Abstract
Metallic ions have been widely investigated and incorporated into bone substitutes for bone regeneration owing to their superior capacity to induce angiogenesis and osteogenesis. Exosomes are key paracrine mediators that play a crucial role in cell-to-cell communication. However, the role of exosomes in metallic ion-induced bone formation and their underlying mechanisms remain unclear. Thus, this review systematically analyzes the effects of metallic ions and metallic ion-incorporated biomaterials on exosome secretion from mesenchymal stem cells (MSCs) and macrophages, as well as the effects of secreted exosomes on inflammation, angiogenesis, and osteogenesis. In addition, possible signaling pathways involved in metallic ion-mediated exosomes, followed by bone regeneration, are discussed. Despite limited investigation, metallic ions have been confirmed to regulate exosome production and function, affecting immune response, angiogenesis, and osteogenesis. Although the underlying mechanism is not yet clear, these insights enrich our understanding of the mechanisms of the metallic ion-induced microenvironment for bone regeneration, benefiting the design of metallic ion-incorporated implants.
1. Introduction
1.1. Metallic Ions and Bone Healing
In recent decades, with rapid economic advancement and the aging population, large bone defects caused by musculoskeletal diseases (e.g., osteoporosis, trauma, bone tumors, and spinal disorders) have affected hundreds of millions of people across the world. Autografts from patients are considered the gold standard for clinical treatment. However, limited donor resources and complications have restricted its widespread application. Presently, orthopedic implants derived from biomaterials are used in therapeutic strategies, such as calcium phosphate as a bone defect filler [1] or titanium alloys as intervertebral cages for spine fusion [2]. Although many bone substitutions are available, inferior healing effects (e.g., poor vascularization and bone bonding) have led researchers to explore more osteoinductive biomaterials that are similar to natural bone.
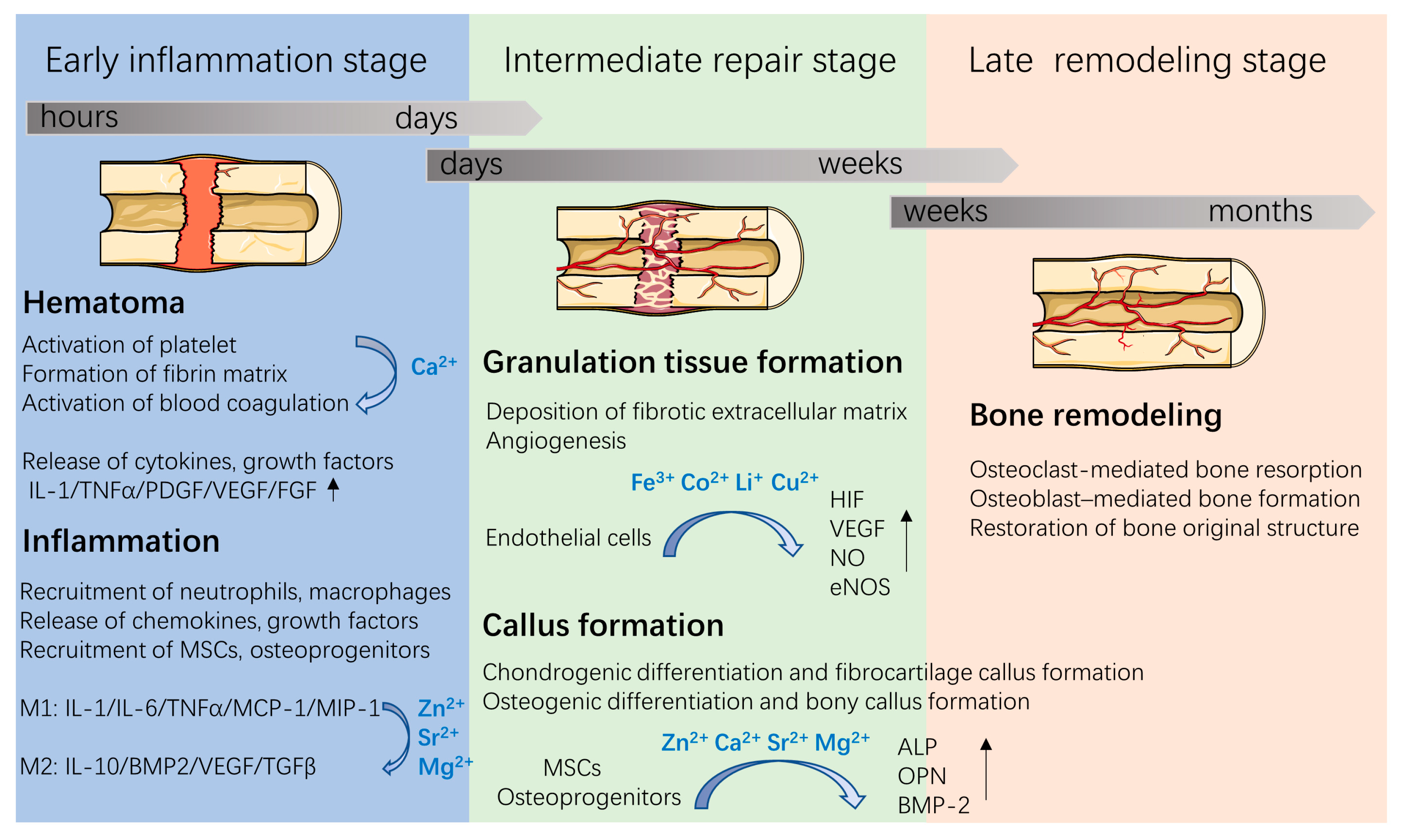
As a part of the musculoskeletal system, bone plays a role in both the support and movement of the body, and is a storage reservoir for calcium, phosphorus, and other trace elements. Bone is mainly composed of inorganic minerals (50–70% calcium phosphate), collagen fibers (20–40%), and water (5–10%). The inorganic minerals in bones are mainly carbo-hydroxyapatite (HA); meanwhile, trace elemental substitutions exist in biological apatite. For example, metallic ions (such as Mg2+, Zn2+, Sr2+) and anions (such as CO32−, SiO32−, F−) are co-incorporated in the apatite structure. Various metallic elements have been proven to play key roles in bone formation and healing processes [3,4,5] (Figure 1). For example, Sr2+, Mg2+, and Ca2+ can enhance osteogenesis, while Cu2+, Co2+, Li+, and Fe3+ can increase neovascularization. Specifically, approximately 99% of Ca is stored in bone in forms of HA. Ca acts as an ionic messenger and participates in a series of cellular process, including exocytosis, apoptosis, and motility. During bone healing processes, Ca2+ plays key roles in the activation and aggregation of platelets, blood clot formation, the stimulation of bone mineralization, and subsequent bone healing [3]. Moreover, approximately 50% of Cu and Mg are stored in the musculoskeletal system while 90% of Sr exists in bone tissue. Although these trace metallic ions are minor components in bone tissues, they act as enzymes cofactors and participate in bone metabolism and remodeling. Sr ions enhance immune suppression and promote osteogenesis [4]. Fe ions regulate various cells (e.g., mesenchymal stem cells (MSCs) and endothelial cells) functions and promote angiogenesis by increasing hypoxia-inducible factor-1α (HIF-1α) levels, vascular endothelial growth factor (VEGF) expression and endothelial NO synthase (eNOS) production [5,6]. Mg ions induce an anti-inflammatory environment and enhance osteogenic differentiation by activating the bone morphogenetic protein 2 (BMP-2) signaling pathway in MSCs [7].
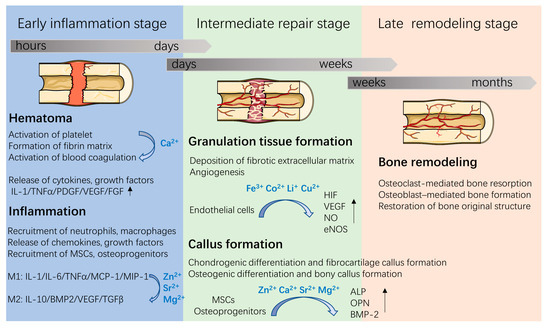
Figure 1.
Three overlapping stages involved in the bone healing process and roles of metallic ions during bone repair. Figure was produced using Servier Medical Art (http://smart.servier.com/ (accessed on 5 January 2022)).
With the evolving understanding of bone remodeling, it has been realized that the bone healing process involves an orchestrated series of biological events (Figure 1). First, after implantation, proteins in the blood and in tissue fluids are immediately adsorbed on the implant surface, a process that is followed by hematoma formation, which is regulated by plasma proteases. These are responsible for fibrin formation and platelet activation [8]. The formed hematomas, as a fibrin scaffold, support cell adhesion and release chemokines, proinflammatory cytokines, and growth factors (e.g., platelet-derived growth factor (PDGF), VEGF, fibroblast growth factor (FGF), and tumor necrosis factor-α (TNFα)) to recruit and activate neutrophils and macrophages. Then, the inflammatory cells release cytokines and recruit MSCs and osteoprogenitors, thus stimulating angiogenesis, extracellular matrix synthesis and bone remodeling at the injury site. Since metallic ions are involved in the above bone healing process, incorporating metallic ions into bone implants has attracted great attention in bone tissue engineering.
1.2. Exosomes and Bone Healing
In bone tissue regeneration, scaffolds both offer mechanical support for cell adhesion, and provide a micro-environment comprising surface structure, chemical composition, and mechanical signal, which regulates cell behavior and tissue healing. The micro-environment provided by the implants has been proven to be a key modulator of cell–material interactions and cell-to-cell communications by stimulating or inhibiting cell-associated signals. Various cells, including MSCs, endothelial cells, osteoblasts, osteoclasts, and immune cells, are involved in the biological healing process around the implant surface. The communications among various cells are vital to the cell functions and tissue regeneration. In addition to direct cell–cell contact, paracrine communication also occurs among cells located far apart from each other via secreted factors. Among the various secreted factors, here, we will focus on the roles of exosomes in cell-to-cell communication and bone regeneration.
As a kind of cell vesicles, exosomes were first discovered in sheep reticulocytes in 1983. Exosomes are extracellular vesicles with diameter of 30–150 nm, which are secreted by a variety of cells (e.g., MSCs, adipocytes, dendritic cells, and epithelial cells) and present in blood, urine, saliva, and other bodily fluids [9,10]. Exosomes, known as important intercellular communication mediators, play an important role in regulating cell-to-cell communication through delivering selected nuclei acids, lipids, and proteins. Meanwhile, exosomes have showed similar biofunctions with their derived cells and low immune rejection [11,12]. Thus, exosomes have been used to promote the process of bone tissue regeneration, avoiding the safety concerns of direct cell transplantation. For instance, MSC-derived exosomes significantly enhanced the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) in distraction osteogenesis-mediated bone regeneration in older rats [13]. Additionally, M2 macrophage-derived exosomes could enhance the osteogenesis and decrease the adipogenesis of BMSCs, exhibiting positive paracrine regulation of bone regeneration [14,15]. Despite these advancements, the clinical application of exosomes is limited by the extraction method and large dose requirements. In addition, the biofunction and production capability of exosomes are relative to various factors, e.g., culture microenvironment and cell types. Therefore, the modification of the cell microenvironment or of cell themselves has potential for achieving exosomes with superior therapeutic effects for regenerative medicine.
It is noteworthy that when biomaterials co-cultured with cells, the various chemical signals derived from implants can directly act on the cells, which is followed by cell behaviors being affected (e.g., through adhesion, proliferation, and differentiation). Additionally, the released chemical signals can change the microenvironment where such cells are located, therefore affecting the exosome secretion from cells. Findings in the literature combined with our previous studies confirmed that metallic ions incorporated with bone substitutes could obviously enhance the angiogenic and osteogenic capacities of various cells and promote new bone formation in vivo [16,17,18]. Due to the fact that exosome production is determined by cell behavior, and exosomes are involved in intercellular paracrine communication, metallic ions released from implants may influence cell behavior by modifying exosome secretion from various cells. Recent research efforts focused on studying the effects of ion signal on exosome secretion and osteogenesis [19]. Therefore, understanding the roles of metallic ions in regulating the exosome secretion of cells is crucial when designing biomaterials for bone regeneration. This review mainly focuses on the roles of metallic ions in the secretion of exosomes from MSCs and macrophages by elaborating on cell–material interactions and bone regeneration.
2. Exosomes Derived from Different Cells Relevant to Bone Regeneration
2.1. MSCs-Derived Exosomes
MSCs are multipotent stromal cells which exist in connective tissues that can differentiate into multiple cell types (e.g., bone, fat, nerves, etc.) and have strong self-renewing abilities. According to their isolated locations, MSCs are mainly divided into adipose tissue-derived MSCs (ADMSCs), bone marrow-derived MSCs (BMSCs), dental pulp-derived MSCs (DPMSCs), umbilical cord-derived MSCs (UCMSCs) and peripheral blood MSCs (PBMSCs), etc. As the most commonly used stem cells, MSCs have been widely used to improve bone tissue repair, including the local injection of MSCs with/without scaffolds [20,21]. However, problems such as risk, immune response and how to achieve stable MSCs rapidly have always been challenging in clinical applications. Many studies proved that the therapeutic effects of MSCs are attributed not only to their differentiation into bone forming-related cells but mainly to their paracrine function, including secreting growth factors, cytokines, and chemokines, and releasing extracellular vesicles such as exosomes [22,23]. Exosomes secreted by MSCs display similar biological functions as those of their parental cells. Therefore, a new therapeutic strategy based on MSCs-derived exosomes has been applied in bone tissue repair as summarized in Table 1.
MSC-derived exosomes have various regenerative capabilities, including guiding immunomodulatory regulation, angiogenesis, and osteogenesis. Thus, MSC-derived exosomes have potential for treating bone diseases, such as osteoarthritis, osteoporosis, and bone fractures, using their biological cargoes (e.g., proteins and miRNAs). Compared with MSC-based therapies in bone regeneration, MSC-derived exosomes show some advantages, such as non-living, stable preservation in the body and high loading capacity for RNAs and proteins. Moreover, exosomes derived from different MSCs exhibit various therapeutic effects. Wang et al. reported that ADMSC-derived exosomes have superior immune regulation capacity, BMSC-derived exosomes have excellent regeneration ability, and UCMSC-derived exosomes play a vital role in tissue damage repair [24]. In addition, another study indicated that DPMSC-derived exosomes show more prominent immune-modulating ability than BMSC-derived exosomes [25]. The different therapeutic effects are highly relevant to the cargoes in the exosomes, including microRNAs and proteins (see Table 1). However, whether these differences have effects, and the question of how these differences can be triggered, require further study.

Table 1.
Exosomes derived from various MSCs and their functions in bone regeneration.
Table 1.
Exosomes derived from various MSCs and their functions in bone regeneration.
| Sources | Markers | Cargoes | Functions | Ref. |
|---|---|---|---|---|
| ADMSCs-derived exosomes | CD9, CD63 | miR-375 overexpression | Enhance the osteogenic differentiation of BMSCs by inhibiting IGFBP3 proteins via miR-375 overexpression in exosomes; | [26] |
| CD9, CD63, Tsg101, CD81 | miR-34, miR-146, miR-21 upregulation | Shift macrophages from M1 to M2 phenotype using exosomes from ADMSCs pr-activated with inflammatory cytokines (IFNγ/TNFα) via miRNA regulation | [27] | |
| BMSCs-derived exosomes | CD9, CD63 | miR-150-3p upregulation | Attenuate osteoporosis by promoting osteoblast proliferation, differentiation and inhibiting apoptosis via miR-150-3p upregulation in exosomes; | [28] |
| CD9, CD63, Hsp70 | miR-26a-5p overexpression | Alleviate osteoarthritis by down-regulation of PTGS2 followed by inhibiting synovial fibroblasts proliferation and inflammation via miR-26a-5p overexpression in exosomes; | [29] | |
| CD63, CD81 | miR-128-3p upregulation | Attenuate osteogenesis and bone fracture healing via upregulation of miR-128-3p in aged-exosomes via targeting Smad5 followed by reducing RUNX2, ALP and Col I | [30] | |
| CD9, CD63, CD81 | Undetected | Enhance osteogenesis, angiogenesis and bone healing process by transplantation of exosomes in vivo via activating BMP-2/Smad1/RUNX2 signaling pathway | [31] | |
| UCMSCs-derived exosomes | CD9, CD81, CD63 | Undetected | Accelerate fracture healing by implantation of exosome via inducing HIF-α and followed angiogenesis; | [32] |
| CD9, CD81, CD63 | Undetected | Enhance bone regeneration using exosomes via promoting osteoblast migration and the expression levels of osteogenic genes (ALP, OCN, COL1A1) | [33] | |
| DPMSCs-derived exosomes | CD9, CD63 | Undetected | Exhibit strong immune-modulating activity by reducing the secretions of pro-inflammatory factors IL-17, TNF-α and IL-17 as well as increasing the anti-inflammatory factors IL-10 and TGF-β | [25] |
2.2. Macrophage-Derived Exosomes
Recently, the crosstalk between bone cells and immune cells has become regarded to be a vital factor in tissue homeostasis and new bone formation. Among these cells, the effect of macrophages on bone metabolism has attracted great attention. Macrophages possess different functional states under various pathophysiological conditions that could affect bone repair. Macrophages can be divided into nonactivated (M0), classically activated (M1), and alternatively activated (M2) subtypes. M1 macrophages can induce a pro-inflammatory response and enhance the host’s defense reaction by producing high levels of reactive oxygen species (ROS), nitric oxide (NO), and proinflammatory cytokines (e.g., TNF-a, interleukin 1 (IL-1), IL-2, IL-6, and IL-12). Conversely, M2 macrophages can produce anti-inflammatory cytokines (e.g., IL-10, CCL-18, and CCL-22) and enhance immune suppression. Meanwhile, M2 macrophages can produce growth factors, including VEGF and BMP-2, and can accelerate bone fracture healing [34]. Both M2 and M0 macrophages can enhance the osteogenic differentiation of MSCs [35].
Similarly, macrophages secrete exosomes that are important for cell-to-cell communication, immune regulation, and tissue regeneration. Exosomes derived from M0, M1, and M2 exert different effects in bone healing. Studies proved that M1 macrophage-derived exosomes have inhibitory effects on bone healing, whereas M2 macrophage-derived exosomes elicit osteoinductive effects in bone regeneration [15]. Moreover, Xia et al. proved that exosomes derived from M0, M1, and M2 all suppressed the chondrogenic differentiation of MSCs [36]. Functional variation may be attributed to different substances in exosomes derived from different types of macrophages (Table 2). For instance, miRNA-5106 was found to be overexpressed in M2 macrophage-derived exosomes, but was found to be decreased in M1 macrophage-derived exosomes [37]. Additionally, miRNA-5106-enriched exosomes could enhance the osteogenic differentiation of BMSCs by inhibiting the expression of salt-inducible kinase 2 (SIK2) and SIK3 genes [37], which are involved in cell cycle regulation and differentiation. In addition, Yu et al. [38] found that M1 macrophage-derived exosomes aggravated bone loss by enhancing miRNA-98 expression in osteoblasts, followed by suppressing DUSP1 and activating the JNK signaling pathway. Moreover, macrophage-derived exosomes possess many alarmins, such as annexin, galectin, and fibronectin [39,40], which are endogenous molecules released upon tissue damage and stimulate inflammation. Annexins, as exosomal membrane-associated proteins, can enhance the phagocytic capacity of macrophages, thus accelerating bone resorption [41]. Galectin, as a lectin enriched in exosomes from young individuals, can improve the osteogenic differentiation of MSCs [40], while fibronectin presented on the exosome surface can regulate the binding of integrin-dependent exosomes to target cells [42]. Despite these potential applications of exosomes in bone regeneration, the knowledge about MSC- or macrophage-derived exosomes is limited. Therefore, further studies are required to determine exosome functions and mechanisms.

Table 2.
Exosomes derived from different macrophages and their functions in bone remodeling.
3. Exosome Secretion upon Metallic Ions Stimulation and the Effects on Bone Regeneration
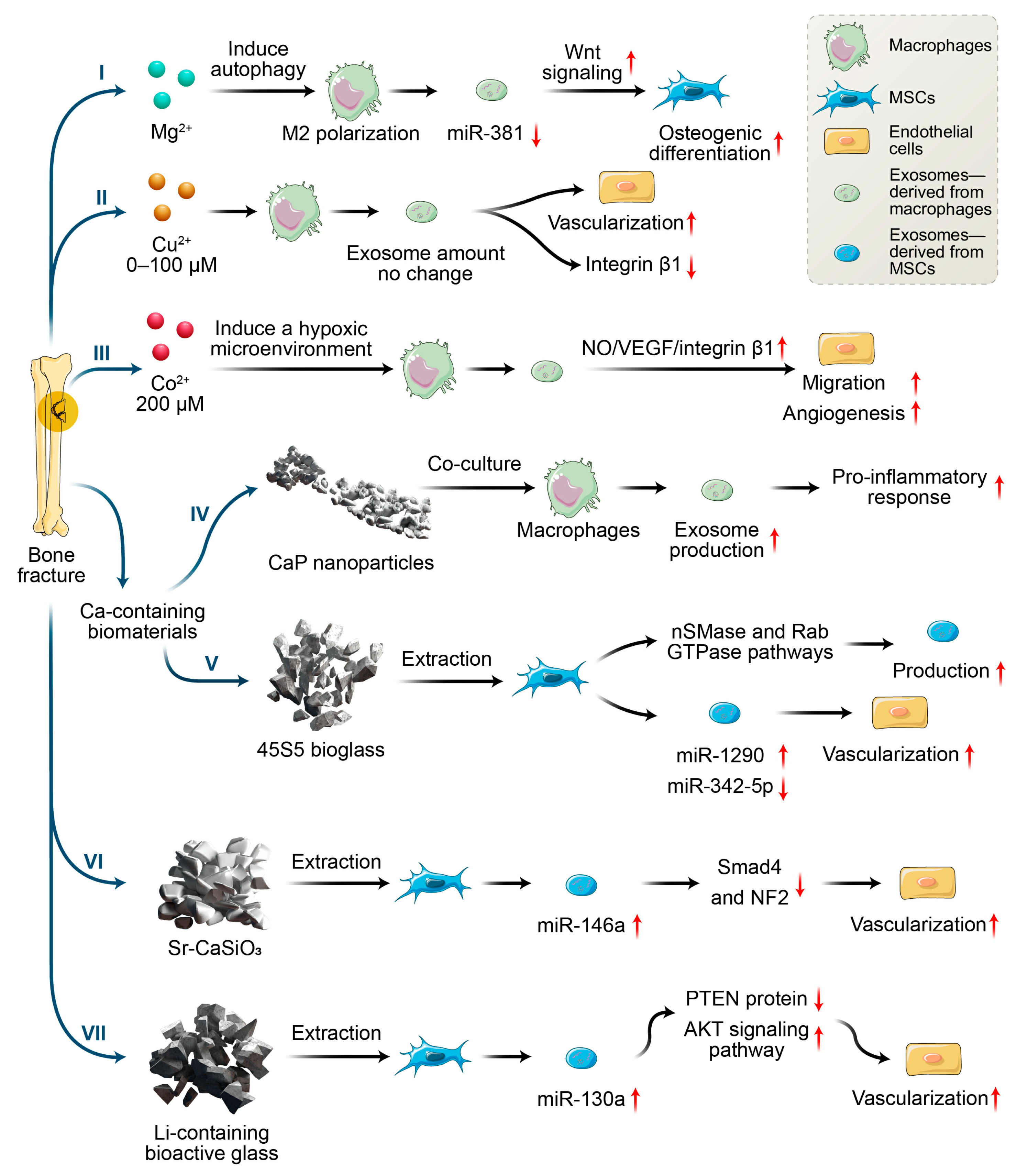
In recent years, the incorporation of metallic ions into biomaterials has been regarded as a promising strategy for inducing osteogenesis and angiogenesis for bone regeneration. Various approaches to incorporate metallic ions into biomaterials have been explored and have been proven to have positive effects on in vitro and in vivo bone healing [43,44]. When biomaterials are in contact with cells, the biomaterials both directly act on cells and affect cell-to-cell communications by changing the microenvironments of the cells’ locations. For instance, Zn2+/Sr2+ released from collagen/HA can enhance the communication between macrophages and BMSCs by inducing an osteoimmune microenvironment [45]. Zn2+/Sr2+ release up-regulated the osteogenic genes (e.g., BMP-2, Wnt10b) of macrophages, and then enhanced the osteogenesis-related gene expression (e.g., OCN, OPN, and ALP) of BMSCs and bone regeneration in vivo. However, the cell-to-cell communication mechanisms involved in the chemical signals derived from biomaterials are complex and are difficult to clarify. In addition to the conventional approaches to investigating mechanisms, exosomes have emerged as potential candidates with paracrine capacity which has been contributed to cell-to-cell communication. In addition, exosomes production and functions are highly dependent on physiological conditions, suggesting that variations in the microenvironment can modify the production of exosomes. However, reports on the effects of metallic ions on exosomes secretion are limited. Nevertheless, recent research efforts focused on this area, and may provide insight for us to design biomaterials for bone regeneration. Here, we mainly focus on the roles of those metallic ions that are widely incorporated into bone substitutes in exosomes production, as well as the biological function in intercellular communication and bone regeneration (Figure 2).
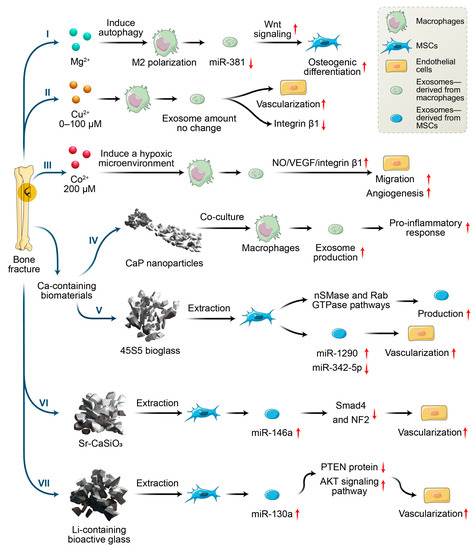
Figure 2.
Effects of metallic ions or metallic ions incorporated with biomaterials on angiogenesis and osteogenesis via macrophages and MSCs secreting exosomes.
3.1. Magnesium Ions
As the fourth most abundant element in the human body, magnesium ions participate in various metabolic activities, such as protein and nuclei acid synthesis, enzymatic reactions, and transmembrane ion transport. Moreover, approximately half of Mg2+ is stored in bone tissue, which directly modifies the bone-calcification process and the generation of new minerals in the human body. Mg2+ deficiency induces the release of inflammatory factors, resulting in a series of clinical disorders such as migraine, metabolic syndrome, hypertension, and atherosclerosis [46]. Additionally, Mg2+ deficiency causes osteoporosis by reducing the activity of osteoblasts and osteoclasts, and inhibiting apatite deposition. In addition, low Mg2+ concentration disturbs the phosphorus acid inositol system and/or reduces adenylate cyclase activity, inducing antiparathyroid hormone effects which are followed by decreased bone strength [47]. Conversely, Mg2+ addition both enhances the expression of the proteins and genes related to osteogenic differentiation and increases nitric oxide production, which can induce angiogenesis as well as prevent inflammation [48,49].
Despite these findings, current researchers have seldom focused on the influence of Mg2+ on bone regeneration from the perspective of exosomes. A study by Zhu et al. [49] was one of the few to study the effects of exosomes derived from Mg2+-treated macrophages on osteogenesis. They found that Mg2+ addition enhanced the M2 polarization of macrophages and increased TGF-β, as well as decreasing the expression of miR-381 in exosomes derived from macrophages through autophagy. Additionally, exosomes with reduced miR-381 were demonstrated to promote the osteogenic differentiation of BMSCs. Previous studies proved that miR-381 can be regarded as a tumor suppressor in various cancers such as breast cancer and osteosarcoma [50,51]. Additionally, another study indicated that miR-381 overexpression inhibited the osteogenic differentiation of BMSCs by suppressing Wnt signaling, while the down-regulation of miR-381 enhanced bone fracture healing [52]. Thus, down-regulation of exosomal miR-381 derived from macrophage upon exogenous Mg2+ stimulation, may provide a new direction for enhancing the osteogenic differentiation of MSCs.
3.2. Copper Ions
As the second essential trace element in humans, copper ions serve as a cofactor and vital component for many enzymes, affecting the development and function of the blood, immune system, and bones, etc. More than 50% of copper is distributed in the muscles and bones. Copper ions participate in the metabolism of connective tissue, bones, and epiphyseal cartilage, which promote angiogenesis and inhibit osteoporosis [53]. In addition, copper has good antibacterial properties against both Gram-positive and Gram-negative bacteria [54]. Ryan et al. [55] combined Cu2+-eluting bioactive glass into collagen scaffolds and found that the scaffolds incorporated with Cu2+ showed antibacterial ability and enhanced angiogenesis and osteogenesis compared with scaffolds without the addition of copper ions. Studies proved that copper ion-induced angiogenesis may be caused by enhancing VEGF secretion and the migration of vascular endothelial cells [56]. Additionally, copper ions can polarize macrophages toward to M1 phenotypes [57,58], and facilitate angiogenesis via VEGF secretion [59]. VEGF secretion may be one of the mechanisms contributing to pro-angiogenesis; however, the effect of copper ion-stimulated exosomes on angiogenesis is still unclear. Wang et al. [58] found that copper ions (concentration: 0–100 µM) have no significant effect on the exosome secretion from macrophages, but the exosomes did reduce the surface integrin β1 of endothelial cells. As the family member of integrins, integrin β1 plays key roles in the adhesion, migration, proliferation, and angiogenesis of endothelial cells. Meanwhile, Tanjore et al. proved that integrin β1 was vital for embryonic angiogenesis but not essential for vasculogenesis. Despite decreased integrin β1, endothelial cells showed enhanced angiogenic capacity when cocultured with the exosomes derived from macrophages upon copper ion stimulation [58]. The results suggested that other factors might promote the angiogenesis of endothelial cells, such as the miRNAs in exosomes. Wang et al. [58] proposed that copper stimulation might up-regulate the pro-angiogenic RNAs and down-regulate anti-angiogenic RNAs in exosomes. Although the mechanism remains to be studied further, the investigation demonstrated that exosomes derived from macrophages upon copper stimulation were pro-angiogenic, which is helpful for the design of copper ion-incorporated bone implants.
3.3. Cobalt Ions
As an essential element in the human body, cobalt ions play a key role in biological activities by complexing with various proteins (e.g., vitamin B12). Cobalt ions serve as hypoxia-mimicking agents for promoting tissue angiogenesis by enhancing the secretion of HIFs. Additionally, studies proved that the hypoxia condition promotes osteogenesis at an early stage by inducing an HIF1a–Twist1 pathway while suppresses osteogenesis maturation at the late stage by inhibiting the HIF1a–Twist1 pathway [60]. Thus, the dose-dependent behavior of cobalt ions, implemented at an appropriate time, is essential for mediating oxygen levels and the bone regeneration that follows. Wu et al. [61] fabricated hypoxia-mimicking bioactive glass scaffolds by incorporating cobalt ions into the scaffolds and found that this incorporation enhanced hypoxia functions through increasing HIF-1α expression and VEGF secretion. This promoted BMSC proliferation and osteogenic gene expression. Another study [62] found that the cobalt-induced osteogenic differentiation of BMSCs was eliminated when macrophages were included. The extraction of cobalt ion-incorporated tricalcium phosphate induced macrophages toward a proinflammatory M1 phenotype; this was accompanied by fibrous encapsulation instead of new bone formation in vivo [62]. However, although cobalt ion-incorporated tricalcium phosphate fails in bone repair, we cannot conclude that cobalt-ion-incorporated implants should be abandoned. Instead, findings have indicated the essential roles of macrophages in the evaluation of biomaterial-induced osteogenesis.
Based on the key roles of exosomes in intercellular communication, the underlying mechanism of exosomes derived from macrophages upon cobalt stimulation on angiogenesis were studied by Zhang et al. [63]. They found that the exosomes could enhance endothelial migration and angiogenesis both in vitro and in vivo when cobalt concentration was 200 µM. These results could possibly be attributed to the enhanced release of nitric oxide (NO), VEGF, and integrin β1 from exosome-regulated endothelial cells upon cobalt stimulation. As an endothelium-derived relaxing factor (EDRF), NO can increase VEGF secretion by enhancing HIF-1α activity through the PI3K-Akt signaling pathways [64]. Conversely, VEGF can increase NO production by enhancing eNOS activation through the PKC- and CaM-Akt signaling pathways [64]. These interactions are beneficial for angiogenesis. However, no significant difference in exosomal VEGF content was observed in the study by Zhang et al. [63]. The authors speculated that this might be attributed to the miRNAs in the exosomes, although no further study has yet been carried out. Admittedly, the presence of miRNAs in exosomes has been considered a critical factor in angiogenesis. For example, exosomal miR-210 and miR-135b have been proven to enhance angiogenesis by targeting HIF-1α [65,66]. Notably, miR-210 is a hypoxia-responsive miRNA, which shows high expression in hypoxic culture while disappearing under normoxic conditions. In contrast, miR-135b expression could be maintained under normoxic conditions. Thus, cobalt ion-driven hypoxia conditions may accelerate angiogenesis by changing exosomal miRNA expression. For integrin β1, exosomes significantly enhance the integrin β1 expression of endothelial cells upon cobalt ions stimulation, which might be beneficial for angiogenesis. Thus, an appropriate concentration of cobalt ions could induce a hypoxic microenvironment and facilitate macrophage secretion of exosomes, promoting angiogenesis. This also indicates the importance of controlling the release of cobalt ions from implants.
3.4. Calcium-Containing Biomaterials
As ionic messengers in cells, calcium ions play key roles in living systems, and are involved in many processes such as apoptosis, movement, signal transduction, gene transcription, and gene differentiation, etc. As essential inorganic components, 99% of calcium ions are stored in bone and present in the form of HA in combination with phosphate. It is important to maintain appropriate concentrations of intracellular and extracellular calcium ions during the formation of mature bone. Low Ca2+ concentrations (2–4 mM) in culture media improve osteoblast proliferation, medium concentrations (6–8 mM) enhance osteoblast differentiation and extracellular matrix mineralization, and high concentrations (>10 mM) are toxic [67]. Additionally, studies proved that calcium ions (3~5 mM) released from CaSO4-containing scaffolds enhance BMSCs migration, osteoblast gene expression, and new bone formation in a concentration-dependent manner by activating the PI3K–AKT pathway [68]. Another study showed that appropriate Ca2+ release from mesoporous silica xerogels could improve osteoblast proliferation and osteogenic differentiation by activating ERK1/2 signaling pathway [69].
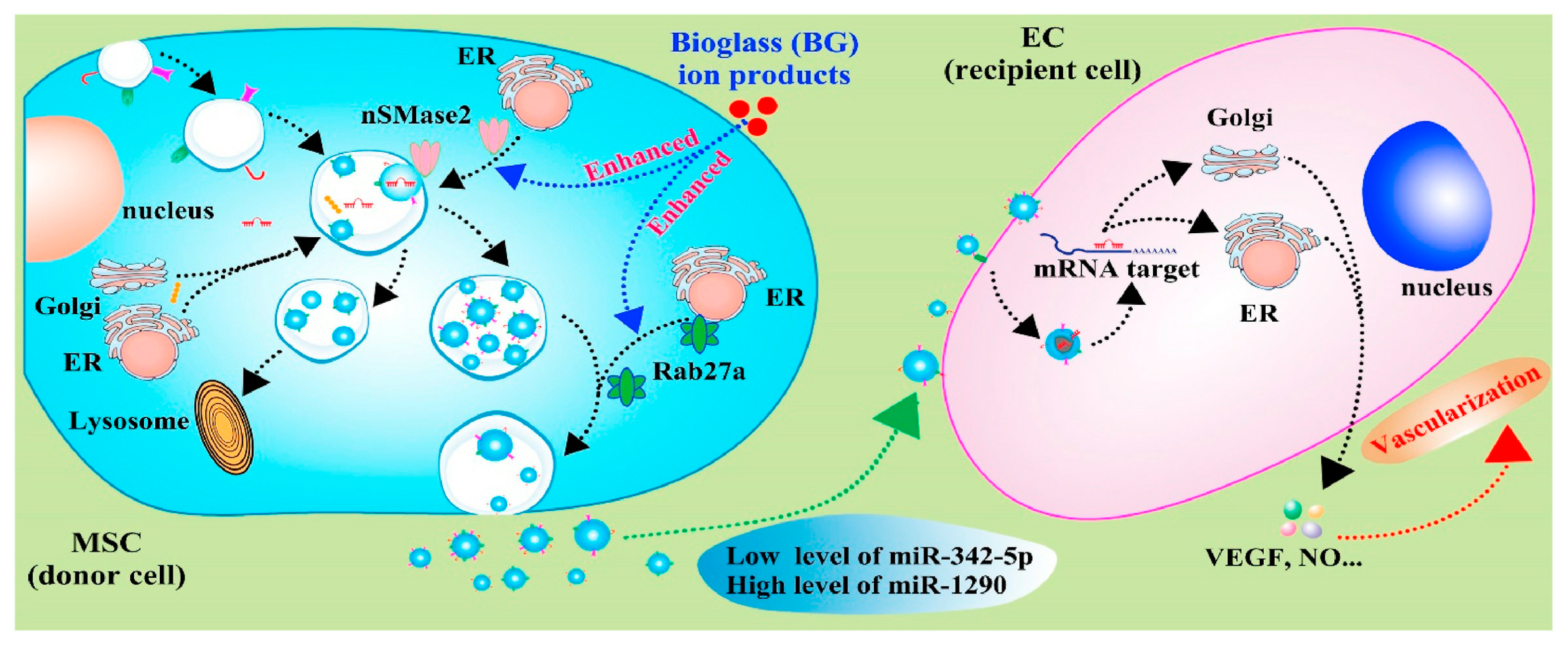
With knowledge of exosomes in intercellular communication developing, the effects of calcium-containing biomaterials on exosomes production and their mechanisms are investigated. As the first bioactive glass introduced by Hench in 1971, 45S5 Bioglass® is high in calcium content and shows good degradable capacity and bioactivity. In particular, 45S5 Bioglass® is composed of 46.1 mol.% SiO2—26.9 mol.% CaO—24.4 mol.% Na2O—2.6 mol.% P2O5, which can form a strong bond with the surrounding bone. The high bioactivity of the material can be attributed to hydroxycarbonate apatite formation on its surface, which occurs due to glass dissolution. The dissolution products of bioglass (e.g., calcium and silica ions) have a positive effect on the proliferation and osteogenic differentiation of bone-forming-related cells, as well as the regulation of macrophage polarization [70,71]. Wu et al. [72] investigated the effects of ion products of 45S5 Bioglass on the exosome production of MSCs and found that ion products induced a two-fold increase in exosome production by up-regulating the expression of sphingomyelinase-2 (nSMase2) and Rab27a. The nSMases family and Rab family play key roles in vesicle formation and membrane traffic. nSMase2 facilitates ceramide formation and the budding of endosomal membranes, leading to enhanced intravesical formation [73]. Rab27a, located and expressed at CD63-positive multivesicular endosomes, regulates vesicle motility by docking into cell-specific compartments, playing a role on membrane-fusion process [74]. Thus, the ion produced by 45S5 Bioglass can affect the formation and release of exosomes by regulating SMase2 and Rab27a expression, which control nSMase and Rab GTPase pathways (Figure 3). In addition, the up-regulation of miR-1290 and the down-regulation of miR-342-5p in exosomes upon the stimulation of ion production have been proven to facilitate the vascularization capability of endothelial cells [72]. Studies have proven that miR-342-5p could repress VEGF-triggered Akt phosphorylation and decrease endoglin expression, which suppress the proliferation of endothelial cells and vascularization [75,76]. Additionally, miR-1290-overexpressing exosomes could promote tumor angiogenesis by attenuating the inhibition of VEGFR2 phosphorylation by targeting SMEK1 [77]. Thus, the exosomes with increased miR-1290 expression and decreased miR-342-5p expression, mediated by ion production, have shown enhanced vascularization capability [72].
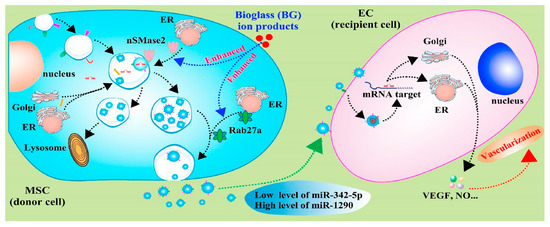
Figure 3.
Schematic diagram indicates the mechanisms of bioglass ion products in regulating the production and function of exosome derived from MSCs, and the effects of secreted exosomes on angiogenesis of endothelial cells [72]. Reprinted with permission from KeAi Publishing.
In addition, calcium ions also regulate the exosome secretion derived from bone-resorbing cells. Calcium phosphate (CaP), as a calcium-rich biomaterial, has good biocompatibility. When amorphous-phase CaP nanoparticles were cocultured with macrophages, it was observed that the particles could increase the number of exosomes more than twofold [78]. However, CaP particle treatment had no significant effect on the calcium concentration in exosomes [78]. CaP particles entered into cells through the endocytosis or phagocytosis pathways and released Ca2+ in the acidic microenvironment (e.g., late endosomes or lysosomes). Then, a Ca2+ increase resulted in the collapse of their membranes and the Ca2+ release into cytosol, which induced the increase in intracellular calcium concentration and exosome release. Thus, CaP particles have potential applications for improving the production efficiency of exosomes, while not affecting the Ca2+ concentration in exosomes. Additionally, calcium oxalate was found to alter the protein expression levels in the macrophage-derived exosomes, which were involved in the immune response, cell migration, transcription regulation, and calcium binding [79,80]. Further study found that these exosomes could enhance IL-8 production and the migration of immune cells [79,80]. All these findings indicate that calcium-enriched microenvironments not only increase the exosome production of macrophages, but also enhance the proinflammatory response via the exosomal pathway.
3.5. Strontium-Containing Biomaterials
As a nonessential element, approximately 98% of strontium ions are stored in human bone tissue, and have strong bone-seeking behavior. Presently, strontium is widely used in the form of strontium ranelate as a treatment for osteoporosis, especially for postmenopausal women. Thus, strontium is incorporated into various biomaterials to enhance bone regeneration, including bioactive glass, calcium phosphate, and metallic implants [81,82]. The incorporation of Sr2+ could enhance ALP activity, osteogenic gene expression in osteoblastic cells, and calcium nodule deposition [82]. Additionally, the addition of Sr2+ enhances the migration and tube formation of endothelial cells, as well as increasing the expression of VEGF and Angiopoietin-1 (Ang-1) [82]. Additionally, Sr2+-containing bioactive microspheres can enhance the transformation of macrophages toward to M2 phenotypes and secrete high levels of PDGF-BB, resulting in the improved angiogenic capacity of endothelial cells and early vascularization in vivo [83]. However, the underlying mechanisms of paracrine communication remain unclear. Liu et al. [19] investigated the influence of the strontium-substituted calcium silicate on exosome-derived BMSCs and found that, after strontium stimulation, exosomes showed superior pro-angiogenic ability. This effect might be attributed to the increased level of miR-146a and the inhibition of Smad4 and NF2 proteins in exosomes. Studies proved that miR-146a is involved in the angiogenic process [84,85]. In addition, it was proposed that Smad4 and NF2 were potential targets of miR-146a. As a member of the Smad family, Smad4 serves as a vital regulator of TGF-β signaling pathways and suppresses tumor progression by inhibiting angiogenesis [86,87]. Additionally, as a component of the Hippo signaling pathway, the knockdown of NF2 can improve the angiogenesis of endothelial cells [88]. Thus, exosomes containing increased miR-146a, derived from BMSCs upon strontium-incorporation stimulation, could enhance angiogenesis by inhibiting the expression of Smad4 and NF2. This was also proven by the knockdown of miR-146a, accompanied by the increased protein expressions of Smad4 and NF2 in a report by Liu et al. [19]. Therefore, exosomes under the stimulation of strontium-incorporated biomaterials play a key role in vascularization and the following bone regeneration. This observation provides new insights into the underlying mechanisms of strontium-incorporated biomaterials in bone regeneration.
3.6. Lithium-Containing Biomaterials
Lithium ions have long been widely used to treat psychiatric disorders and bipolar disease. Due to its association with hyperparathyroidism, lithium has attracted great interest in bone tissue treatment. Zamani et al. [89] found that 75 patients treated with lithium carbonate had significantly greater bone density compared with normal patients, indicating the potential of lithium therapy for preserving or enhancing bone mass. Another study has proved that Li+ addition can enhance the proliferation of BMSCs by glycogen synthase-3β (GSK-3β) inhibition, and the β-catenin/Wnt activation that follows [90]. GSK-3β is a multifunctional protein kinase involved in diverse cellular metabolic processes such as cell proliferation, migration, and cell cycles. Inhibition of GSK-3β increased BMSCs migration through enhancing β-catenin expression, which was followed by the activation of Wnt-responsive genes [91]. Moreover, Li+ inhibits GSK-3, activating HIF-1 that induces vasculogenesis [92,93]. Thus, Li+ is a new additive for incorporation into bone substitutes due to its stimulating effects on vasculogenesis and bone formation. Since high glucose inhibits the migration of BMSCs through the activation of GSK-3β, Chen et al. [94] prepared Li+-containing bioactive glasses and found that Li+ addition could reverse the suppression of migration, proliferation, and osteogenic differentiation of BMSCs, which is induced by high glucose, by activating the β-catenin/Tcf7/Ccn4 signaling pathway. Moreover, the Li+ released from bioactive glasses has been proven to facilitate the proliferation and tubules formation of endothelial cells by activating the canonical Wnt/β-catenin pathway and increasing the expressions of proangiogenic cytokines including insulin-like growth factor 1 (IGF-1) and TGFβ [95].
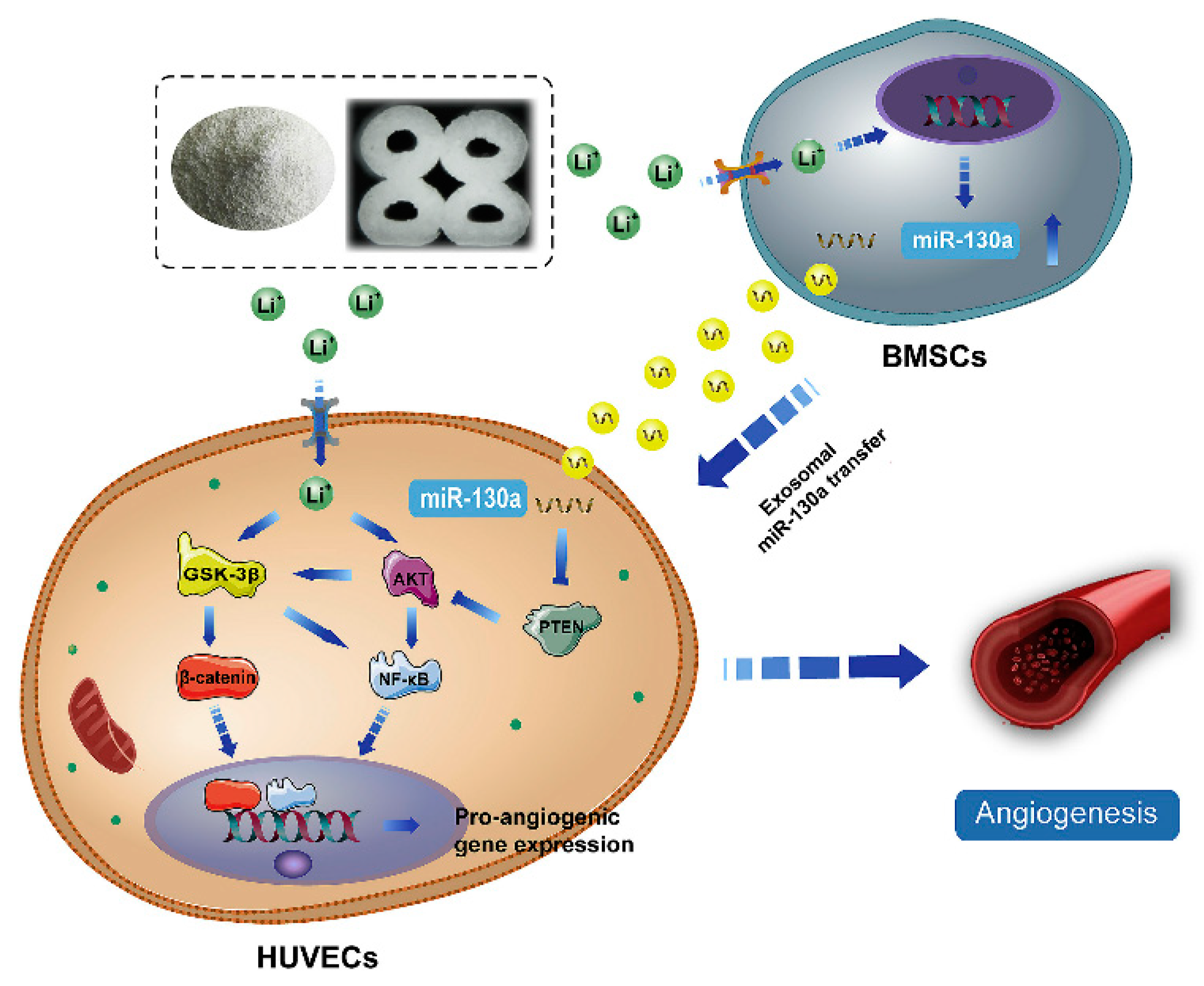
Despite advances in the exploration of the roles of Li+ in bone regeneration, the underlying mechanisms between intercellular communications remain unclear. During the bone healing process, the communication between BMSCs and endothelial cells is very important for vascularized bone regeneration. Liu et al. [96] found that Li-containing bioactive glass could promote the angiogenic capacity of endothelial cells by enhancing the expressions of miR-130a in exosomes derived from BMSCs and subsequently down-regulating the PTEN protein and activating the AKT signaling pathway (Figure 4). miR-130a plays a vital role in angiogenesis. First, miR-130a can maintain normal autophagy behavior and improve the survival of endothelial cells by the regulation of Beclin1 and Bcl-2 expression [97]. Second, miR-130a has been proven to enhance angiogenesis and tumor growth through targeting Runx3 [98]. More importantly, miR-130a has been proven to target the PTEN protein, and can thus prevent cerebral damage by activating the PI3K/AKT signaling pathway through the inhibition of PTEN [99]. Thus, exosomal miR-130a upon stimulation by Li+-containing bioactive glass is beneficial for communication between BMSCs and endothelial cells through paracrine secretion. Subsequently, it enhances angiogenesis by the activation of the PTEN/AKT signaling pathway, which can up-regulate HIF-1α expression and VEGF secretion [96].
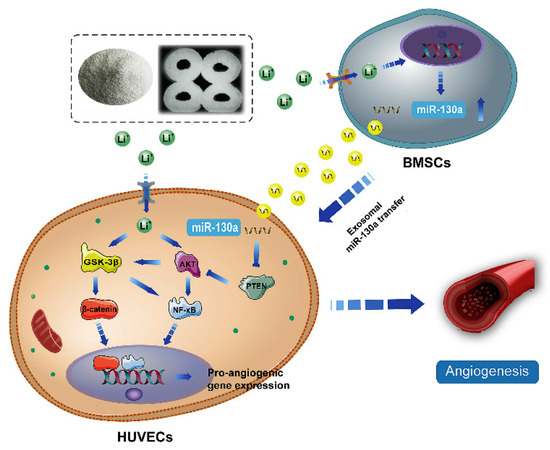
Figure 4.
The potential mechanism of exosomes derived BMSCs upon stimulation of Li+-containing biomaterials in promoting angiogenesis of endothelial cells [96]. Reprinted with permission from Elsevier.
4. Conclusions and Future Directions
During skeletal development and remodeling, exosomes derived from cells including immune cells and bone-forming-related cells, play crucial roles in regulating cell-to-cell communication and followed angiogenesis and osteogenesis. Moreover, metallic ions, either alone or incorporated with bone substitutes, have been proven to affect the amount or cargoes of exosomes, subsequent modification of cell adhesion, proliferation, and differentiation as well as bone regeneration (Table 3). Although limited studies have investigated the detailed mechanisms of the effects of metallic ions on angiogenesis and osteogenesis through their influence on exosome secretion, there is no doubt that metallic ions-mediated paracrine secretion-based methods present promising strategies for determining the angiogenic and osteogenic behavior of bone forming-related cells and the subsequent in vivo fate of bone implants. Future biomaterial designs should focus on modifying direct cell-material interactions as well as on generating a favorable microenvironment for enhancing cell behavior through paracrine communication. However, the underlying mechanism of exosome-mediated bone regeneration upon metallic ions stimulation remains unclear and require further study.

Table 3.
Exosomes derived from different cells upon metallic ions stimulation and their effects on bone repair.
For an investigation of the effect of metallic ions on exosome secretion, the following issues must be resolved: First, the number of exosomes obtained in cell culture mediums is always not sufficient. Two possible approaches are in use at present: one is to use commercial isolation kits to increase the rate of recovery from the culture medium; the other is to stimulate the secretion of cell-derived exosomes as much as possible. Thus, the modification of bone substitutes by the incorporation of metallic ions may be a promising method for the creation of favorable microenvironments that can enhance the amount and biofunction of cell-secreted exosomes. Second, since our understanding of the underlying mechanism of the effect of (implanted) metallic ion-mediated exosomes on bone regeneration is still in its infancy, more efforts should be devoted into investigating the mechanism by how chemical signals derived from biomaterials affect exosomes, and how these exosomes affect the subsequent immune reaction, angiogenesis, and osteogenesis. To achieve this, the types and release kinetics of the metallic ion-incorporated bone substitutes should be further investigated so that we can work toward fabricating tailor-made biomaterials that generate an ideal microenvironment, enhancing exosome biofunctions and bone regeneration. Moreover, investigations into the roles of metallic ion-incorporated bone substitutes in relation to exosome secretion are currently focused on bioglasses and other bioceramics. Metallic alloy- and polymer-based bone substitutes should also be taken into consideration in future studies. Moreover, since exosomes play an important role in cell-to-cell communications, the effects of metallic ion-mediated exosomes on the interactions among different cells should be further studied. We do not currently fully understand how metallic ions regulate cell response by paracrine secretion, or how these responses affect the subsequent bone formation; however, through careful research design, it will be possible to investigate the role of exosomes in inflammation, angiogenesis, and osteogenesis. Such research advances would enrich our understanding of the biological mechanism of metallic ion-mediated bone regeneration, and would be beneficial for the design of metallic ion-incorporated bone substitutes. Similarly, in addition to chemical composition, the surface structure and mechanical signals derived from biomaterials should be explored when investigating the stimulation effects on exosomes secretions. Finally, due to their low immune rejection and high therapeutic effects, metallic ion-stimulated functional exosomes can serve as potential drugs for various clinical diseases.
Author Contributions
Conceptualization and supervision, D.X. and G.W.; writing—original draft preparation, X.L.; investigation and graphics, C.Z. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (82002289), Natural Science Foundation of Sichuan Province (2022NSFSC0685, 2022NSFSC0609), the Medical Research Project Plan of Sichuan Province (S20012), the Medical Technology Project of Sichuan Provincial Health Commission (21PJ196), the College-City Cooperation Project of Nanchong City (20SXQT0335, 20SXQT0323).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lindsay, E.; Ce, A.C.A.; Copley, L. Proximal Femoral Unicameral Cyst Resolved by Single Injection of a Calcium Phosphate Bone Void Filler without Instrumentation. Int. J. Biol. Instrum. 2018, 1, 2631–5025. [Google Scholar] [CrossRef][Green Version]
- Hoppe, S.; Albers, C.E.; Elfiky, T.; Deml, M.C.; Milavec, H.; Bigdon, S.F.; Benneker, L.M. First Results of a New Vacuum Plasma Sprayed (VPS) Titanium-Coated Carbon/PEEK Composite Cage for Lumbar Interbody Fusion. J. Funct. Biomater. 2018, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Yang, Y.; Zheng, B.; Yan, F.; Wei, F.; Friis, T.E.; Crawford, R.W.; Xiao, Y. Alteration of clot architecture using bone substitute biomaterials (beta-tricalcium phosphate) significantly delays the early bone healing process. J. Mater. Chem. B 2018, 6, 8204–8213. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Y.; Yang, X.; He, J.; Zhong, Q.; Guo, X. The anti-inflammation effect of strontium ranelate on rat chondrocytes with or without IL-1β in vitro. Exp. Ther. Med. 2022, 23, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Yang, S.; Zeng, S.; Liu, X.; Zhang, J.; Zhang, J.; Wu, T.; Ye, X.; Yu, T.; Zhou, C.; et al. Enhanced angiogenesis of biodegradable iron-doped octacalcium phosphate/poly (lactic-co-glycolic acid) scaffold for potential cancerous bone regeneration. Appl. Mater. Today 2019, 15, 100–114. [Google Scholar] [CrossRef]
- Mehta, K.J. Role of iron and iron-related proteins in mesenchymal stem cells: Cellular and clinical aspects. J. Cell. Physiol. 2021, 236, 7266–7289. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Mao, X. Magnesium Enhances Osteogenesis of BMSCs by Tuning Osteoimmunomodulation. BioMed Res. Int. 2019, 2019, 7908205. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials Regulating Bone Hematoma for Osteogenesis. Adv. Health Mater. 2020, 9, 2000726. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S.J.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Phinney, D.; Pittenger, M.J.S.C. MSC-derived exosomes for cell-free therapy stem cells. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- He, N.; Zhang, Y.; Zhang, S.; Wang, D.; Ye, H. Exosomes: Cell-Free Therapy for Cardiovascular Diseases. J. Cardiovasc. Transl. Res. 2020, 13, 713–721. [Google Scholar] [CrossRef]
- Jia, Y.; Qiu, S.; Xu, J.; Kang, Q.; Chai, Y. Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction osteogenesis in older rats. Calcif. Tissue Int. 2020, 106, 509–517. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, S.; Li, Y. Exosomes Derived from M2 Macrophages Facilitate Osteogenesis and Reduce Adipogenesis of BMSCs. Front. Endocrinol. 2021, 12, 783. [Google Scholar] [CrossRef]
- Kang, M.; Huang, C.-C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone regeneration is mediated by macrophage extracellular vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Xiao, D.; Yang, F.; Zhao, Q.; Chen, S.; Shi, F.; Xiang, X.; Deng, L.; Sun, X.; Weng, J.; Feng, G. Fabrication of a Cu/Zn co-incorporated calcium phosphate scaffold-derived GDF-5 sustained release system with enhanced angiogenesis and osteogenesis properties. RSC Adv. 2018, 8, 29526–29534. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, F.; Xiao, D.; Zhao, Q.; Chen, S.; Liu, K.; Zhang, B.; Feng, G.; Duan, K. Repair of segmental rabbit radial defects with Cu/Zn co-doped calcium phosphate scaffolds incorporating GDF-5 carrier. RSC Adv. 2020, 10, 1901–1909. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, A.-T.; Wang, D.-D.; Lin, G.-F.; Liu, T.; He, F.-M. The effects of Sr-incorporated micro/nano rough titanium surface on rBMSC migration and osteogenic differentiation for rapid osteointegration. Biomater. Sci. 2018, 6, 1946–1961. [Google Scholar] [CrossRef]
- Liu, L.; Yu, F.; Li, L.; Zhou, L.; Zhou, T.; Xu, Y.; Lin, K.; Fang, B.; Xia, L. Bone marrow stromal cells stimulated by strontium-substituted calcium silicate ceramics: Release of exosomal miR-146a regulates osteogenesis and angiogenesis. Acta Biomater. 2020, 119, 444–457. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Alom, N.; Amer, M.; White, L.; Shakesheff, K.M.; Tatullo, M. Decellularized bone extracellular matrix and human dental pulp stem cells as a construct for bone regeneration. J. Biomater. Sci. Polym. Ed. 2017, 28, 730–748. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Eslaminejad, M.B. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Teo, K.; Zhang, S.; Chuah, S.; Lai, R.; Lim, S.; Toh, W. MSC exosomes alleviate osteoarthritis through restoration of matrix homeostasis. Cytotherapy 2019, 21, S52. [Google Scholar] [CrossRef]
- Norouzi-Barough, L.; Shirian, S.; Gorji, A.; Sadeghi, M. Therapeutic potential of mesenchymal stem cell-derived exosomes as a cell-free therapy approach for the treatment of skin, bone, and cartilage defects. Connect. Tissue Res. 2021, 63, 83–96. [Google Scholar] [CrossRef]
- Wang, Z.-G.; He, Z.-Y.; Liang, S.; Yang, Q.; Cheng, P.; Chen, A.-M. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 511. [Google Scholar] [CrossRef]
- Ji, L.; Bao, L.; Gu, Z.; Zhou, Q.; Liang, Y.; Zheng, Y.; Xu, Y.; Zhang, X.; Feng, X. Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells. Immunol. Res. 2019, 67, 432–442. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef]
- Domenis, R.; Cifù, A.; Quaglia, S.; Pistis, C.; Moretti, M.; Vicario, A.; Parodi, P.C.; Fabris, M.; Niazi, K.R.; Soon-Shiong, P.; et al. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci. Rep. 2018, 8, 13325. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Zhai, S.; Fu, Q.; Liu, D. Bone marrow mesenchymal stem cells-derived exosomal microRNA-150-3p promotes osteoblast proliferation and differentiation in osteoporosis. Hum. Gene Ther. 2021, 32, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ren, J.; Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2020, 78, 105946. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Luo, Y.; Wang, J.; Zhang, N.; Gu, C.; Li, L.; Qian, D.; Cai, W.; Fan, J.; Yin, G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J. Nanobiotechnol. 2020, 18, 47. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2018, 52, e12570. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, B.; Yin, P.; Zhao, L.; Wang, Y.; Fu, Z.; Dang, R.; Xu, J.; Zhang, J.; Wen, N. Integration of human umbilical cord mesenchymal stem cells-derived exosomes with hydroxyapatite-embedded hyaluronic acid-alginate hydrogel for bone regeneration. Acs. Biomater. Sci. Eng. 2020, 6, 1590–1602. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Luo, J.; Zhao, H.; Zhou, X.; Gu, Q.; Yang, H.; Zhu, X.; Cui, W.; Shi, Q. Immunopolarization-regulated 3D printed-electrospun fibrous scaffolds for bone regeneration. Biomaterials 2021, 276, 121037. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Yin, Y.; Wu, R.-X.; Xu, X.-Y.; Chen, F.-M. The effects of conditioned media generated by polarized macrophages on the cellular behaviours of bone marrow mesenchymal stem cells. J. Cell. Mol. Med. 2017, 22, 1302–1315. [Google Scholar] [CrossRef]
- Xia, Y.; He, X.-T.; Xu, X.-Y.; Tian, B.-M.; An, Y.; Chen, F.-M. Exosomes derived from M0, M1 and M2 macrophages exert distinct influences on the proliferation and differentiation of mesenchymal stem cells. PeerJ 2020, 8, e8970. [Google Scholar] [CrossRef]
- Xiong, Y.; Chen, L.; Yan, C.; Zhou, W.; Yu, T.; Sun, Y.; Cao, F.; Xue, H.; Hu, Y.; Chen, D.; et al. M2 Macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J. Nanobiotechnol. 2020, 18, 66. [Google Scholar] [CrossRef]
- Yu, L.; Hu, M.; Cui, X.; Bao, D.; Luo, Z.; Li, D.; Li, L.; Liu, N.; Wu, Y.; Luo, X.; et al. M1 macrophage-derived exosomes aggravate bone loss in postmenopausal osteoporosis via a microRNA-98/DUSP1/JNK axis. Cell Biol. Int. 2021, 45, 2452–2463. [Google Scholar] [CrossRef]
- Chen, K.; Jiao, Y.; Liu, L.; Huang, M.; He, C.; He, W.; Hou, J.; Yang, M.; Luo, X.; Li, C. Communications Between Bone Marrow Macrophages and Bone Cells in Bone Remodeling. Front. Cell Dev. Biol. 2020, 8, 1608. [Google Scholar] [CrossRef]
- Weilner, S.; Keider, V.; Winter, M.; Harreither, E.; Salzer, B.; Weiss, F.; Schraml, E.; Messner, P.; Pietschmann, P.; Hildner, F.; et al. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging 2016, 8, 16–30. [Google Scholar] [CrossRef]
- Stukes, S.; Coelho, C.; Rivera, J.; Jedlicka, A.E.; Hajjar, K.A.; Casadevall, A. The Membrane Phospholipid Binding Protein Annexin A2 Promotes Phagocytosis and Nonlytic Exocytosis of Cryptococcus neoformans and Impacts Survival in Fungal Infection. J. Immunol. 2016, 197, 1252–1261. [Google Scholar] [CrossRef]
- Osawa, S.; Kurachi, M.; Yamamoto, H.; Yoshimoto, Y.; Ishizaki, Y. Fibronectin on extracellular vesicles from microvascular endothelial cells is involved in the vesicle uptake into oligodendrocyte precursor cells. Biochem. Biophys. Res. Commun. 2017, 488, 232–238. [Google Scholar] [CrossRef]
- Rivera, L.R.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact. Mater. 2020, 6, 1479–1490. [Google Scholar] [CrossRef]
- Han, H.; Jun, I.; Seok, H.; Lee, K.; Lee, K.; Witte, F.; Mantovani, D.; Kim, Y.; Glyn-Jones, S.; Edwards, J.R. Biodegradable Magnesium Alloys Promote Angio-Osteogenesis to Enhance Bone Repair. Adv. Sci. 2020, 7, 2000800. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, X.; Wang, Y.; Li, M.; Li, Y.; Liu, X.; Zhang, X.; Lan, Z.; Wang, J.; Du, Y.; et al. Zn/Sr dual ions-collagen co-assembly hydroxyapatite enhances bone regeneration through procedural osteo-immunomodulation and osteogenesis. Bioact. Mater. 2021, 10, 195–206. [Google Scholar] [CrossRef]
- Rosanoff, A.; Weaver, C.M.; Rude, R.K. Suboptimal magnesium status in the United States: Are the health consequences underestimated? Nutr. Rev. 2012, 70, 153–164. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and Hormonal Effects of Magnesium Deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, J.; Zhang, X.; Liang, G.; Xu, T.; Niu, W. Three-dimensional Printed Mg-Doped β-TCP Bone Tissue Engineering Scaffolds: Effects of Magnesium Ion Concentration on Osteogenesis and Angiogenesis In Vitro. Tissue Eng. Regen. Med. 2019, 16, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, S.; Cheng, L.; Lin, Z.; Zeng, M.; Ruan, Z.; Sun, B.; Luo, Z.; Tang, Y.; Long, H. Mg2+-mediated autophagy-dependent polarization of macrophages mediates the osteogenesis of bone marrow stromal stem cells by interfering with macrophage-derived exosomes containing miR-381. J. Orthop. Res. 2021, 40, 1563–1576. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Yu, Z.; Chen, J.; She, X.; Li, P.; Liu, C.; Zhang, Y.; Feng, J.; Fu, H.; et al. Low expression of miR-381 is a favorite prognosis factor and enhances the chemosensitivity of osteosarcoma. Oncotarget 2016, 7, 68585–68596. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, W.; Zhao, W.; Wang, W.; Zhang, D.; Wu, P. miR-381 inhibited breast cancer cells proliferation, epithelial-to-mesenchymal transition and metastasis by targeting CXCR4. Biomed. Pharmacother. 2017, 86, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Zhu, Y.; Lin, Z.; Wan, J.; Cheng, L.; Zeng, M.; Tang, Y.; Zhao, R. miR-381 modulates human bone mesenchymal stromal cells (BMSCs) osteogenesis via suppressing Wnt signaling pathway during atrophic nonunion development. Cell Death Dis. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-dependent osteogenic and angiogenic biological performances of calcium phosphate cement modified with copper ions. Mater. Sci. Eng. C 2019, 99, 1199–1212. [Google Scholar] [CrossRef]
- Li, X.; Xiao, D.; Zhao, Q.; Chen, S.; Bai, Y.; Liu, K.; Gang, F.; Ke, D. Preparation and properties of copper-loaded antibacterial functional film on titanium surface. Chin. J. Tissue Eng. Res. 2021, 25, 553. [Google Scholar]
- Ryan, E.J.; Ryan, A.J.; González-Vázquez, A.; Philippart, A.; Ciraldo, F.E.; Hobbs, C.; Nicolosi, V.; Boccaccini, A.R.; Kearney, C.J.; O’Brien, F.J. Collagen scaffolds functionalised with copper-eluting bioactive glass reduce infection and enhance osteogenesis and angiogenesis both in vitro and in vivo. Biomaterials 2019, 197, 405–416. [Google Scholar] [CrossRef]
- Zhao, S.; Li, L.; Wang, H.; Zhang, Y.; Cheng, X.; Zhou, N.; Rahaman, M.N.; Liu, Z.; Huang, W.; Zhang, C. Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 2015, 53, 379–391. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y.; Zhao, Y.; Zhang, Y.; Yao, X.; Hang, R. Exosomes secreted by macrophages upon copper ion stimulation can promote angiogenesis. Mat. Sci. Eng. C 2021, 123, 111981. [Google Scholar] [CrossRef]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef]
- Chen, X.; Gu, S.; Chen, B.-F.; Shen, W.-L.; Yin, Z.; Xu, G.-W.; Hu, J.-J.; Zhu, T.; Li, G.; Wan, C.; et al. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials 2015, 53, 239–250. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2011, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuen, J.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials 2015, 61, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, Y.; Zhang, Y.; Hang, R.; Yao, X.; Hang, R. Exosomes derived from macrophages upon cobalt ion stimulation promote angiogenesis. Colloids Surf. B Biointerfaces 2021, 203, 111742. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Esumi, H.J.A.B.P. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50, 49–59. [Google Scholar] [CrossRef]
- Umezu, T.; Tadokoro, H.; Azuma, K.; Yoshizawa, S.; Ohyashiki, K.; Ohyashiki, J.H. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 2014, 124, 3748–3757. [Google Scholar] [CrossRef]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022; in press. [Google Scholar] [CrossRef]
- Maeno, S.; Niki, Y.; Matsumoto, H.; Morioka, H.; Yatabe, T.; Funayama, A.; Toyama, Y.; Taguchi, T.; Tanaka, J. The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 2005, 26, 4847–4855. [Google Scholar] [CrossRef]
- Aquino-Martínez, R.; Angelo, A.P.; Pujol, F.V. Calcium-containing scaffolds induce bone regeneration by regulating mesenchymal stem cell differentiation and migration. Stem Cell Res. Ther. 2017, 8, 1–10. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, J.; Wu, X.; Shi, J.; Liu, C.; Jia, J.; Dai, C.; Gan, Q. The bio-functional role of calcium in mesoporous silica xerogels on the responses of osteoblasts in vitro. J. Mater. Sci. Mater. Electron. 2010, 21, 2175–2185. [Google Scholar] [CrossRef]
- Vuornos, K.; Ojansivu, M.; Koivisto, J.T.; Häkkänen, H.; Belay, B.; Montonen, T.; Huhtalag, H.; Kääriäinenh, M.; Hupai, L.; Kellomäkic, M.; et al. Bioactive glass ions induce efficient osteogenic differentiation of human adipose stem cells encapsulated in gellan gum and collagen type I hydrogels. Mat. Sci. Eng. C 2019, 99, 905–918. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Z.; Kong, L.; He, Y.; Chan, H.F.; Li, H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials 2020, 256, 120216. [Google Scholar] [CrossRef]
- Wu, Z.; He, D.; Li, H. Bioglass enhances the production of exosomes and improves their capability of promoting vascularization. Bioact. Mater. 2021, 6, 823–835. [Google Scholar] [CrossRef]
- Guo, B.B.; Bellingham, S.A.; Hill, A.F. The Neutral Sphingomyelinase Pathway Regulates Packaging of the Prion Protein into Exosomes. J. Biol. Chem. 2015, 290, 3455–3467. [Google Scholar] [CrossRef]
- Tsuboi, T.; Fukuda, M. Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J. Cell Sci. 2006, 119, 2196–2203. [Google Scholar] [CrossRef]
- Yan, X.C.; Cao, J.; Liang, L.; Wang, L.; Gao, F.; Yang, Z.Y.; Duan, J.; Chang, T.; Deng, S.; Liu, Y.; et al. miR-342-5p is a Notch downstream molecule and regulates multiple angiogenic pathways including Notch, vascular endothelial growth factor and transforming growth factor β signaling. J. Am. Heart Assoc. 2016, 5, e003042. [Google Scholar] [CrossRef]
- Barnett, J.M.; Suarez, S.; Mccollum, G.W.; Penn, J.S. Endoglin Promotes Angiogenesis in Cell- and Animal-Based Models of Retinal Neovascularization. Investig. Opthalmology Vis. Sci. 2014, 55, 6490–6498. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, G.; Niu, L.; Zhao, S.; Li, J.; Zhang, Z.; Jiang, H.; Zhang, Q.; Wang, H.; Sun, P.; et al. Exosomal MiR-1290 Promotes Angiogenesis of Hepatocellular Carcinoma via Targeting SMEK1. J. Oncol. 2021, 2021, 6617700. [Google Scholar] [CrossRef]
- Shyong, Y.J.; Chang, K.C.; Lin, F.H. Calcium phosphate particles stimulate exosome secretion from phagocytes for the enhancement of drug delivery. Colloid. Surface. B Biointerfaces 2018, 171, 391–397. [Google Scholar] [CrossRef]
- Singhto, N.; Thongboonkerd, V. Exosomes derived from calcium oxalate-exposed macrophages enhance IL-8 production from renal cells, neutrophil migration and crystal invasion through extracellular matrix. J. Proteom. 2018, 185, 64–76. [Google Scholar] [CrossRef]
- Singhto, N.; Kanlaya, R.; Nilnumkhum, A.; Thongboonkerd, V. Roles of macrophage exosomes in immune response to calcium oxalate monohydrate crystals. Front. Immunol. 2018, 9, 316. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human mesenchymal stem cells differentiate into an osteogenic lineage in presence of strontium containing bioactive glass nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Z.; Wu, K.; Bai, Y.; Lin, X.; Yang, H.; Yang, Q.; Wang, Z.; Ni, X.; Liu, H.; et al. Strontium–calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J. Mater. Chem. B 2021, 9, 5982–5997. [Google Scholar] [CrossRef]
- Zhao, F.; Lei, B.; Li, X.; Mo, Y.; Wang, R.; Chen, D.; Chen, X. Promoting in vivo early angiogenesis with sub-micrometer strontium-contained bioactive microspheres through modulating macrophage phenotypes. Biomaterials 2018, 178, 36–47. [Google Scholar] [CrossRef]
- Xu, M.; Su, X.; Xiao, X.; Yu, H.; Li, X.; Keating, A.; Wang, S.; Zhao, R.C. Hydrogen Peroxide-Induced Senescence Reduces the Wound Healing-Promoting Effects of Mesenchymal Stem Cell-Derived Exosomes Partially via miR-146a. Aging Dis. 2021, 12, 102–115. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Wei, X.; Li, H.; Yu, Z.; Zhang, H.; Liu, W. LPS induces HUVEC angiogenesis in vitro through miR-146a-mediated TGF-β1 inhibition. Am. J. Transl. Res. 2017, 9, 591–600. [Google Scholar]
- Ahmed, S.; Bradshaw, A.-D.; Gera, S.; Dewan, M.Z.; Xu, R. The TGF-β/Smad4 Signaling Pathway in Pancreatic Carcinogenesis and Its Clinical Significance. J. Clin. Med. 2017, 6, 5. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhou, J.; Shen, F.; Shi, X.; Chen, Y. CircATRNL1 activates Smad4 signaling to inhibit angiogenesis and ovarian cancer metastasis via miR-378. Mol. Oncol. 2020, 15, 1217–1233. [Google Scholar] [CrossRef]
- Seo, H.-H.; Lee, S.-Y.; Lee, C.Y.; Kim, R.; Kim, P.; Oh, S.; Lee, H.; Lee, M.Y.; Kim, J.; Kim, L.K.; et al. Exogenous miRNA-146a Enhances the Therapeutic Efficacy of Human Mesenchymal Stem Cells by Increasing Vascular Endothelial Growth Factor Secretion in the Ischemia/Reperfusion-Injured Heart. J. Vasc. Res. 2017, 54, 100–108. [Google Scholar] [CrossRef]
- Zamani, A.; Omrani, G.R.; Nasab, M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, J.; Guan, J.; Hu, B.; Niu, X.; Jin, D.; Wang, Y.; Zhang, C. Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3β-dependent β-catenin/Wnt pathway activation. FEBS J. 2014, 281, 5371–5389. [Google Scholar] [CrossRef]
- Kim, Y.S.; Noh, M.Y.; Kim, J.Y.; Yu, H.-J.; Kim, K.S.; Kim, S.H.; Koh, S.-H. Direct GSK-3β Inhibition Enhances Mesenchymal Stromal Cell Migration by Increasing Expression of Beta-PIX and CXCR4. Mol. Neurobiol. 2013, 47, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, X.; Yang, Z.; Wang, C.; Wei, Z.; Kang, P. Enhanced bone defect repairing effects in glucocorticoid-induced osteonecrosis of the femoral head using a porous nano-lithium-hydroxyapatite/gelatin microsphere/erythropoietin composite scaffold. Biomater. Sci. 2018, 6, 519–537. [Google Scholar] [CrossRef] [PubMed]
- E Kast, R. How lithium treatment generates neutrophilia by enhancing phosphorylation of GSK-3, increasing HIF-1 levels and how this path is important during engraftment. Bone Marrow Transplant. 2007, 41, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, L.; Wang, Y.; Lin, K.; Liu, J. Lithium-containing bioactive glasses enhanced 3D-printed PLGA scaffolds for bone regeneration in diabetes. Compos. Part B-Eng. 2022, 230, 109550. [Google Scholar] [CrossRef]
- Haro Durand, L.A.; Vargas, G.E.; Vera-Mesones, R.; Baldi, A.; Zago, M.P.; Fanovich, M.A.; Boccaccini, A.R.; Gorustovich, A. In vitro human umbilical vein endothelial cells response to ionic dissolution products from lithium-containing 45S5 bioactive glass. Materials 2017, 10, 740. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Feng, C.; Chang, J.; Fu, R.; Wu, T.; Yu, F.; Wang, X.; Xia, L.; Wu, C.; et al. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials 2019, 192, 523–536. [Google Scholar] [CrossRef]
- Xu, Q.; Meng, S.; Liu, B.; Li, M.Q.; Li, Y.; Fang, L.; Li, Y.G. MicroRNA-130a regulates autophagy of endothelial progenitor cells through Runx3. Clin. Exp. Pharmacol. Physiol. 2014, 41, 351–357. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, Y.D.; Choi, Y.S.; Lee, Y.M. Targeting of RUNX3 by miR-130a and miR-495 cooperatively increases cell proliferation and tumor angiogenesis in gastric cancer cells. Oncotarget 2015, 6, 33269–33278. [Google Scholar] [CrossRef]
- Zheng, T.; Shi, Y.; Zhang, J.; Peng, J.; Zhang, X.; Chen, K.; Chen, Y.; Liu, L. MiR-130a exerts neuroprotective effects against ischemic stroke through PTEN/PI3K/AKT pathway. Biomed. Pharmacother. 2019, 117, 109117. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).