Evaluation of Bone Response to a Nano HA Implant Surface on Sinus Lifting Procedures: Study in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Implant Surfaces Preparation
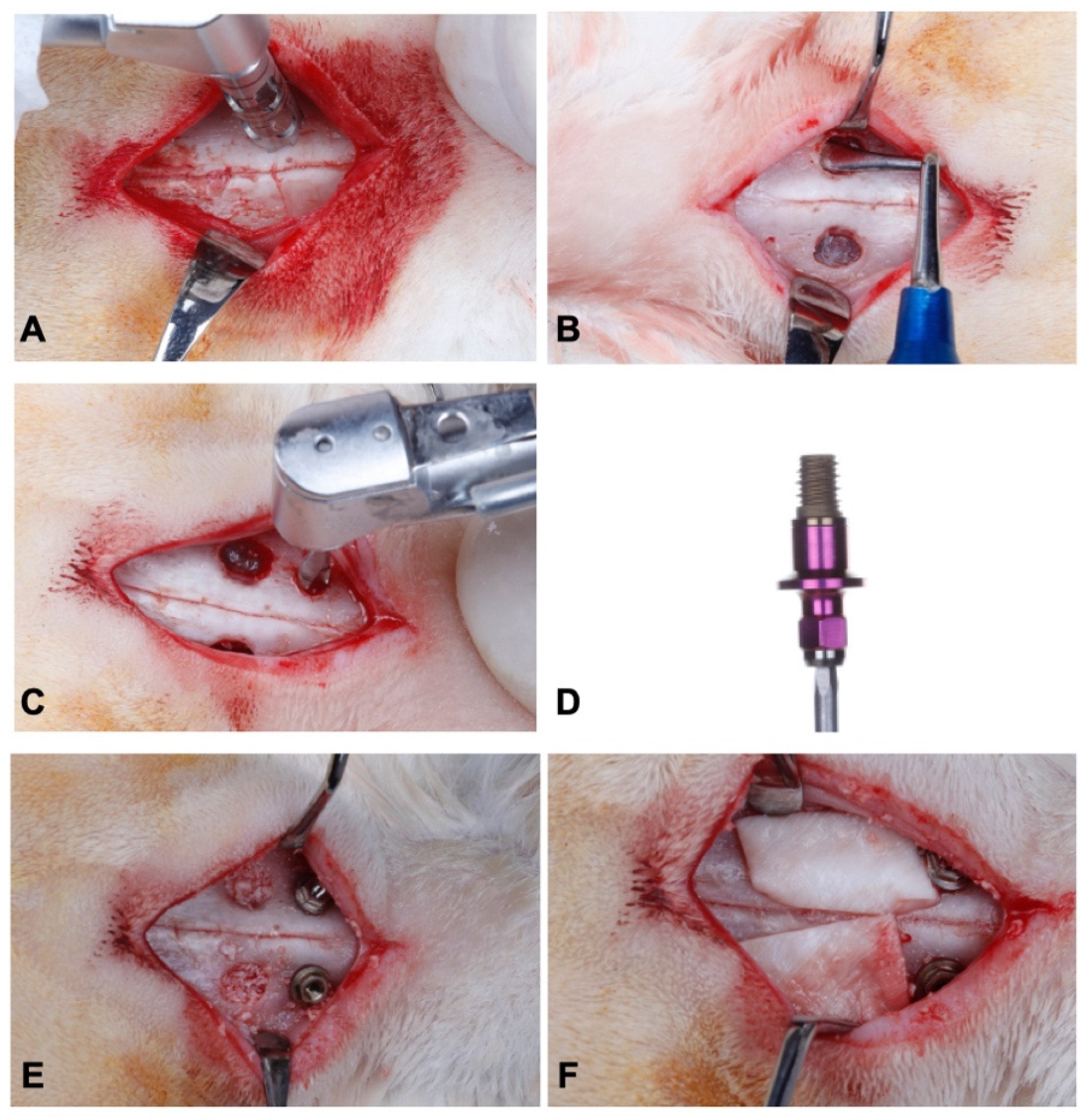
2.3. Sinus Lift and Installation of Implants
2.4. Microtomographic Analysis
2.5. Histomorphometric Analysis
2.6. Statistical Analysis
3. Results
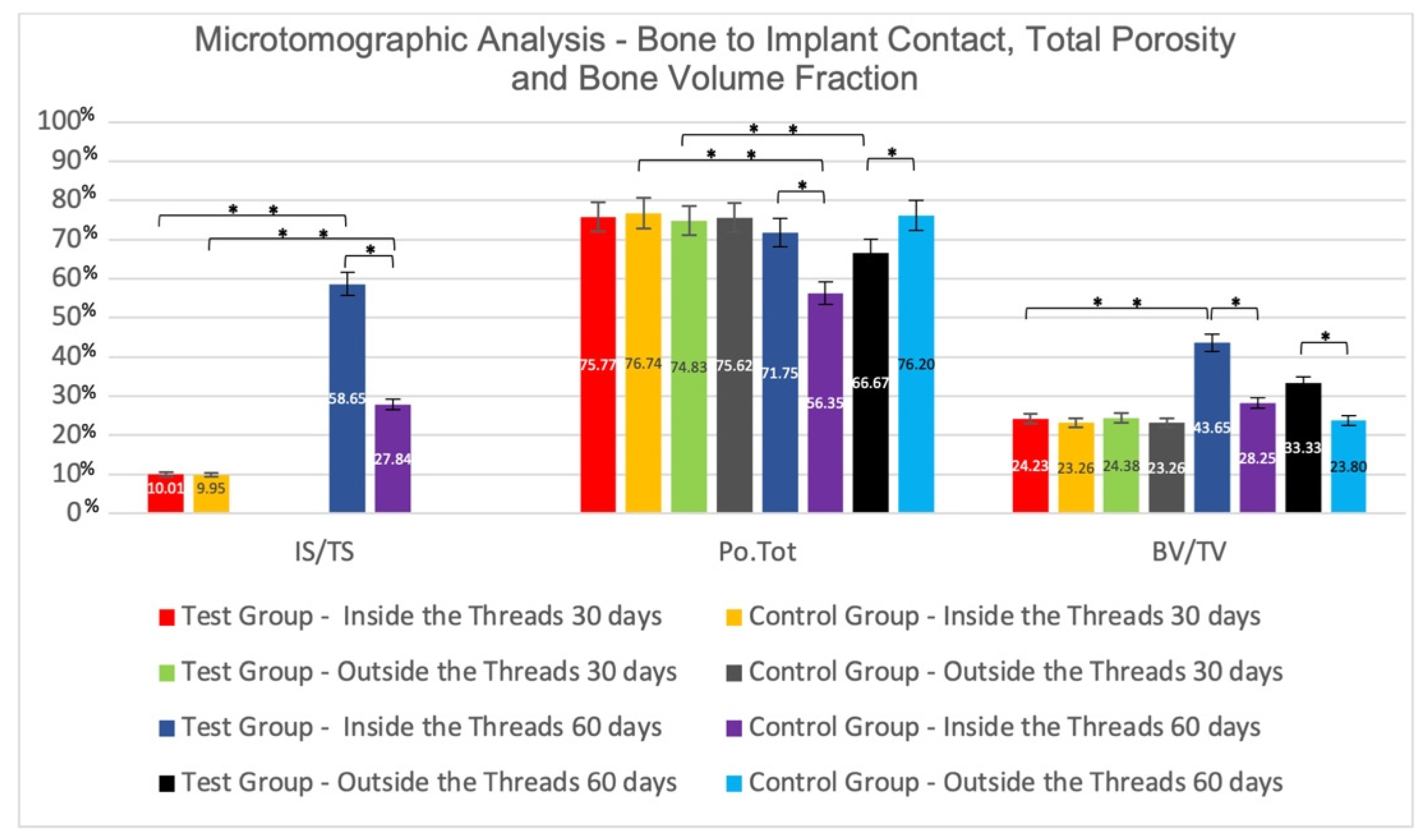
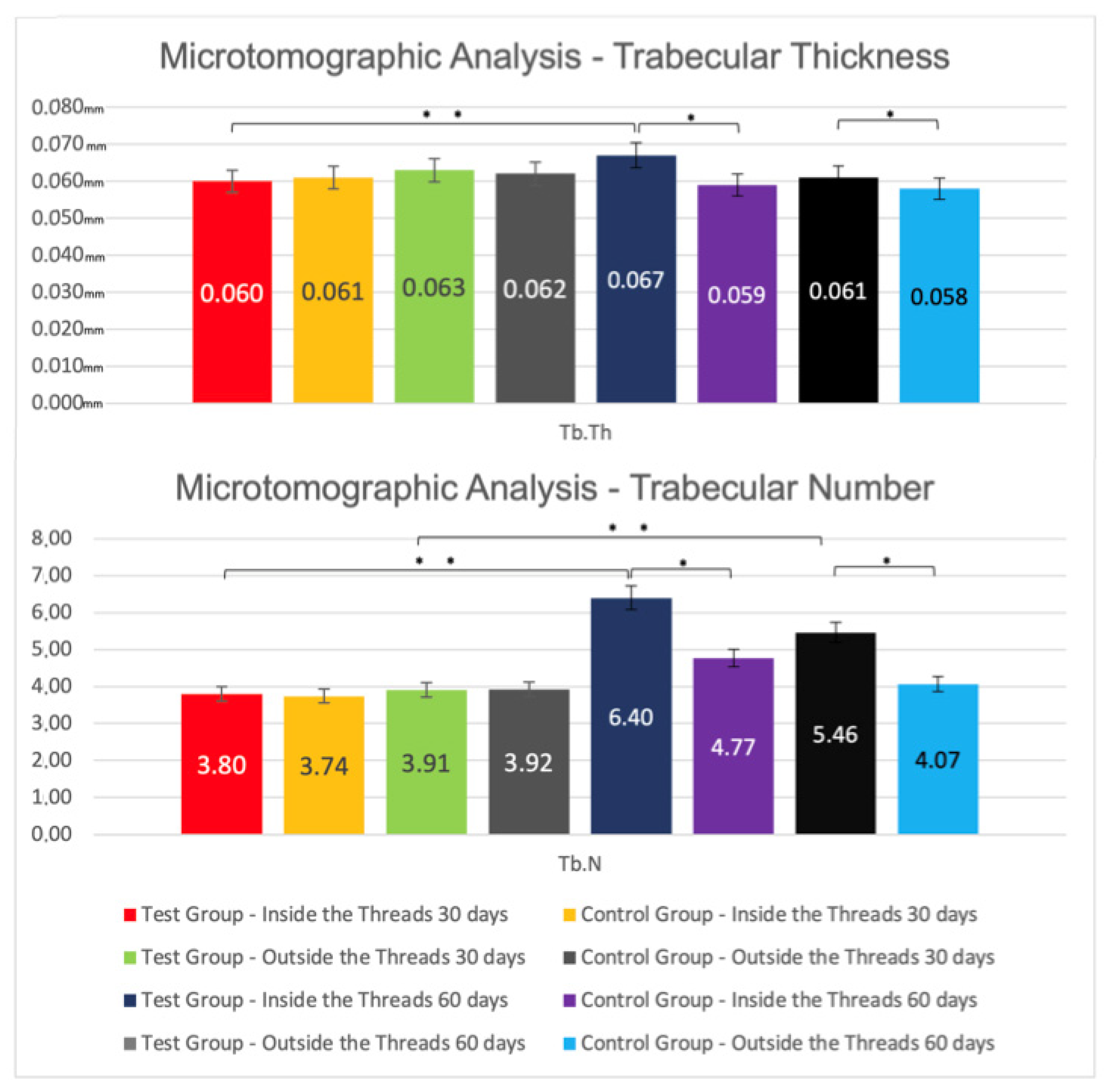
3.1. Microtomographic Results
Inter-Group Analysis
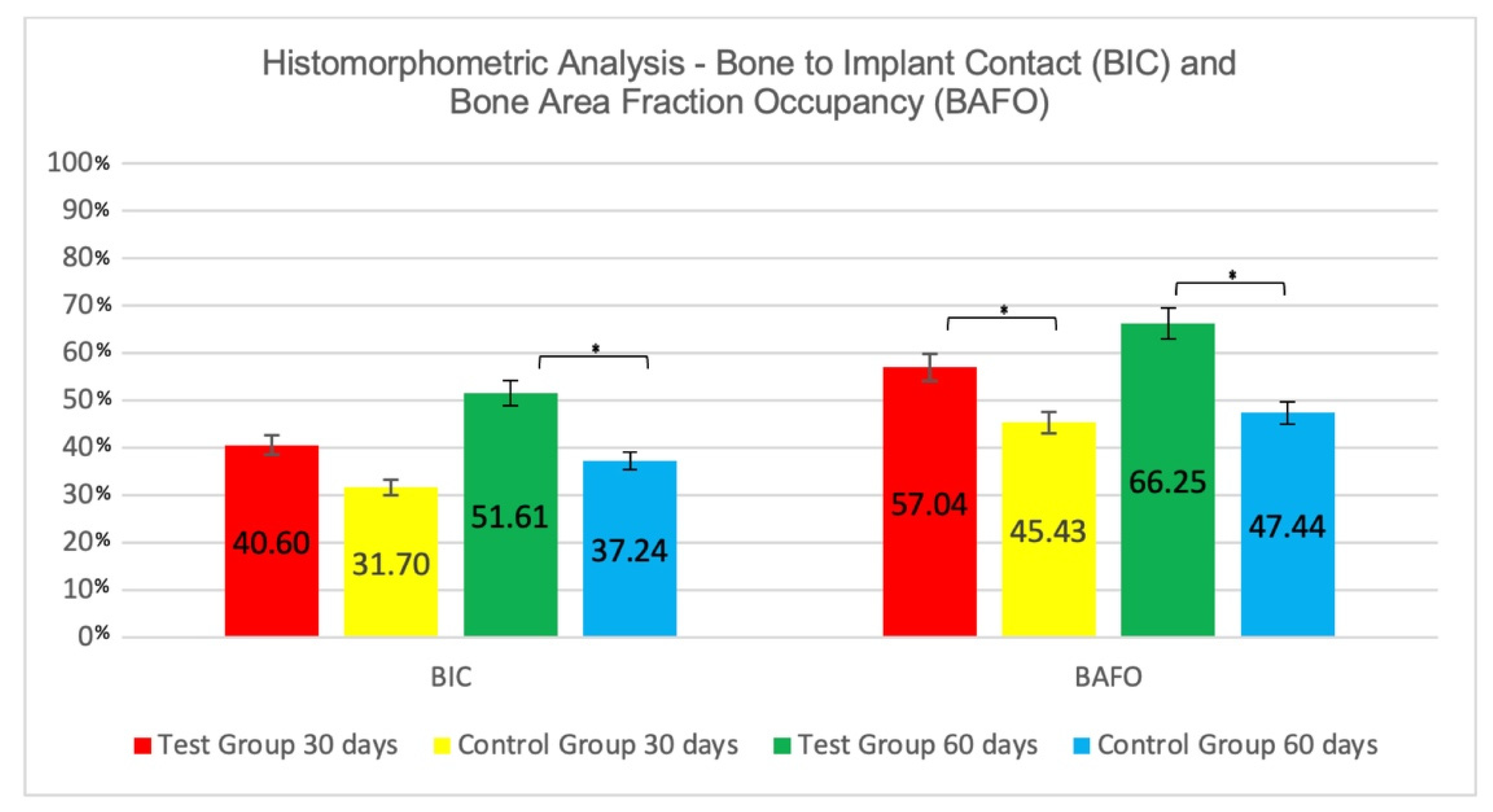
3.2. Histomorphometric Results
3.2.1. Intra-Group Analysis
3.2.2. Inter-Group Analysis
3.3. Analysis of Pre-Existing Cortical Bone versus Grafted Bone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlegel, K.A.; Prechtl, C.; Möst, T.; Seidl, C.; Lutz, R.; Von Wilmowsky, C. Osseointegration of SLActive implants in diabetic pigs. Clin. Oral Implant. Res. 2013, 24, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Donnell, A.M.; Kaval, N.; Al-Rjoub, M.F.; Augsburger, J.J.; Banerjee, R.K. Improved design and characterization of PLGA/PLA-coated Chitosan based micro-implants for controlled release of hydrophilic drugs. Int. J. Pharm. 2018, 547, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; De Groot, K.; Hunziker, E.B. Osteoinductive Implants: The Mise-en-scène for Drug-Bearing Biomimetic Coatings. Ann. Biomed. Eng. 2004, 32, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R. Osseointegration of metallic devices: Current trends based on implant hardware design. Arch. Biochem. Biophys. 2014, 561, 99–108. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; de Souza, S.L.; de Barros, R.R.; Pereira, K.K.; Iezzi, G.; Piattelli, A. Influence of implant surfaces on osseointegration. Braz. Dent. J. 2010, 21, 471–481. [Google Scholar] [CrossRef]
- Elias, C.N.; Meirelles, L. Improving osseointegration of dental implants. Expert Rev. Med. Devices 2010, 7, 241–256. [Google Scholar] [CrossRef]
- Brunette, D.M.; Chehroudi, B. The Effects of the Surface Topography of Micromachined Titanium Substrata on Cell Behavior in Vitro and in Vivo. J. Biomech. Eng. 1999, 121, 49–57. [Google Scholar] [CrossRef]
- Braceras, I.; De Maeztu, M.; Alava, J.; Gay-Escoda, C. In vivo low-density bone apposition on different implant surface materials. Int. J. Oral Maxillofac. Surg. 2009, 38, 274–278. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20, 172–184. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Wennerberg, A.; Albrektsson, T. Current challenges in successful rehabilitation with oral implants. J. Oral Rehabil. 2010, 38, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 2--Review focusing on clinical knowledge of different surfaces. Int. J. Prosthodont. 2004, 17, 544–564. [Google Scholar] [PubMed]
- Albrektsson, T.; Wennerberg, A. Oral implant surfaces: Part 1--Review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int. J. Prosthodont. 2004, 17, 536–543. [Google Scholar] [PubMed]
- Coelho, P.G.; Freire, J.N.; Granato, R.; Marin, C.; Bonfante, A.E.; Gil, J.N.; Chuang, S.-K.; Suzuki, M. Bone mineral apposition rates at early implantation times around differently prepared titanium surfaces: A study in beagle dogs. Int. J. Oral Maxillofac. Implant. 2011, 26, 63–69. [Google Scholar]
- Coelho, P.G.; Takayama, T.; Yoo, D.; Jimbo, R.; Karunagaran, S.; Tovar, N.; Janal, M.N.; Yamano, S. Nanometer-scale features on micrometer-scale surface texturing: A bone histological, gene expression, and nanomechanical study. Bone 2014, 65, 25–32. [Google Scholar] [CrossRef]
- Webster, T.J.; Ahn, E.S. Nanostructured Biomaterials for Tissue Engineering Bone. Adv. Biochem. Eng. Biotechnol. 2007, 103, 275–308. [Google Scholar] [CrossRef]
- Albrektsson, T.; Wennerberg, A. On osseointegration in relation to implant surfaces. Clin. Implant. Dent. Relat. Res. 2019, 21, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano hydroxyapatite structures influence early bone formation. J. Biomed. Mater. Res. A 2008, 87, 299–307. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Xia, W.; Wang, Z.; Zhang, P.; Xia, L.; Lin, K.; Zhu, M. Nano-Structure Designing Promotion Osseointegration of Hydroxyapatite Coated Ti–6Al–4V Alloy Implants in Diabetic Model. J. Biomed. Nanotechnol. 2019, 15, 1701–1713. [Google Scholar] [CrossRef]
- Tsukimura, N.; Yamada, M.; Iwasa, F.; Minamikawa, H.; Att, W.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.-A.; Ogawa, T. Synergistic effects of UV photofunctionalization and micro-nano hybrid topography on the biological properties of titanium. Biomaterials 2011, 32, 4358–4368. [Google Scholar] [CrossRef]
- Jiang, N.; Guo, Z.; Sun, D.; Ay, B.; Li, Y.; Yang, Y.; Tan, P.; Zhang, L.; Zhu, S. Exploring the mechanism behind improved osteointegration of phosphorylated titanium implants with hierarchically structured topography. Colloids Surf. B Biointerfaces 2019, 184, 110520. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Xue, Y.; Hayashi, M.; Schwartz-Filho, H.O.; Andersson, M.; Mustafa, K.; Wennerberg, A. Genetic Responses to Nanostructured Calcium-phosphate-coated Implants. J. Dent. Res. 2011, 90, 1422–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimbo, R.; Sotres, J.; Johansson, C.; Breding, K.; Currie, F.; Wennerberg, A.; Jimbo, R.; Sotres, J.; Johansson, C.; Breding, K.; et al. The biological response to three different nanostructures applied on smooth implant surfaces. Clin. Oral Implant. Res. 2011, 23, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.G.F.P.; Coelho, P.G.; Bergamo, E.T.P.; Witek, L.; Borges, C.A.; Bezerra, F.B.; Novaes, J.A.B.; Souza, S.L.S. Histological and Nanomechanical Properties of a New Nanometric Hydroxiapatite Implant Surface. An In Vivo Study in Diabetic Rats. Materials 2020, 13, 5693. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.F.P.; Soares, M.S.D.M.; e Souza, A.M.M.S.; Taba, M., Jr.; Palioto, D.B.; Messora, M.R.; Ghiraldini, B.; Nunes, F.A.D.S.; de Souza, S.L.S. Influence of nano-hydroxyapatite coating implants on gene expression of osteogenic markers and micro-CT parameters. An in vivo study in diabetic rats. J. Biomed. Mater. Res. A 2020, 109, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Browaeys, H.; Bouvry, P.; De Bruyn, H. A Literature Review on Biomaterials in Sinus Augmentation Procedures. Clin. Implant Dent. Relat. Res. 2007, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J. Cranio-Maxillofac. Surg. 2012, 40, 321–328. [Google Scholar] [CrossRef]
- Palma, V.C.; Magro-Filho, O.; De Oliveria, J.A.; Lundgren, S.; Salata, L.A.; Sennerby, L. Bone Reformation and Implant Integration following Maxillary Sinus Membrane Elevation: An Experimental Study in Primates. Clin. Implant Dent. Relat. Res. 2006, 8, 11–24. [Google Scholar] [CrossRef]
- Srouji, S.; Ben-David, D.; Lotan, R.; Riminucci, M.; Livne, E.; Bianco, P. The innate osteogenic potential of the maxillary sinus (Schneiderian) membrane: An ectopic tissue transplant model simulating sinus lifting. Int. J. Oral Maxillofac. Surg. 2010, 39, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Srouji, S.; Kizhner, T.; Ben David, D.; Riminucci, M.; Bianco, P.; Livne, E. The Schneiderian Membrane Contains Osteoprogenitor Cells: In Vivo and In Vitro Study. Calcif. Tissue Int. 2009, 84, 138–145. [Google Scholar] [CrossRef]
- Pérez-Martínez, S.; Martorell-Calatayud, L.; Penarrocha-Oltra, D.; García-Mira, B.; Penarrocha-Diago, M. Indirect sinus lift without bone graft material: Systematic review and meta-analysis. J. Clin. Exp. Dent. 2015, 7, e316–e319. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Cha, J.; Lim, H.; Lee, J.; Choi, S.; Jung, U. De novo bone formation underneath the sinus membrane supported by a bone patch: A pilot experiment in rabbit sinus model. Clin. Oral Implant. Res. 2016, 28, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Yoon, S.-R.; Cha, J.-K.; Lim, H.-C.; Lee, J.-S.; Choi, S.-H.; Jung, U.-W. Sinus floor elevation using implants coated with recombinant human bone morphogenetic protein-2: Micro-computed tomographic and histomorphometric analyses. Clin. Oral Investig. 2018, 22, 829–837. [Google Scholar] [CrossRef]
- Choi, Y.; Yun, J.-H.; Kim, C.-S.; Choi, S.-H.; Chai, J.-K.; Jung, U.-W. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli -expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: A radiographic and histologic analysis. Clin. Oral Implant. Res. 2012, 23, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.-S.; Kim, Y.-J.; Kim, M.-S.; Choi, S.-H.; Cho, K.-S.; Jung, U.-W. Recombinant Human Bone Morphogenetic Protein-2 Stimulates the Osteogenic Potential of the Schneiderian Membrane: A Histometric Analysis in Rabbits. Tissue Eng. A 2013, 19, 1994–2004. [Google Scholar] [CrossRef]
- Coelho, P.G.; Marin, C.; Granato, R.; Giro, G.; Suzuki, M.; Bonfante, E.A. Biomechanical and histologic evaluation of non-washed resorbable blasting media and alumina-blasted/acid-etched surfaces. Clin. Oral Implant. Res. 2012, 23, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Gil, L.; Sarendranath, A.; Neiva, R.; Marão, H.; Tovar, N.; Bonfante, E.; Janal, M.; Castellano, A.; Coelho, P. Bone Healing Around Dental Implants: Simplified vs Conventional Drilling Protocols at Speed of 400 rpm. Int. J. Oral Maxillofac. Implant. 2017, 32, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Jinno, Y.; Jimbo, R.; Tovar, N.; Teixeira, H.S.; Witek, L.; Coelho, P.G. In Vivo Evaluation of Dual Acid-Etched and Grit-Blasted/Acid-Etched Implants with Identical Macrogeometry in High-Density Bone. Implant Dent. 2017, 26, 815–819. [Google Scholar] [CrossRef]
- Stellino, G.; Landi, L. A 6-year unloaded hydroxyapatite-coated dental implant placed into an extraction socket in conjunction with nonresorbable hydroxyapatite grafting material: Histologic evaluation. Int. J. Periodontics Restor. Dent. 2002, 22, 575–581. [Google Scholar]
- Mendonça, G.; Mendonça, D.B.; Aragão, F.J.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef]
- Jimbo, R.; Coelho, P.; Bryington, M.; Baldassarri, M.; Tovar, N.; Currie, F.; Hayashi, M.; Janal, M.; Andersson, M.; Ono, D.; et al. Nano Hydroxyapatite-coated Implants Improve Bone Nanomechanical Properties. J. Dent. Res. 2012, 91, 1172–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bougas, K.; Jimbo, R.; VanDeWeghe, S.; Hayashi, M.; Bryington, M.; Kozai, Y.; Schwartz-Filho, H.; Tovar, N.; Adolfsson, E.; Ono, D.; et al. Bone apposition to laminin-1 coated implants: Histologic and 3D evaluation. Int. J. Oral Maxillofac. Surg. 2013, 42, 677–682. [Google Scholar] [CrossRef]
- Coelho, P.G.; Bonfante, E.A.; Pessoa, R.S.; Marin, C.; Granato, R.; Giro, G.; Witek, L.; Suzuki, M. Characterization of Five Different Implant Surfaces and Their Effect on Osseointegration: A Study in Dogs. J. Periodontol. 2011, 82, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Granato, R.; Marin, C.; Bonfante, E.A.; Janal, M.N.; Suzuki, M. Biomechanical and bone histomorphologic evaluation of four surfaces on plateau root form implants: An experimental study in dogs. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Rangel, I.G., Jr.; De Oliveira, J.A.; Okamoto, R.; Lang, N.P. Early healing after elevation of the maxillary sinus floor applying a lateral access: A histological study in monkeys. Clin. Oral Implant. Res. 2010, 21, 1320–1326. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Ferreira, S.; Rangel Garcia, I., Jr.; Caneva, M.; Botticelli, D. Healing at implants installed concurrently to maxillary sinus floor elevation with Bio-Oss® or autologous bone grafts. A histo-morphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 503–511. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, S.H.L.; Cadore, U.B.; Novaes, A.B., Jr.; Messora, M.R.; Ghiraldini, B.; Bezerra, F.J.B.; Botticelli, D.; de Souza, S.L.S. Evaluation of Bone Response to a Nano HA Implant Surface on Sinus Lifting Procedures: Study in Rabbits. J. Funct. Biomater. 2022, 13, 122. https://doi.org/10.3390/jfb13030122
Martins SHL, Cadore UB, Novaes AB Jr., Messora MR, Ghiraldini B, Bezerra FJB, Botticelli D, de Souza SLS. Evaluation of Bone Response to a Nano HA Implant Surface on Sinus Lifting Procedures: Study in Rabbits. Journal of Functional Biomaterials. 2022; 13(3):122. https://doi.org/10.3390/jfb13030122
Chicago/Turabian StyleMartins, Sergio H. L., Uislen B. Cadore, Arthur B. Novaes, Jr., Michel R. Messora, Bruna Ghiraldini, Fabio J. B. Bezerra, Daniele Botticelli, and Sergio L. S. de Souza. 2022. "Evaluation of Bone Response to a Nano HA Implant Surface on Sinus Lifting Procedures: Study in Rabbits" Journal of Functional Biomaterials 13, no. 3: 122. https://doi.org/10.3390/jfb13030122
APA StyleMartins, S. H. L., Cadore, U. B., Novaes, A. B., Jr., Messora, M. R., Ghiraldini, B., Bezerra, F. J. B., Botticelli, D., & de Souza, S. L. S. (2022). Evaluation of Bone Response to a Nano HA Implant Surface on Sinus Lifting Procedures: Study in Rabbits. Journal of Functional Biomaterials, 13(3), 122. https://doi.org/10.3390/jfb13030122







