The Anticancer Efficacy of Thiourea-Mediated Reduced Graphene Oxide Nanosheets against Human Colon Cancer Cells (HT-29)
Abstract
:1. Introduction
2. Methodology
2.1. Synthesis of GO
2.2. Synthesis of rGO
2.3. Characterization of rGO and T-rGO
2.4. Cell Line and Culture
2.5. Reagents
2.6. WST-8-Cell Viability Assay
2.7. Cell Cycle Arrest Analysis
2.8. DNA Fragmentation
2.9. Cell Morphology
3. Results and Discussion
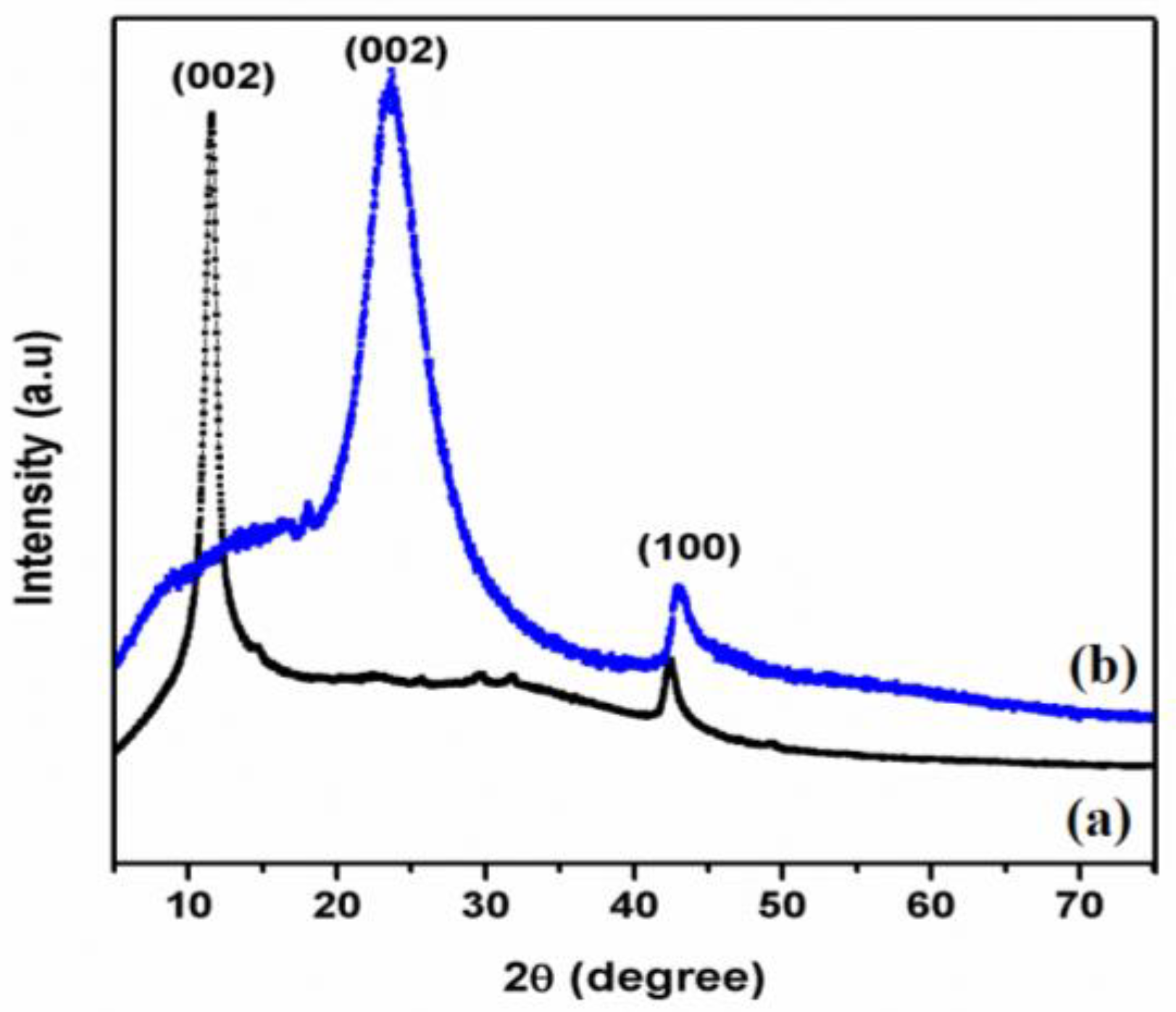
3.1. XRD Analysis of GO and T-rGO
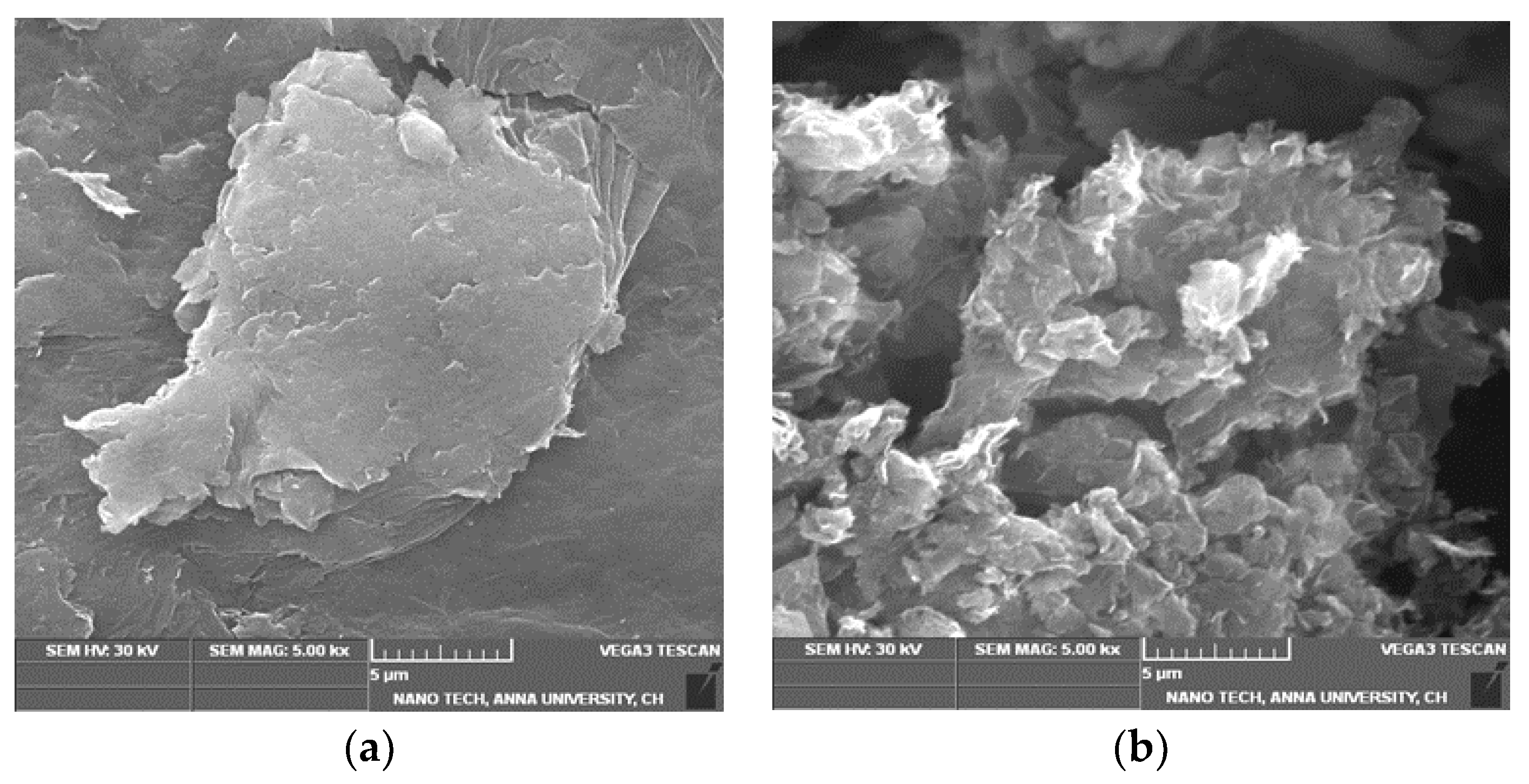
3.2. Morphological Observations of GO and T-rGO
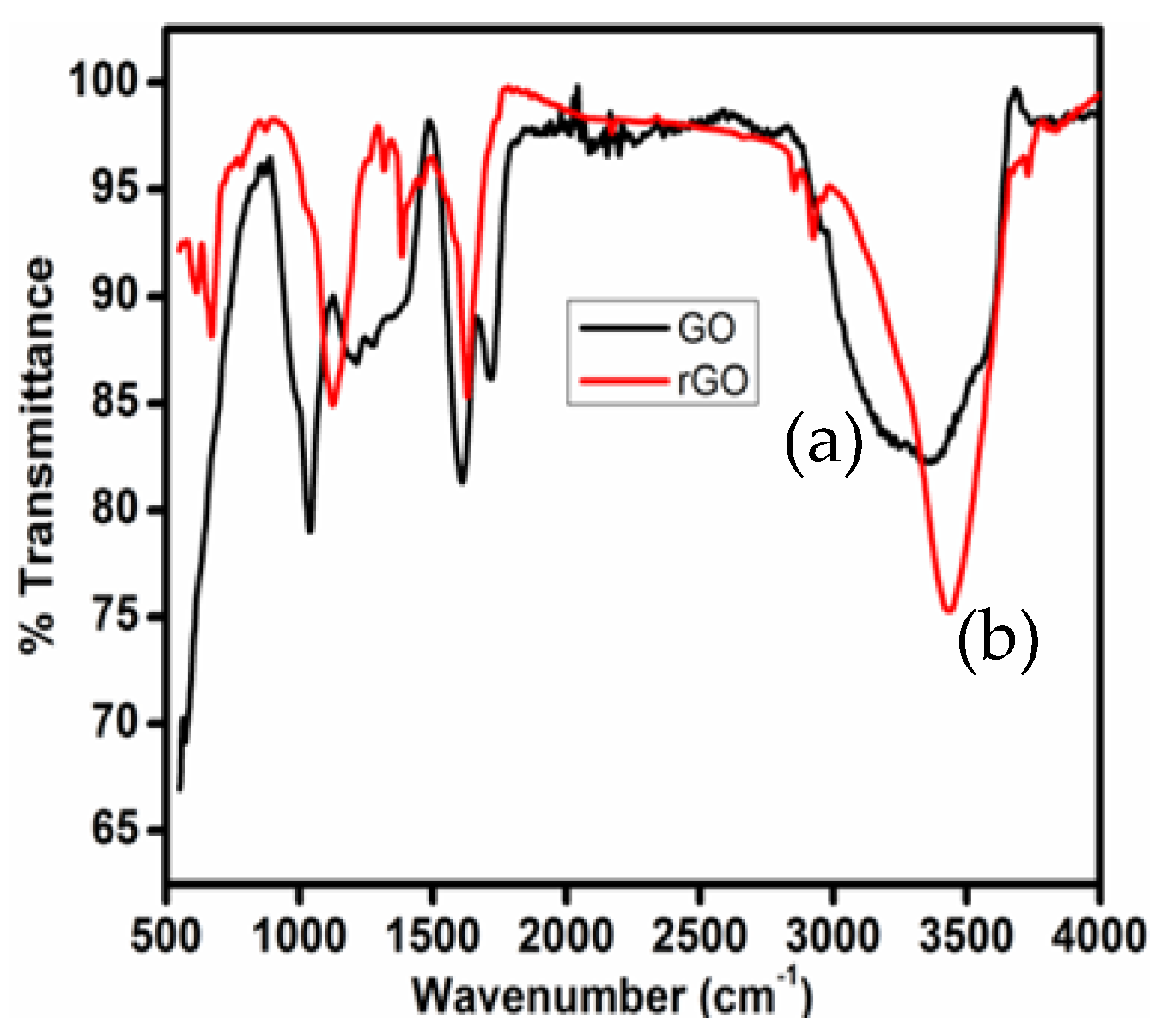
3.3. FTIR Analysis of GO and T-rGO
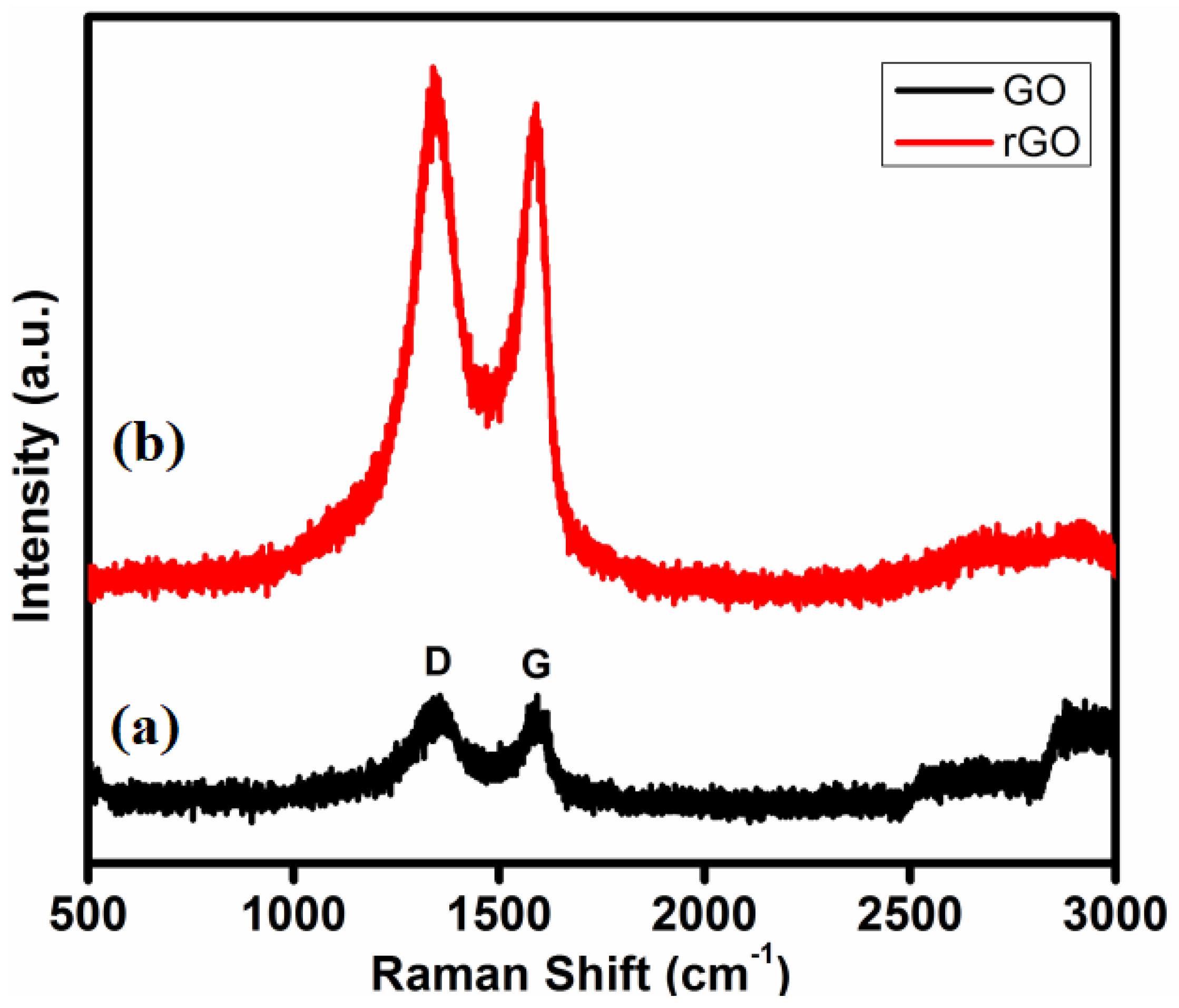
3.4. Raman Analysis of GO and T-rGO
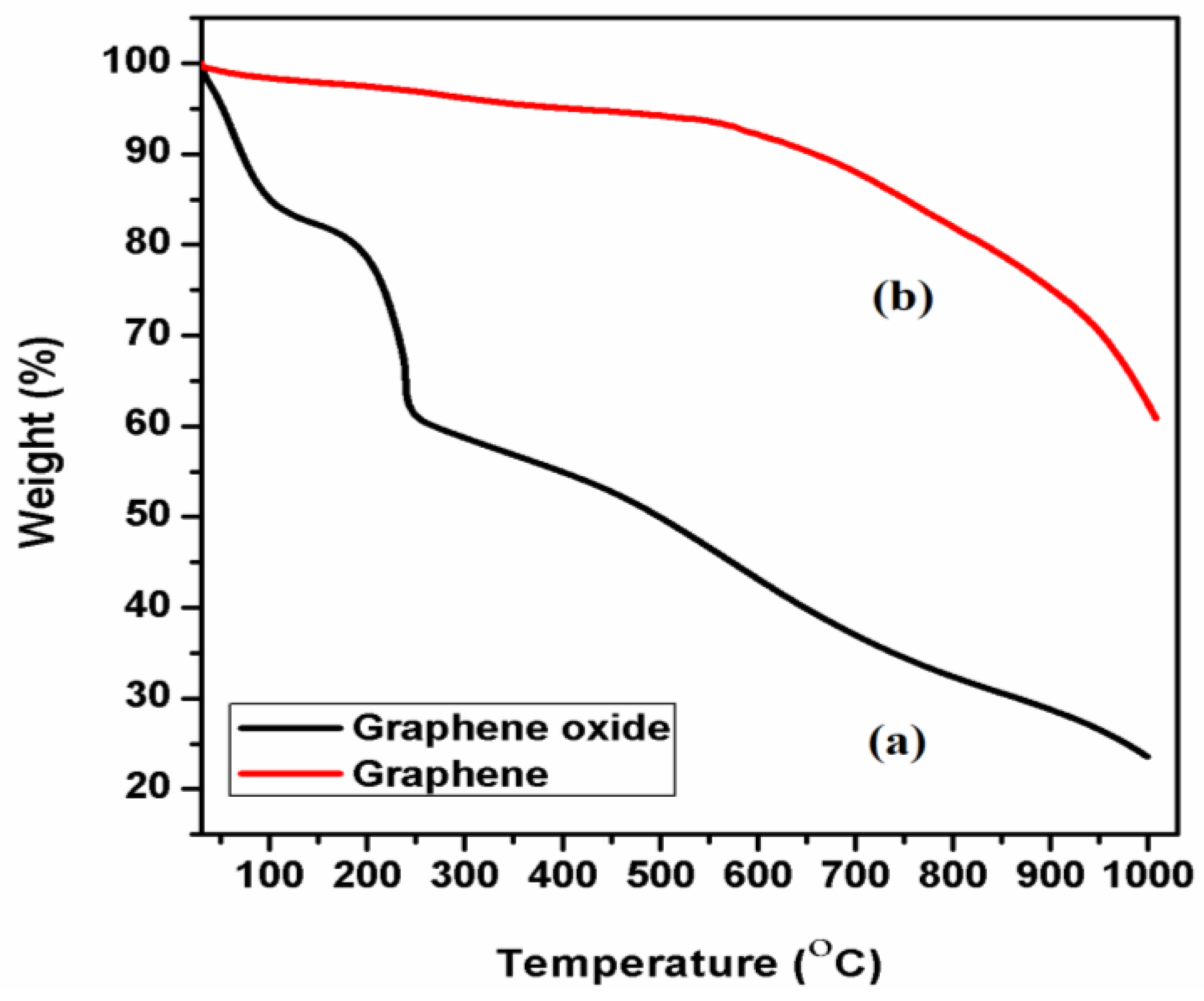
3.5. Thermal Analysis of GO and T-rGO
3.6. Impact of GO on the Potentiality of HT-29 Cells
3.7. Graphene-Induced Arrest at the Sub-G1 and G1 Phases of the Cell Cycle
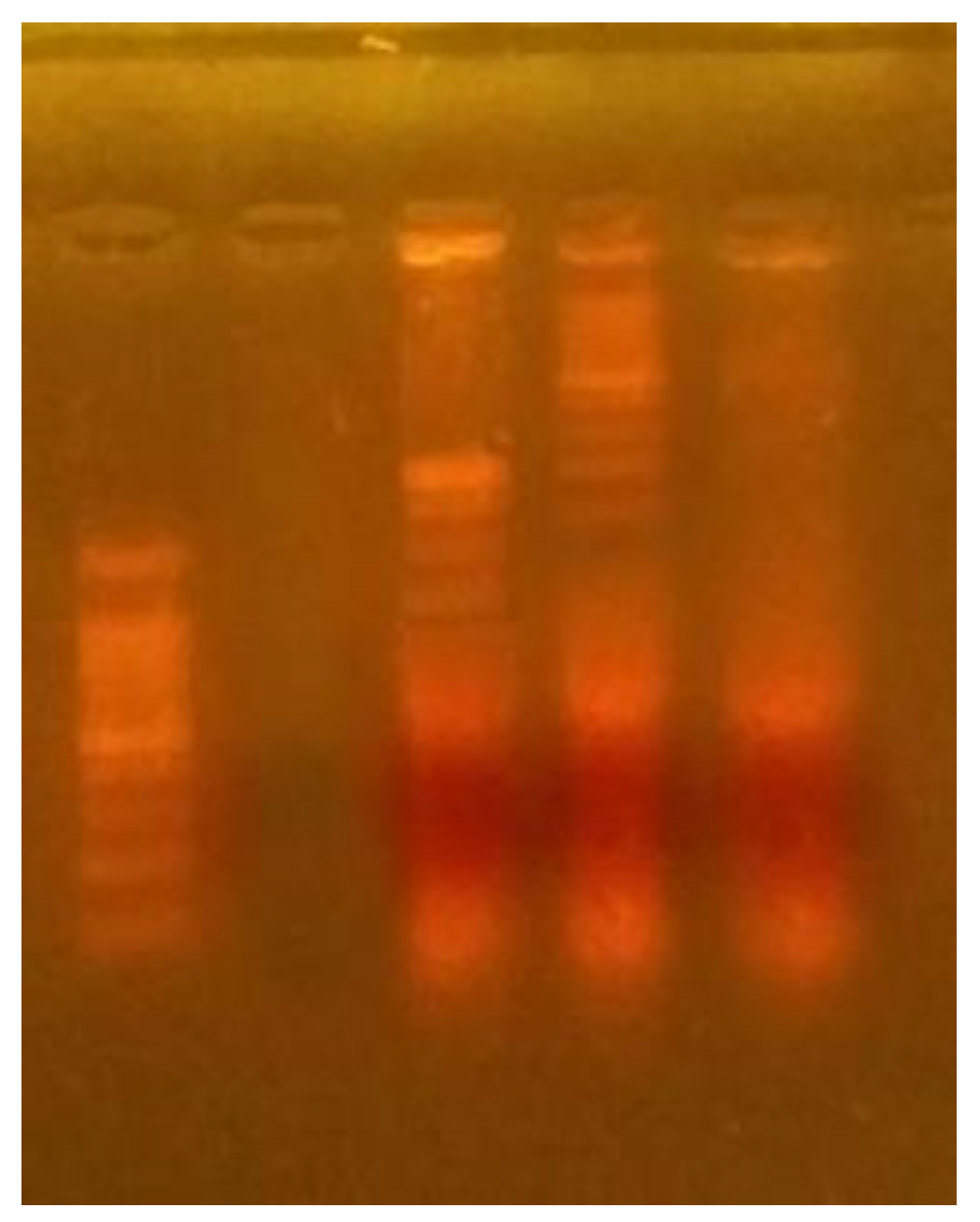
3.8. DNA Fragmentation
3.9. Effect of GO on the Morphology of Cell
4. Discussion and Conclusions
5. Perspective and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Kim, M.H.; Min, D.H. Biocompatible reduced graphene oxide prepared by using dextran as a multifunctional reducing agent. Chem. Commun. 2011, 47, 3195–3197. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphene. Nat. Nano Technol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.; Kim, J.H. Humanin: A novel functional molecule for the green synthesis of graphene. Colloids Surf. B Biointerfaces 2013, 111, 376–383. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.; Park, J.H.; Kim, J.H. An in vitro evaluation of graphene oxide reduced by Ganoderma spp. in Human breast cancer cells (MDA-MB-231). Int. J. Nano Med. 2014, 9, 1783–1797. [Google Scholar] [CrossRef]
- Li, X.L.; Wang, X.R.; Zhang, L.; Lee, S.W.; Dai, H.J. Chemically derived, ultrasmooth grapheme nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Avouris, P.; Chen, Z.; Perebeinas, V. Carbon-based electronics. Nat. Nano Technol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Half metallic grapheme nanoribbon. Nature 2006, 444, 347–349. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.M.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual Gas molecules adsorbed on Graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef]
- Sakhaee-Pour, A.; Ahmadian, M.; Vafai, A. Potential application of single-layered graphene sheet as strain sensor. Solid State Commun. 2008, 147, 336–340. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Pinen, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Watcharotone, S.; Dikin, D.A.; Stankovich, S.; Piner, R.; Jung, I.; Mommett, G.H.B.; Wu, S.E.; Chen, S.F.; Liu, C.P.; Nguyen, S.T.; et al. Graphene-silica composite thin film as transparent conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef] [PubMed]
- Takamura, T.; Endo, K.; Fu, L.; Wu, Y.; Lee, K.J.; Matsumoto, T. Identification of Nano-sized Holes by TEM in the Graphene layer of graphite and the High rate Discharge capability of Li-ion Battery Anodes. Electrochim. Acta 2007, 53, 1055–1061. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed]
- Sattari, S.; Adeli, M.; Beyranvand, S.; Nemati, M. Functionalized graphene platforms for anticancer drug delivery. Int. J. Nanomed. 2021, 16, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Croitoru, A.M.; Moroșan, A.; Tihăuan, B.; Oprea, O. Novel Graphene Oxide/Quercetin and Graphene Oxide/Juglone Nanostructured Platforms as effective drug delivery systems with biomedical applications. Nanomaterials 2022, 12, 1943. [Google Scholar] [CrossRef]
- –Collado, J.L.A.; Garcia-San-Martin, N.; Molina-Mateo, J.; Cabanilles, C.T.; Quiles, V.D.; Serrano-Aroca, A.; Serra, R.S.I. Electroactive calcium-alginate/polycaprolactone/reduced graphene oxide naohybrid hydrogels for skeletal muscle tissue engineering. Colloids Surf. B Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef]
- Lekshmi, G.S.; Tamilselvi, R.; Geethalakshmi, R.; Kirupha, D.; Mandhakini, M. Multifunctional oil-produced reduced graphene oxide—Silver oxide composites with photocatalytic, antioxidant, and antibacterial activities. J. Colloid Interface Sci. 2022, 608, 294–305. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, Y.; Shen, L.; Ma, H.; Sun, T.; Lv, F.; Zhu, N. Oil-water self-assembly engineering of Prussian blue/quantum dots decorated graphene film for wearable textile biosensors and photoelectronic unit. Chem. Eng. J. 2022, 424, 131824. [Google Scholar] [CrossRef]
- Leon, C.; Melink, R. Machine Learning for Shape Memory Graphene Nanoribbons and applications in Biomedical engineering. Bioengineering 2022, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Bousiakou, G.L.; Qindeel, R.; L-Dossary, M.O.A.; Kalkani, H. Symthesis and characterization of graphene oxide for pathogen inhibition: Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J. King Saud Univ.-Sci. 2022, 34, 102002. [Google Scholar] [CrossRef]
- Szabo, T.; Berkesi, O.; Fergo, P.; Jose, P.O.; Vits, K.; Sanakis, Y.; Petridis, D.; Dekany, I. Evaluation of surface functional Groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Chen, H.; Müller, M.B.; Gilmore, K.J.; Wallace, G.G.; Li, D. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 2008, 20, 3557–3561. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhou, X.; Ye, F.; He, Q.; Chen, G.C.K.; Soo, J.; Boey, F.; Zhang, H.; Chen, P. Interfacing live cells with nanocarbon substrates. Langmuir 2010, 26, 2244–2247. [Google Scholar] [CrossRef]
- Park, S.; Mohanty, N.; Suk, J.W.; Nagaraja, A.; An, J.; Piner, R.D.; Cai, W.; Dreyer, D.R.; Berry, V.; Ruoff, R.S. Biocompatible, Robust Free-Standing Paper Composed of a TWEEN/Graphene Composite. Adv. Mater. 2010, 22, 1736–1740. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.; Ma, N.; Wang, Z.; Zhang, X. Environment-Friendly Method To Produce Graphene That Employs Vitamin C and Amino Acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Aghayee, S.; Fereydooni, Y.; Talebi, A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012, 22, 13773–13781. [Google Scholar] [CrossRef]
- Esfandiar, A.; Akhavan, O.; Irajizad, A. Melatonin as a powerful bio-antioxidant for reduction of graphene oxide. J. Mater. Chem. 2011, 21, 10907–10914. [Google Scholar] [CrossRef]
- Gonçalves, G.; Vila, M.; Portolés, M.-T.; Vallet-Regi, M.; Gracio, J.; Marques, P.A.A.P. Nano-graphene oxide: A potential multifunctional platform for cancer therapy. Adv. Healthc. Mater. 2013, 2, 1072–1090. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Kim, J.-H. Green synthesis of graphene and its cytotoxic effects in human breast cancer cells. Int. J. Nanomed. 2013, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Gurunathan, S.; Kim, J.-H. Graphene oxide-silver nanocomposites enhances cytotooxic and apoptotic potential of Salinomycin in human ovarian cancer stem cells:A novel approach for cancer therapy. Int. J. Mol. Sci. 2018, 19, 710. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylatedgraphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA: A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.; Jemal, A. Cancer Statistics, 2015. CA A Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA A Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Li, Z.-F.; Wang, Z.-D.; Ji, Y.-Y.; Zhang, S.; Huang, C.; Li, J.; Xia, X.-M. Induction of apoptosis and cell cycle arrest in human HCC MHCC97H cells with Chrysanthemum indicum extract. World J. Gastroenterol. 2009, 15, 4538–4546. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Modifiable risk factors for colon cancer. Gastroenterol. Clin. N. Am. 2002, 31, 925–943. [Google Scholar]
- Labianca, R.; Beretta, G.D.; Kildani, B.; Milesi, L.; Merlin, F.; Mosconi, S.; Pessi, M.A.; Prochilo, T.; Quadri, A.; Gatta, G.; et al. Colon cancer. Crit. Rev. Oncol. Hematol. 2010, 74, 106–133. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef]
- Kumari, S.; Sharma, P.; Rawat, P.; Yadav, S. A novel synthesis of the graphene oxide silver nanocomposites for unique physiochemical application. ACS Omega 2020, 5, 5041–5047. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.G.; Gurunathan, S. Combination of graphene oxide-silvernanoparticle nanocomposites and Cisplatin enhances apoptosis and autophagy in human cervical cancer. Int. J. Nanomed. 2017, 12, 6537–6558. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, J.; Wu, J.; Yin, Q.; Chen, A. Graphene oxide-reduced graphene oxide induced neural pheochromocytoma –derived PC12 cell lines apoptosis and cellcycle alterationsvia the ERK signaling pathway. Int. J. Nanomed. 2017, 12, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Grassi, G.; Scaggiante, B.; Farra, R.; Dapas, B.; Agostini, F.; Baiz, D.; Rosso, N.; Tiribelli, C. The expression levels of the translational factors eEF1A 1/2 correlate with cell growth but not apoptosis in hepatocellular carcinoma cell lines with different differentiation grade. Biochimie 2007, 89, 1544–1552. [Google Scholar] [CrossRef]
- Wyllie, A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284, 555–556. [Google Scholar] [CrossRef]
- Wyllie, A.; Kerr, J.; Currie, A. Cell Death: The Significance of Apoptosis. Int. Rev. Cytol. 1980, 68, 251–306. [Google Scholar]
- Gurunathan, S.; Han, J.W.; Kim, J.H. Green chemistry approach for the synthesis of biocompatible graphene. Int. J. Nanomed. 2013, 8, 2719–2732. [Google Scholar] [CrossRef]
- Tienne, L.G.P.; Candido, L.d.; Simao, R.A. Reduced graphene oxide synthesis by a new modified hummers method for enhancing thermal and crystallinity properties of poly(vinylidene fluoride). J. Mater. Res. Technol. 2022, 18, 4871–4893. [Google Scholar] [CrossRef]
- Abers, W.P.; Leich, V.; Ramirez-Cuesta, A.J.; Cheng, Y.; Honig, J.; Parker, F.S. The characterization of commercial 2D carbons: Graphene, graphene oxide and reduced graphene oxide. Mater. Adv. 2022, 3, 2810–2826. [Google Scholar] [CrossRef]
- Ngidi, P.D.N.B.; Mucuweni, E.; Nyamori, O.V. Synthesis and characterization of heteroatom-doped reduced graphene oxide bismuth oxide nanocomposites and their application as photoanodes in DSScs. RSC Adv. 2022, 12, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Aragaw, B.A. Reduced graphene oxide intercalated graphene oxide nano-hybrid for enhanced photoelectrochemical water reduction. J. Nano Struc. Chem. 2020, 10, 9–18. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene chiral liquid crystals and macroscopic assembled fibres. Nat. Commun. 2011, 2, 571. [Google Scholar] [CrossRef] [PubMed]
- Jirickova, A.; Sofer, O.J.; Sedmidubsky, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Chauhan, D.; Kumar, Y.; Chandra, R.; Kumar, S. Nanostructured zirconia reduced graphene oxide based ultraefficient nanobiosensing for food toxin detection. Sens. Diagn. 2022, 1, 550. [Google Scholar] [CrossRef]
- Elbasuney, S.; Al-Hazmi, M.Y.S.l.E.N.; Tantawy, H. Potential impact of reduced graphene oxide incorporated metal oxide nanocomposites as antimicrobial, and antibioflim agents against pathogenic microbes: Bacterial protein leakage reaction mechanism. J. Clust. Sci. 2022, 1–18. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Xie, F.; Ma, X.; Niu, B.; Chen, M.; Zhang, H. General symthesis of ultrafine metal oxide/reduced graphene oxide nanocomposites for ultrahigh-flux nanofilteration membrane. Nat. Commun. 2022, 13, 471. [Google Scholar] [CrossRef]
- Scari, J.; Nidheesh, P.V. Magnetic–reduced graphene oxide nanocomposites as an efficient heteatrogeneous Fenton catalyst for the degradation of tetracycline antibiotics. Environ. Sci. Water Res. Tech. 2022, 8, 1261–1276. [Google Scholar] [CrossRef]
- Rahmani, M.H.; Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B. Introducing GO-based 2D –platform modified via phytic acid molecules decorated by zeolite imidazole ZIF-9 MOFs for designing multi-functional polymeric anticorrosive system;DFT-D computations and experimental studies. J. Mol. Liq. 2022, 364, 119945. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Han, T.H.; Hong, J.; Kim, J.E.; Lee, S.H.; Kim, H.W.; Kim, S.O. Noncovalent functionalizatiion of graphene with end-functional polymers. J. Mater. Chem. 2010, 20, 1907–1912. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Xu, Z.; Li, Y.; Zhou, B.; Shan, M.; Wang, Z.; Guo, Q.; Qian, X. Preparing graphene with notched edges and nanopore defects by γ-ray etching of graphite oxide. Mater. Lett. 2012, 89, 226–228. [Google Scholar] [CrossRef]
- Fu, D.; Han, G.; Chang, Y.; Dong, J. The synthesis and properties of ZnO–graphenenano hybrid for photodegradation of organic pollutant in water. Mater. Chem. Phys. 2012, 132, 673–681. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Ni, Z.H.; Yu, T.; Shen, Z.X.; Wang, H.M.; Wu, Y.H.; Chen, W.; Wee, A.T.S. Raman Studies of Monolayer Graphene: The Substrate Effect. J. Phys. Chem. C 2008, 112, 10637–10640. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef]
- Jeong, H.-K.; Lee, Y.P.; Lahaye, R.J.W.E.; Park, M.-H.; An, K.H.; Kim, I.J.; Yang, C.-W.; Park, C.Y.; Ruoff, A.R.S.; Lee, Y.H. Evidence of Graphitic AB Stacking Order of Graphite Oxides. J. Am. Chem. Soc. 2008, 130, 1362–1366. [Google Scholar] [CrossRef]
- Faivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric analysis of graphene materials: Effect of particle size of graphene, graphene oxide and graphite on thermal parameters. Carbon 2021, 7, 41. [Google Scholar]
- Coros, M.; Pogacean, F.; Turzaial, A.; Dan, M.; Pana, L.-O. Green synthesis, characterization and potential application of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostructures 2020, 119, 113971. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Kim, E.; Han, J.; Kim, J.-H.; Gurunathan, S. A novel Biomolecule-mediated reduction of graphene oxide A multifunctional anti-cancer agent. Molecules 2016, 21, 375. [Google Scholar] [CrossRef]
- Senwoo, H.; Choung, H.; Chung, J.H.; Garg, P.; Park, C.; Lee, J. Reduced graphene oxide incorporated calcium phosphate cements with pulsed electromagnetic fields for bone regeneration. RSC Adv. 2022, 12, 5557–5570. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, J.; Straonjny, B.; Chwalibog, E.S.; Jaworski, S. Effect of reduced graphene oxide on apoptosis and cell cycle of Glioblastoma multiforme. Int. J. Mol. Sci. 2018, 19, 3939. [Google Scholar] [CrossRef] [PubMed]
- Kretowski, R.; Tyrypuc, A.J.; Cechowska-Pasko, M. The Preliminary study on the proapoptotic effect of reduced graphene oxide in Breast cancer cell lines. Int. J. Mol. Sci. 2021, 22, 12593. [Google Scholar] [CrossRef] [PubMed]
- Adil, S.F.; Shaik, M.R.; Nasar, A.F.; Alqahtani, S.A.A.; Ahmed, Z.M. Etnhanced apoptosis by functionalized graphene oxide and gold nanocomposites in MCF-7 Breast cancer cells. ACS Omega 2021, 6, 15147–15155. [Google Scholar] [CrossRef]
- Yaghoubi, F.; Motlagh, N.S.H.; Naghib, S.M.; Jaliani, H.Z.; Naghib, A. A functionalized graphene oxide with improved cytocompatability for Stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment. Sci. Rep. 2022, 12, 2341. [Google Scholar] [CrossRef]
- Jaworski, S.; Sawosz, E.; Grodzik, M.; Winnicka, A.; Prasek, M.; Wierzbicki, M.; Chwalibog, A. In vitro evaluation of the effects of graphene platelets on glioblastomamultiforme cells. Int. J. Nanomed. 2013, 8, 413–420. [Google Scholar]
- Zhang, G.; Chang, H.; Amatore, C.; Chen, Y.; Jiang, H.; Wang, X. Apoptosis induction and inhibition of drug resistant tumor growth in vivo involving daunorubicin-loaded graphene-gold composites. J. Mater. Chem. B 2013, 1, 493–499. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Kim, J.H. Reduced graphene oxide-silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, H.-Y.; Wang, J.-Q.; Wu, G.-D.; Wang, L. Graphene oxide and Reduced graphene oxide exhibit cardio toxicity through the regulation of lipid peroxidation oxidative stress and mitochondrial Dysfunction. Front. Cell. Dev. Biol. 2021, 9, 616888. [Google Scholar] [CrossRef]
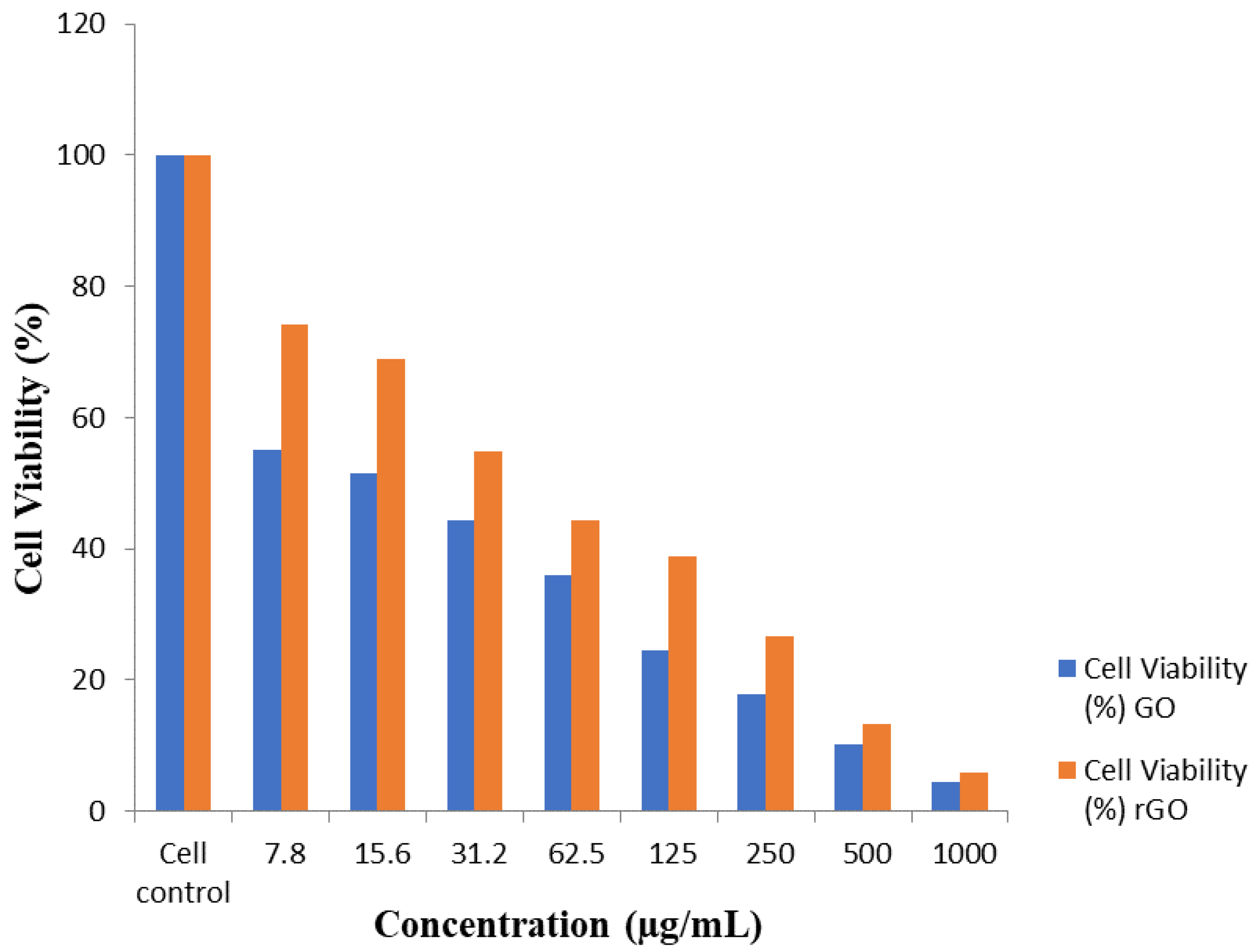



| Scheme | Concentration (µg/mL) | Dilution | Absorbance (O.D) | Cell Viability (%) |
|---|---|---|---|---|
| 1 | 1000 | Neat | 0.042 ± 0.01 | 4.44 |
| 2 | 500 | 1:1 | 0.10 ± 0.01 | 10.13 |
| 3 | 250 | 1:2 | 0.17 ± 0.01 | 17.89 |
| 4 | 125 | 1:4 | 0.29 ± 0.01 | 24.40 |
| 5 | 62.5 | 1:8 | 0.35 ± 0.01 | 35.88 |
| 6 | 31.2 | 1:16 | 0.43 ± 0.01 | 44.36 |
| 7 | 15.6 | 1:32 | 0.50 ± 0.01 | 51.60 |
| 8 | 7.8 | 1:64 | 0.53 ± 0.01 | 55.01 |
| 9 | Cell control | - | 0.96 ± 0.01 | 100 |
| Sample | Concentration (µg/mL) | Dilution | Absorbance (O.D) | Cell Viability (%) |
|---|---|---|---|---|
| 1 | 1000 | Neat | 0.057 + 0.01 | 5.89 |
| 2 | 500 | 1:1 | 0.13 ± 0.01 | 13.23 |
| 3 | 250 | 1:2 | 0.26 ± 0.01 | 26.68 |
| 4 | 125 | 1:4 | 0.375 ± 0.01 | 38.78 |
| 5 | 62.5 | 1:8 | 0.43 ± 0.01 | 44.26 |
| 6 | 31.2 | 1:16 | 0.53 ± 0.01 | 54.80 |
| 7 | 15.6 | 1:32 | 0.67 ± 0.01 | 68.97 |
| 8 | 7.8 | 1:64 | 0.72 ± 0.01 | 74.14 |
| 9 | Cell control | - | 0.97 ± 0.01 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vimalanathan, B.; Vijaya, J.J.; Mary, B.C.J.; Ignacimuthu, S.; Daniel, M.; Jayavel, R.; Bououdina, M.; Bellucci, S. The Anticancer Efficacy of Thiourea-Mediated Reduced Graphene Oxide Nanosheets against Human Colon Cancer Cells (HT-29). J. Funct. Biomater. 2022, 13, 130. https://doi.org/10.3390/jfb13030130
Vimalanathan B, Vijaya JJ, Mary BCJ, Ignacimuthu S, Daniel M, Jayavel R, Bououdina M, Bellucci S. The Anticancer Efficacy of Thiourea-Mediated Reduced Graphene Oxide Nanosheets against Human Colon Cancer Cells (HT-29). Journal of Functional Biomaterials. 2022; 13(3):130. https://doi.org/10.3390/jfb13030130
Chicago/Turabian StyleVimalanathan, Babu, J. Judith Vijaya, B. Carmel Jeeva Mary, Savarimuthu Ignacimuthu, Magesh Daniel, Ramasamy Jayavel, Mohamed Bououdina, and Stefano Bellucci. 2022. "The Anticancer Efficacy of Thiourea-Mediated Reduced Graphene Oxide Nanosheets against Human Colon Cancer Cells (HT-29)" Journal of Functional Biomaterials 13, no. 3: 130. https://doi.org/10.3390/jfb13030130
APA StyleVimalanathan, B., Vijaya, J. J., Mary, B. C. J., Ignacimuthu, S., Daniel, M., Jayavel, R., Bououdina, M., & Bellucci, S. (2022). The Anticancer Efficacy of Thiourea-Mediated Reduced Graphene Oxide Nanosheets against Human Colon Cancer Cells (HT-29). Journal of Functional Biomaterials, 13(3), 130. https://doi.org/10.3390/jfb13030130








