] [CrossRef]
- Hu, Y.; Cong, W. A review on laser deposition-additive manufacturing of ceramics and ceramic reinforced metal matrix composites. Ceram. Int. 2018, 44, 20599–20612. [Google Scholar] [CrossRef]
- Kunieda, M.; Nakagawa, T. Manufacturing of Laminated Deep Drawing Dies by Laser Beam Cutting. Proc. of ICTP 1984, 1, 520. [Google Scholar]
- Zhang, Y.; He, X.; Du, S.; Zhang, J. Al2O3 ceramics preparation by LOM (laminated object manufacturing). Int. J. Adv. Manuf. Technol. 2001, 17, 531–534. [Google Scholar] [CrossRef]
- Lewis, J.A.; Smay, J.E.; Stuecker, J.; Cesarano, J. Direct ink writing of three-dimensional ceramic structures. J. Am. Ceram. Soc. 2006, 89, 3599–3609. [Google Scholar] [CrossRef]
- Feilden, E. Additive Manufacturing of Ceramics and Ceramic Composites via Robocasting. Ph.D. Thesis, Imperial College London, London, UK, 2017. [Google Scholar]
- Pezzotti, G.; Yamamoto, K. Artificial hip joints: The biomaterials challenge. J. Mech. Behav. Biomed. Mater. 2014, 31, 3–20. [Google Scholar] [CrossRef]
- Cigada, A. Biomaterials, tissue engineering, gene therapy. J. Appl. Biomater. Biomech. 2008, 6, 127–131. [Google Scholar]
- Piconi, C. Bioinert Ceramics. State-of-the-Art; KEM 758, 3-13. 2017. 2017. Available online: https://doi.org/10.4028/www.scientific.net/kem.7 (accessed on 5 September 2022). [CrossRef]
- De Aza, P.N.; De Aza, A.H.; De Aza, S. Crystaline Bioceramic Materials. Boletín de la sociedad española de Cerámica y Vidrio. Bol. Soc. Esp. Ceram 2005, 44, 135–145. [Google Scholar] [CrossRef]
- Hsu, S.K.; Tian, J.; Ho, W.F.; Hsu, H.C.; Liao, H.J.; Chen, Y.F.; Wu, S.C. Enhancing the bioactivity of yttria-stabilized zirconia immobilized with adhesive peptide using L-dopa as cross-linker. Thin Solid Films 2016, 620, 145–149. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G. Zirconia as a ceramic biomaterial. Biomaterials 1999, 20, 1–25. [Google Scholar] [CrossRef]
- Pilathadka, S.; Vahalová, D.; Vosáhlo, T. The Zirconia: A new dental ceramic material. An overview. Prague Med. Rep. 2007, 108, 5–12. [Google Scholar]
- Emsley, J. Nature’s Building Blocks: An A–Z Guide to the Elements; Oxford University Press: New York, NY, USA, 2011; ISBN 0199605637. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 97th ed.; CRC Press: London, UK; New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Helmer, J.D.; Driskell, T.D. Symposium on Use of Ceramics as Surgical Implants. In Research on Bioceramics; Clemson Unviersity: Clemson, SC, USA, 1969. [Google Scholar]
- Belli, R.; Petschelt, A.; Lohbauer, U. Thermal-induced residual stresses affect the fractographic patterns of zirconia-veneer dental prostheses. J. Mech. Behav. Biomed. Mater. 2013, 21, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Garvie, R.C.; Nicholson, P.S. Phase Analysis in Zirconia Systems. J. Am. Ceram. Soc. 1972, 55, 303–305. [Google Scholar] [CrossRef]
- Cristache, C.M.; Burlibaşa, M.; Cristache, G.; Drafta, S.; Popovici, I.A.; Iliescu, A.A.; Zisi, S.; Burlibaşa, L. Zirconia and its biomedical applications. Metal. Int. 2011, 16, 18–23. [Google Scholar]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef]
- Osman, R.B.; van der Veen, A.J.; Huiberts, D.; Wismeijer, D.; Alharbi, N. 3D-printing zirconia implants; A dream or a reality? An in-vitro study evaluating the dimensional accuracy, surface topography and mechanical properties of printed zirconia implant and discs. J. Mech. Behav. Biomed. Mater. 2017, 75, 521–528. [Google Scholar] [CrossRef]
- Jiang, L.; Liao, Y.; Wan, Q.; Li, W. Effects of sintering temperature and particle size on the translucency of zirconium dioxide dental ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 2429–2435. [Google Scholar] [CrossRef]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalt. Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- 70. Maziero Volpato, C.A.; D’Altoé Garbelotto, L.G.; Fredel, M.C.; Bondioli, F. Application of Zirconia in Dentistry: Biological, Mechanical and Optical Considerations. In Ceramics-Electric and Magnetic Ceramics Bioceramics; Ceramics nd Environment; Intech: London, UK, 2011; (Ebook). [Google Scholar]
- Bearden, L.J.; Cooke, F.W. Growth inhibition of cultured fibroblasts by cobalt and nickel. J. Biomed. Mater. Res. 1980, 14, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Bergman, B.; Söremark, R. Tissue accumulation of nickel released due to electrochemical corrosion of non-precious dental casting alloys. J. Oral Rehabil. 1980, 7, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Moussi, J.; Drury, J.L.; Wataha, J.C. Zirconia in biomedical applications. Expert Rev. Med. Devices 2016, 13, 945–963. [Google Scholar] [CrossRef]
- Barry, T.S.; Uysal, T.; Birinci, M.; Erdemoğlu, M. Thermal and Mechanical Activation in Acid Leaching Processes of Non-bauxite Ores Available for Alumina Production—A Review. Min. Metall. Explor. 2019, 36, 557–569. [Google Scholar] [CrossRef]
- Rock, M. Kunstliche Ersatzteile fur das Innere und Augere des Menschlichen und Tierischen Korpers. DRP 583589, 1933. [Google Scholar]
- Morgan, V.J. The Bicon Short Implant. Stomatol. Edu. J. 2019, 6, 202. [Google Scholar] [CrossRef]
- Boutin, P. otal hip arthroplasty using a ceramic prosthesis. Rev. Chir. Orthop 1972, 58, 230–246. [Google Scholar]
- Steiner, C.J.; Hasselman, D.P.H.; Spriggs, R.M. Kinetics of the Gamma-to-Alpha Alumina Phase Transformation. J. Am. Ceram. Soc. 1971, 54, 412–413. [Google Scholar] [CrossRef]
- Abyzov, A.M. Aluminum Oxide and Alumina Ceramics (review). Part 1. Properties of Al2O3 and Commercial Production of Dispersed Al2O3 . Refract. Ind. Ceram. 2019, 60, 24–32. [Google Scholar] [CrossRef]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data; Springer: Cham, Switzerland, 2006. [Google Scholar]
- Fu, L.; Huang, A.; Gu, H.; Ni, H. Properties and microstructures of lightweight alumina containing different types of nano-alumina. Ceram. Int. 2018, 44, 17885–17894. [Google Scholar] [CrossRef]
- Rahmati, M.; Mozafari, M. Biocompatibility of alumina-based biomaterials—A review. J. Cell. Physiol. 2019, 234, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, H.; Liu, Y.; Liu, Y.; Hu, K.; Wang, N.; Lu, Z.; Liang, J.; He, S. Effect of Holding Time During Sintering on Microstructure and Properties of 3D Printed Alumina Ceramics. Front. Mater. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Wu, H.; Li, D.; Tang, Y.; Sun, B.; Xu, D. Rapid fabrication of alumina-based ceramic cores for gas turbine blades by stereolithography and gelcasting. J. Mater. Process. Technol. 2009, 209, 5886–5891. [Google Scholar] [CrossRef]
- Boutillier, S.; Fourmentin, S.; Laperche, B. History of titanium dioxide regulation as a food additive: A review. Environ. Chem. Lett. 2022, 20, 1017–1033. [Google Scholar] [CrossRef]
- Ahn, T.K.; Lee, D.H.; Kim, T.; Jang, G.; Choi, S.J.; Oh, J.B.; Ye, G.; Lee, S. Modification of Titanium Implant and Titanium Dioxide for Bone Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 355–368. [Google Scholar] [CrossRef]
- Chen, T.; Sun, A.; Chu, C.; Wu, H.; Wang, J.; Wang, J.; Li, Z.; Guo, J.; Xu, G. Rheological behavior of titania ink and mechanical properties of titania ceramic structures by 3D direct ink writing using high solid loading titania ceramic ink. J. Alloys Compd. 2019, 783, 321–328. [Google Scholar] [CrossRef]
- Muranyi, P.; Schraml, C.; Wunderlich, J. Antimicrobial efficiency of titanium dioxide-coated surfaces. J. Appl. Microbiol. 2010, 108, 1966–1973. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; Von Goetz, N. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Nadeem, M.; Tungmunnithum, D.; Hano, C.; Abbasi, B.H.; Hashmi, S.S.; Ahmad, W.; Zahir, A. The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem. Lett. Rev. 2018, 11, 492–502. [Google Scholar] [CrossRef]
- Khaskhoussi, A.; Calabrese, L.; Currò, M.; Lentile, R.; Bouaziz, J.; Proverbio, E. Effect of the Compositions on the Biocompatibility of New Alumina–Zirconia–Titania Dental Ceramic Composites. Materials 2020, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Gahlert, M.; Roehling, S. Peri-implantitis and ceramic implants: First clinical observations. Implantologie 2015, 23, 305–310. [Google Scholar]
- Wu, H.; Liu, W.; Lin, L.; Li, L.; Li, Y.; Tian, Z.; Zhao, Z.; Ji, X.; Xie, Z.; Wu, S. Preparation of alumina-toughened zirconia via 3D printing and liquid precursor infiltration: Manipulation of the microstructure, the mechanical properties and the low temperature aging behavior. J. Mater. Sci. 2019, 54, 7447–7459. [Google Scholar] [CrossRef]
- Dehurtevent, M.; Robberecht, L.; Thuault, A.; Deveaux, E.; Leriche, A.; Petit, F.; Denis, C.; Hornez, J.C.; Béhin, P. Effect of build orientation on the manufacturing process and the properties of stereolithographic dental ceramics for crown frameworks. J. Prosthet. Dent. 2021, 125, 453–461. [Google Scholar] [CrossRef]
- Zandinejad, A.; Revilla-León, M.; Methani, M.M.; Khanlar, L.N.; Morton, D. The fracture resistance of additively manufactured monolithic zirconia vs. Bi-layered alumina toughened zirconia crowns when cemented to zirconia abutments. evaluating the potential of 3d printing of ceramic crowns: An in vitro study. Dent. J. 2021, 9, 115. [Google Scholar] [CrossRef]
- Lian, Q.; Sui, W.; Wu, X.; Yang, F.; Yang, S. Additive manufacturing of ZrO2 ceramic dental bridges by stereolithography. Rapid Prototyp. J. 2018, 24. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Liu, Y.; Jiang, X.; Gao, B. Trueness analysis of zirconia crowns fabricated with 3-dimensional printing. J. Prosthet. Dent. 2019, 121, 285–291. [Google Scholar] [CrossRef]
- Coppola, B.; Lacondemine, T.; Tardivat, C.; Montanaro, L.; Palmero, P. Designing alumina-zirconia composites by DLP-based stereolithography: Microstructural tailoring and mechanical performances. Ceram. Int. 2021, 47, 13457–13468. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jiang, C.P. Development of a three-dimensional slurry printing system using dynamic mask projection for fabricating zirconia dental implants. Mater. Manuf. Process. 2015, 30, 1498–1504. [Google Scholar] [CrossRef]
- Anssari Moin, D.; Hassan, B.; Wismeijer, D. A novel approach for custom three-dimensional printing of a zirconia root analogue implant by digital light processing. Clin. Oral Implants Res. 2017, 28, 668–670. [Google Scholar] [CrossRef]
- Arnesano, A.; Kunjalukkal Padmanabhan, S.; Notarangelo, A.; Montagna, F.; Licciulli, A. Fused deposition modeling shaping of glass infiltrated alumina for dental restoration. Ceram. Int. 2020, 46, 2206–2212. [Google Scholar] [CrossRef]
- Li, W.; Ghazanfari, A.; McMillen, D.; Leu, M.C.; Hilmas, G.E.; Watts, J. Characterization of zirconia specimens fabricated by ceramic on-demand extrusion. Ceram. Int. 2018, 44, 12245–12252. [Google Scholar] [CrossRef]
- Özkol, E. Rheological characterization of aqueous 3Y-TZP inks optimized for direct thermal ink-jet printing of ceramic components. J. Am. Ceram. Soc. 2013, 96, 1124–1130. [Google Scholar] [CrossRef]
- Khanlar, L.N.; Rios, A.S.; Tahmaseb, A.; Zandinejad, A. Additive Manufacturing of Zirconia Ceramic and Its Application in Clinical Dentistry: A Review. Dent. J. 2021, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Baura, G. Medical Device Technologies, 2nd ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Merola, M.; Affatato, S. Materials for hip prostheses: A review of wear and loading considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Lavigne, M.; Roy, A.; Massé, V.; Vendittoli, P.A.; Beaulieu, Y.; Puliero, B.; Blakeney, W.G. Excellent results of large-diameter ceramic-on-ceramic bearings in total hip arthroplasty: Is squeaking related to head size? Bone Jt. J. 2018, 100B, 1434–1441. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, K.; Deng, J.; Ye, J.; Ai, F.; Ouyang, H.; Wu, T.; Jia, J.; Cheng, X.; Wang, X. 3D printed zirconia ceramic hip joint with precise structure and broad-spectrum antibacterial properties. Int. J. Nanomedicine 2019, 14, 5977–5987. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Gopal, V.; McNallan, M.; Manivasagam, G.; Mathew, M.T. Enhanced Tribocorrosion Resistance of Hard Ceramic Coated Ti-6Al-4V Alloy for Hip Implant Application: In-Vitro Simulation Study. ACS Biomater. Sci. Eng. 2019, 5, 4817–4824. [Google Scholar] [CrossRef]
- Stanciuc, A.M.; Sprecher, C.M.; Adrien, J.; Roiban, L.I.; Alini, M.; Gremillard, L.; Peroglio, M. Robocast zirconia-toughened alumina scaffolds: Processing, structural characterisation and interaction with human primary osteoblasts. J. Eur. Ceram. Soc. 2018, 38, 845–853. [Google Scholar] [CrossRef]
- Nájera, S.E.; Michel, M.; Kim, N.S. 3D Printed PLA/PCL/TiO2 Composite for Bone Replacement and Grafting. MRS Adv. 2018, 3, 2373–2378. [Google Scholar] [CrossRef]
- Nakamura, S.; Ito, H.; Nakamura, K.; Kuriyama, S.; Furu, M.; Matsuda, S. Long-Term Durability of Ceramic Tri-Condylar Knee Implants: A Minimum 15-Year Follow-Up. J. Arthroplasty 2017, 32, 1874–1879. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kobayashi, M.; Ito, H.; Nakamura, K.; Ueo, T.; Nakamura, T. The Bi-Surface total knee arthroplasty: Minimum 10-year follow-up study. Knee 2010, 17, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Bergschmidt, P.; Bader, R.; Ganzer, D.; Hauzeur, C.; Lohmann, C.H.; Krüger, A.; Rüther, W.; Tigani, D.; Rani, N.; Esteve, J.L.; et al. Prospective multi-centre study on a composite ceramic femoral component in total knee arthroplasty: Five-year clinical and radiological outcomes. Knee 2015, 22, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Meier, E.; Gelse, K.; Trieb, K.; Pachowsky, M.; Hennig, F.F.; Mauerer, A. First clinical study of a novel complete metal-free ceramic total knee replacement system. J. Orthop. Surg. Res. 2016, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brehm, P. BPK-S INTEGRATION Ceramic Knee Prosthesis. Available online: https://www.peter-brehm.de/en/products/knee/bpk-s-integration/ (accessed on 5 September 2022).
- Schwarzer, E.; Götz, M.; Markova, D.; Stafford, D.; Scheithauer, U.; Moritz, T. Lithography-based ceramic manufacturing (LCM)—Viscosity and cleaning as two quality influencing steps in the process chain of printing green parts. J. Eur. Ceram. Soc. 2017, 37, 5329–5338. [Google Scholar] [CrossRef]
- Manotham, S.; Channasanon, S.; Nanthananon, P.; Tanodekaew, S.; Tesavibul, P. Photosensitive binder jetting technique for the fabrication of alumina ceramic. J. Manuf. Process. 2021, 62, 313–322. [Google Scholar] [CrossRef]
- Curodeau, A.; Sachs, E.; Caldarise, S. Design and fabrication of cast orthopedic implants with freeform surface textures from 3-D printed ceramic shell. J. Biomed. Mater. Res. 2000, 53, 525–535. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Domínguez-Fernández, A.; Gómez-Gejo, A. Effect of printing parameters on dimensional error and surface roughness obtained in direct ink writing (DIW) processes. Materials 2020, 13, 2157. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J.U.; Kim, G.H. Biomimetic gelatin/HA biocomposites with effective elastic properties and 3D-structural flexibility using a 3D-printing process. Addit. Manuf. 2020, 36, 101616. [Google Scholar] [CrossRef]
- Cesarano, J., III; Segalman, R.; Calvert, P. Robocasting provides moldless fabrication from slurry deposition. Ceram. Ind. 1998, 148, 94–96. [Google Scholar]
- Ghazanfari, A.; Li, W.; Leu, M.; Watts, J.; Hilmas, G. Mechanical characterization of parts produced by ceramic on-demand extrusion process. Int. J. Appl. Ceram. Technol. 2017, 14, 486–494. [Google Scholar] [CrossRef]
- Buj-corral, I.; Vidal, D.; Tejo-otero, A.; Padilla, A.; Xuriguera, E.; Fenollosa-art, F. Characterization of 3D Printed Yttria-Stabilized Zirconia Parts for Use in Prostheses. Nanomaterials 2021, 11, 2942. [Google Scholar] [CrossRef] [PubMed]
- Peng, E.; Wei, X.; Garbe, U.; Yu, D.; Edouard, B.; Liu, A.; Ding, J. Robocasting of dense yttria-stabilized zirconia structures. J. Mater. Sci. 2018, 53, 247–273. [Google Scholar] [CrossRef]
- Tejo-otero, A.; Colly, A.; Courtial, E.; Lyon, U.; Fenollosa-artés, F.; Buj-corral, I.; Marquette, C.A.; Lyon, U. Soft-tissue-mimicking using silicones for the manufacturing of soft phantoms by fresh 3D printing. Rapid Prototyp. J. 2021, 28. [Google Scholar] [CrossRef]
- Bernal, P.N.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef]

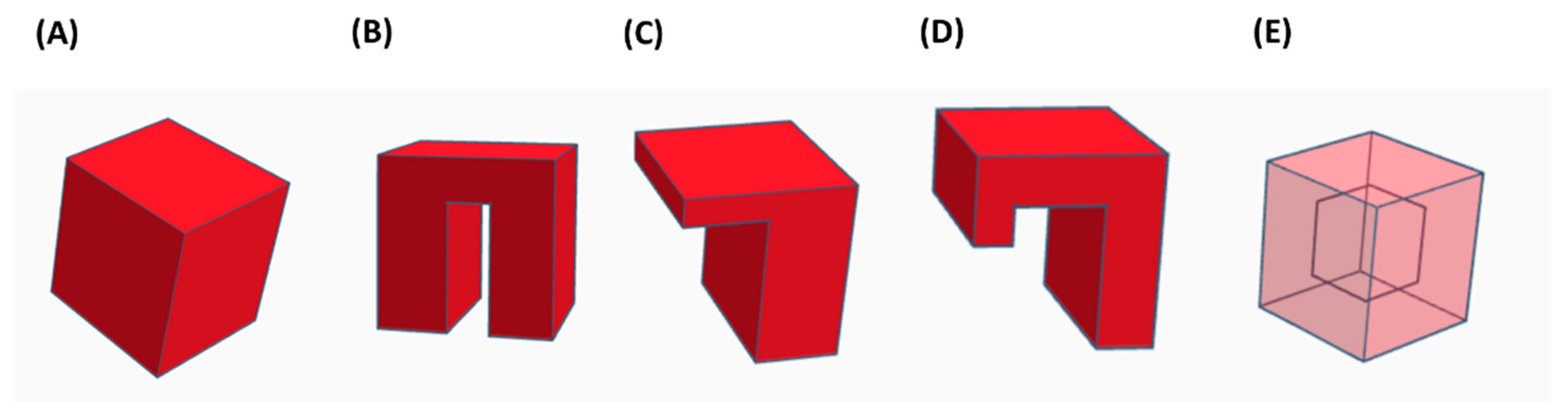

| Feedstock Form | AM Technology | Abbreviation |
|---|---|---|
| Slurry-Based | Vat photopolymerization (Stereolithography) | SLA |
| Vat photopolymerization (Digital Light Processing) | DLP | |
| Material extrusion (Direct Ink Writing or Robocasting) | DIW or RC | |
| Material Jetting (Inkjet Printing) | IJP | |
| Binder Jetting | BJ | |
| Powder-Based | Powder Bed Fusion (Selective Laser Sintering) | SLS |
| Powder Bed Fusion (Selective Laser Melting) | SLM | |
| Bulk-Based | Directed Energy Deposition | DED |
| Material Extrusion (Fused Deposition Modelling) | FDM |
| AM Group | 3D Printing Technique | Features | ||||
|---|---|---|---|---|---|---|
| Filling | Spanning | Overhanging | Floating | Closed Cavity | ||
| VAT Photopolymerization | SLA | |||||
| DLP | ||||||
| ME | DIW | |||||
| FDM | ||||||
| MJ | IPJ | |||||
| BJ | BJ | |||||
| PBF | SLS | |||||
| SLM | ||||||
| DED | DED | |||||
| LOM | LOM | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buj-Corral, I.; Tejo-Otero, A. 3D Printing of Bioinert Oxide Ceramics for Medical Applications. J. Funct. Biomater. 2022, 13, 155. https://doi.org/10.3390/jfb13030155
Buj-Corral I, Tejo-Otero A. 3D Printing of Bioinert Oxide Ceramics for Medical Applications. Journal of Functional Biomaterials. 2022; 13(3):155. https://doi.org/10.3390/jfb13030155
Chicago/Turabian StyleBuj-Corral, Irene, and Aitor Tejo-Otero. 2022. "3D Printing of Bioinert Oxide Ceramics for Medical Applications" Journal of Functional Biomaterials 13, no. 3: 155. https://doi.org/10.3390/jfb13030155
APA StyleBuj-Corral, I., & Tejo-Otero, A. (2022). 3D Printing of Bioinert Oxide Ceramics for Medical Applications. Journal of Functional Biomaterials, 13(3), 155. https://doi.org/10.3390/jfb13030155








