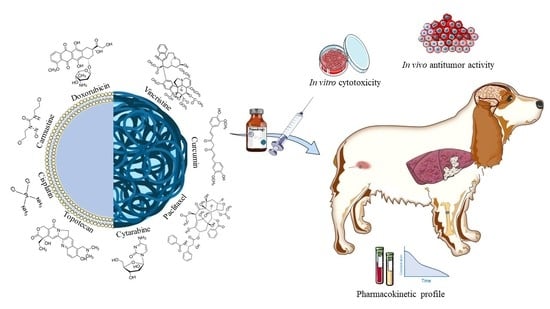
Application of Biocompatible Drug Delivery Nanosystems for the Treatment of Naturally Occurring Cancer in Dogs
Abstract
1. Introduction
2. Comparative Oncology: How the Canine Model Can Help Research in Cancer Treatment
3. Drug Delivery Systems for the Treatment of Canine Tumors
3.1. (Phospho) Lipid-Based Nanosystems
3.1.1. Liposomes
3.1.2. Non Phospholipid-based Nanoparticles
3.2. Non Lipid Nanoparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E.L. Mortality in North American Dogs from 1984 to 2004: An Investigation into Age-, Size-, and Breed-related Causes of Death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.W.; Ostrander, E.A. Domestic Dogs and Cancer Research: A Breed-Based Genomics Approach. ILAR J. 2014, 55, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chibuk, J.; Flory, A.; Kruglyak, K.M.; Leibman, N.; Nahama, A.; Dharajiya, N.; Van den Boom, D.; Jensen, T.J.; Friedman, J.S.; Shen, M.R. Horizons in Veterinary Precision Oncology: Fundamentals of Cancer Genomics and Applications of Liquid Biopsy for the Detection, Characterization, and Management of Cancer in Dogs. Front. Vet. Sci. 2021, 8, 664718. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of New Cancer Treatments from Pet Dogs to Humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A.; Dreger, D.L.; Evans, J.M. Canine Cancer Genomics: Lessons for Canine and Human Health. Annu. Rev. Anim. Biosci. 2019, 7, 449–472. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, T.; Xu, Y.; Dong, Q.; Xiao, J.; Xu, Y.; Li, Q.; Zhang, C.; Gao, J.; Liu, L. A Comprehensive Overview of Oncogenic Pathways in Human Cancer. Brief. Bioinform. 2020, 21, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current Challenges in Cancer Treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Du, Z.; Lovly, C.M. Mechanisms of Receptor Tyrosine Kinase Activation in Cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef]
- Vineis, P.; Wild, C.P. Global Cancer Patterns: Causes and Prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. 2016 AAHA Oncology Guidelines for Dogs and Cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef]
- Biller, B. Metronomic Chemotherapy in Veterinary Patients with Cancer: Rethinking the Targets and Strategies of Chemotherapy. Vet. Clin. Small Anim. Pract. 2014, 44, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Chariou, P.L.; Ortega-Rivera, O.A.; Steinmetz, N.F. Nanocarriers for the Delivery of Medical, Veterinary, and Agricultural Active Ingredients. ACS Nano 2020, 14, 2678–2701. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-Modified Polymeric Nanoparticles for Drug Delivery to Cancer Cells. Expert Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Shi, X.; Shen, M. Intelligent Design of Ultrasmall Iron Oxide Nanoparticle-Based Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 45119–45129. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Liposomes Modified with Bio-Substances for Cancer Treatment. Biomater. Sci. 2020, 8, 6442–6468. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Breen, M. Comparative Oncology: What Dogs and Other Species Can Teach Us about Humans with Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef]
- Alvarez, C.E. Naturally Occurring Cancers in Dogs: Insights for Translational Genetics and Medicine. ILAR J. 2014, 55, 16–45. [Google Scholar] [CrossRef]
- Burton, J.; Khanna, C. The Role of Clinical Trials in Veterinary Oncology. Vet. Clin. Small Anim. Pract. 2014, 44, 977–987. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.N. Improving Human Cancer Therapy through the Evaluation of Pet Dogs. Nat. Rev. Cancer 2020, 20, 727–742. [Google Scholar] [CrossRef]
- Harman, R.M.; Das, S.P.; Bartlett, A.P.; Rauner, G.; Donahue, L.R.; Van de Walle, G.R. Beyond Tradition and Convention: Benefits of Non-Traditional Model Organisms in Cancer Research. Cancer Metastasis Rev. 2021, 40, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Diessner, B.J.; Marko, T.A.; Scott, R.M.; Eckert, A.L.; Stuebner, K.M.; Hohenhaus, A.E.; Selting, K.A.; Largaespada, D.A.; Modiano, J.F.; Spector, L.G. A Comparison of Risk Factors for Metastasis at Diagnosis in Humans and Dogs with Osteosarcoma. Cancer Med. 2019, 8, 3216–3226. [Google Scholar] [CrossRef] [PubMed]
- Koutros, S.; Silverman, D.T.; Alavanja, M.C.R.; Andreotti, G.; Lerro, C.C.; Heltshe, S.; Lynch, C.F.; Sandler, D.P.; Blair, A.; Beane Freeman, L.E. Occupational Exposure to Pesticides and Bladder Cancer Risk. Int. J. Epidemiol. 2016, 45, 792–805. [Google Scholar] [CrossRef] [PubMed]
- Hayes JR, H.M.; Hoover, R.; Tarone, R.E. Bladder Cancer in Pet Dogs: A Sentinel for Environmental Cancer? Am. J. Epidemiol. 1981, 114, 229–233. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes in Biology and Medicine. Bio-Appl. Nanoparticles 2007, 602, 117–128. [Google Scholar]
- Gabizon, A.A.; Shmeeda, H.; Zalipsky, S. Pros and Cons of the Liposome Platform in Cancer Drug Targeting. J. Liposome Res. 2006, 16, 175–183. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Russo, D.; Fresta, M. Liposomes as Multicompartmental Carriers for Multidrug Delivery in Anticancer Chemotherapy. Drug Deliv. Transl. Res. 2011, 1, 66–75. [Google Scholar] [CrossRef]
- Krauss, A.C.; Gao, X.; Li, L.; Manning, M.L.; Patel, P.; Fu, W.; Janoria, K.G.; Gieser, G.; Bateman, D.A.; Przepiorka, D. FDA Approval Summary:(Daunorubicin and Cytarabine) Liposome for Injection for the Treatment of Adults with High-Risk Acute Myeloid LeukemiaFDA Approval:(Daunorubicin and Cytarabine). Clin. Cancer Res. 2019, 25, 2685–2690. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Pasut, G.; Paolino, D.; Celia, C.; Mero, A.; Joseph, A.S.; Wolfram, J.; Cosco, D.; Schiavon, O.; Shen, H.; Fresta, M. Polyethylene Glycol (PEG)-Dendron Phospholipids as Innovative Constructs for the Preparation of Super Stealth Liposomes for Anticancer Therapy. J. Control. Release 2015, 199, 106–113. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or Not to PEGylate: Immunological Properties of Nanomedicine’s Most Popular Component, Poly (Ethylene) Glycol and Its Alternatives. Adv. Drug Deliv. Rev. 2021, 180, 114079. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Alving, C.R.; Rosivall, L.; Bünger, R.; Baranyi, L.; Bedöcs, P.; Tóth, M.; Barenholz, Y. Animal Models of Complement-Mediated Hypersensitivity Reactions to Liposomes and Other Lipid-Based Nanoparticles. J. Liposome Res. 2007, 17, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for Cancer-Targeted Drug Delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The First FDA-Approved Nano-Drug: Lessons Learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Pal, A.; Khan, S.; Wang, Y.-F.; Kamath, N.; Sarkar, A.K.; Ahmad, A.; Sheikh, S.; Ali, S.; Carbonaro, D.; Zhang, A. Preclinical Safety, Pharmacokinetics and Antitumor Efficacy Profile of Liposome-Entrapped SN-38 Formulation. Anticancer Res. 2005, 25, 331–341. [Google Scholar]
- Hauck, M.L.; LaRue, S.M.; Petros, W.P.; Poulson, J.M.; Yu, D.; Spasojevic, I.; Pruitt, A.F.; Klein, A.; Case, B.; Thrall, D.E. Phase I Trial of Doxorubicin-Containing Low Temperature Sensitive Liposomes in Spontaneous Canine Tumors. Clin. Cancer Res. 2006, 12, 4004–4010. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Wang, S. A Novel Method to Load Topotecan into Liposomes Driven by a Transmembrane NH4EDTA Gradient. Eur. J. Pharm. Biopharm. 2012, 80, 332–339. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, Y.; Li, J.; Wei, Y. Preparation and the In-Vivo Evaluation of Paclitaxel Liposomes for Lung Targeting Delivery in Dogs. J. Pharm. Pharmacol. 2011, 63, 80–86. [Google Scholar] [CrossRef]
- Li, C.; Wang, C.; Yang, H.; Zhao, X.; Wei, N.; Cui, J. Liposomal Topotecan Formulation with a Low Polyethylene Glycol Grafting Density: Pharmacokinetics and Antitumour Activity. J. Pharm. Pharmacol. 2012, 64, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.S.T.; Lucci, C.M.; Longo, J.P.F.; Galera, P.D.; Simioni, A.R.; Lacava, Z.G.M.; Tedesco, A.C.; Azevedo, R.B. Aluminum-Chloride-Phthalocyanine Encapsulated in Liposomes: Activity against Naturally Occurring Dog Breast Cancer Cells. J. Biomed. Nanotechnol. 2012, 8, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo®(Vincristine Sulfate Liposome Injection) Improves the Pharmacokinetics and Pharmacodynamics of Vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, L.; Li, N.; Qiu, Z.; Zhou, S.; Li, C.; Zhao, J.; Song, H.; Chen, X. Pharmacokinetics and Biodistribution Study of Paclitaxel Liposome in Sprague-Dawley Rats and Beagle Dogs by Liquid Chromatography-Tandem Mass Spectrometry. Drug Res. (Stuttg.) 2013, 63, 603–606. [Google Scholar] [CrossRef]
- Zhong, J.; Mao, W.; Shi, R.; Jiang, P.; Wang, Q.; Zhu, R.; Wang, T.; Ma, Y. Pharmacokinetics of Liposomal-Encapsulated and Un-Encapsulated Vincristine after Injection of Liposomal Vincristine Sulfate in Beagle Dogs. Cancer Chemother. Pharmacol. 2014, 73, 459–466. [Google Scholar] [CrossRef]
- Li, K.; Wang, S. Preparation, Pharmacokinetic Profile, and Tissue Distribution Studies of a Liposome-Based Formulation of SN-38 Using an UPLC–MS/MS Method. Aaps Pharmscitech 2016, 17, 1450–1456. [Google Scholar] [CrossRef][Green Version]
- Vazquez Fuster, I.B.; Taylor, A.R.; Smith, A.N.; Duran, S.H.; Ravis, W.R.; Jasper, S.L.; Arnold, R.D. Pharmacokinetics of Multivesicular Liposomal Encapsulated Cytarabine When Administered Subcutaneously in Dogs. J. Vet. Intern. Med. 2020, 34, 1563–1569. [Google Scholar] [CrossRef]
- Bredlau, A.L.; Motamarry, A.; Chen, C.; McCrackin, M.A.; Helke, K.; Armeson, K.E.; Bynum, K.; Broome, A.-M.; Haemmerich, D. Localized Delivery of Therapeutic Doxorubicin Dose across the Canine Blood–Brain Barrier with Hyperthermia and Temperature Sensitive Liposomes. Drug Deliv. 2018, 25, 973–984. [Google Scholar] [CrossRef]
- Reckelhoff, C.R.; Lejeune, A.; Thompson, P.M.; Shiomitsu, K. In Vitro Effects of the Chemotherapy Agent Water-soluble Micellar Paclitaxel (Paccal Vet) on Canine Hemangiosarcoma Cell Lines. Vet. Comp. Oncol. 2019, 17, 32–41. [Google Scholar] [CrossRef]
- Audrey, G.; Claire, L.-C.; Joel, E. Effect of the NFL-TBS. 40-63 Peptide on Canine Glioblastoma Cells. Int. J. Pharm. 2021, 605, 120811. [Google Scholar] [CrossRef]
- Yagi, N.K.; Wi, J.; Hashimoto, Y.; Kong, L.Y.; Gabrusiewicz, K.; Nduom, E.K.; Ling, X.; Huang, N.; Zhou, S.; Kerrigan, B.C.P. Immune Modulatory Nanoparticle Therapeutics for Intracerebral Glioma. Neuro. Oncol. 2017, 19, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Kisseberth, W.C.; MacEwen, E.G.; Helfand, S.C.; Vail, D.M.; London, C.L.; Keller, E. Response to Liposome-encapsulated Doxorubicin (TLC D-99) in a Dog with Myeloma. J. Vet. Intern. Med. 1995, 9, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Kravis, L.D.; Cooley, A.J.; Chun, R.; MacEwen, E.G. Preclinical Trial of Doxorubicin Entrapped in Sterically Stabilized Liposomes in Dogs with Spontaneously Arising Malignant Tumors. Cancer Chemother. Pharmacol. 1997, 39, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Teske, E.; Rutteman, G.R.; Kirpenstein, J.; Hirschberger, J. A Randomized Controlled Study into the Efficacy and Toxicity of Pegylated Liposome Encapsulated Doxorubicin as an Adjuvant Therapy in Dogs with Splenic Haemangiosarcoma. Vet. Comp. Oncol. 2011, 9, 283–289. [Google Scholar] [CrossRef]
- Sorenmo, K.; Samluk, M.; Clifford, C.; Baez, J.; Barrett, J.S.; Poppenga, R.; Overley, B.; Skorupski, K.; Oberthaler, K.; Winkle, T. Van Clinical and Pharmacokinetic Characteristics of Intracavitary Administration of Pegylated Liposomal Encapsulated Doxorubicin in Dogs with Splenic Hemangiosarcoma. J. Vet. Intern. Med. 2007, 21, 1347–1354. [Google Scholar] [CrossRef]
- Hueso, L.; Sanmartín, O.; Nagore, E.; Botella-Estrada, R.; Requena, C.; Llombart, B.; Serra-Guillén, C.; Alfaro-Rubio, A.; Guillén, C. Chemotherapy-Induced Acral Erythema: A Clinical and Histopathologic Study of 44 Cases. Actas Dermo-Sifiliográficas (Engl. Ed.) 2008, 99, 281–290. [Google Scholar] [CrossRef]
- Vail, D.M.; Chun, R.; Thamm, D.H.; Garrett, L.D.; Cooley, A.J.; Obradovich, J.E. Efficacy of Pyridoxine to Ameliorate the Cutaneous Toxicity Associated with Doxorubicin Containing Pegylated (Stealth) Liposomes: A Randomized, Double-Blind Clinical Trial Using a Canine Model. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1998, 4, 1567–1571. [Google Scholar]
- Vail, D.M.; Kurzman, I.D.; Glawe, P.C.; O’Brien, M.G.; Chun, R.; Garrett, L.D.; Obradovich, J.E.; Fred, R.M.; Khanna, C.; Colbern, G.T. STEALTH Liposome-Encapsulated Cisplatin (SPI-77) versus Carboplatin as Adjuvant Therapy for Spontaneously Arising Osteosarcoma (OSA) in the Dog: A Randomized Multicenter Clinical Trial. Cancer Chemother. Pharmacol. 2002, 50, 131–136. [Google Scholar] [CrossRef]
- Ogilvie, G.K.; Krawiec, D.R.; Gelberg, H.B.; Twardock, A.R.; Reschke, R.W.; Richardson, B.C. Evaluation of a Short-Term Saline Diuresis Protocol for the Administration of Cisplatin. Am. J. Vet. Res. 1988, 49, 1076–1078. [Google Scholar]
- Sayour, E.J.; Grippin, A.; De Leon, G.; Stover, B.; Rahman, M.; Karachi, A.; Wummer, B.; Moore, G.; Castillo-Caro, P.; Fredenburg, K. Personalized Tumor RNA Loaded Lipid-Nanoparticles Prime the Systemic and Intratumoral Milieu for Response to Cancer Immunotherapy. Nano Lett. 2018, 18, 6195–6206. [Google Scholar] [CrossRef]
- Kamstock, D.; Guth, A.; Elmslie, R.; Kurzman, I.; Liggitt, D.; Coro, L.; Fairman, J.; Dow, S. Liposome–DNA Complexes Infused Intravenously Inhibit Tumor Angiogenesis and Elicit Antitumor Activity in Dogs with Soft Tissue Sarcoma. Cancer Gene Ther. 2006, 13, 306–317. [Google Scholar] [CrossRef] [PubMed]
- MacEwen, E.G.; Kurzman, I.D.; Helfand, S.; Vail, D.; London, C.; Kisseberth, W.; Rosenthal, R.C.; Fox, L.E.; Keller, E.T.; Obradovich, J. Current Studies of Liposome Muramyl Tripeptide (CGP 19835A Lipid) Therapy for Metastasis in Spontaneous Tumors: A Progress Review. J. Drug Target. 1994, 2, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kurzman, I.D.; MacEwen, E.G.; Rosenthal, R.C.; Fox, L.E.; Keller, E.T.; Helfand, S.C.; Vail, D.M.; Dubielzig, R.R.; Madewell, B.R.; Rodriguez Jr, C.O. Adjuvant Therapy for Osteosarcoma in Dogs: Results of Randomized Clinical Trials Using Combined Liposome-Encapsulated Muramyl Tripeptide and Cisplatin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1995, 1, 1595–1601. [Google Scholar]
- Vail, D.M.; MacEwen, E.G.; Kurzman, I.D.; Dubielzig, R.R.; Helfand, S.C.; Kisseberth, W.C.; London, C.A.; Obradovich, J.E.; Madewell, B.R.; Rodriguez Jr, C.O. Liposome-Encapsulated Muramyl Tripeptide Phosphatidylethanolamine Adjuvant Immunotherapy for Splenic Hemangiosarcoma in the Dog: A Randomized Multi-Institutional Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1995, 1, 1165–1170. [Google Scholar]
- Teske, E.; Rutteman, G.R.; Ingh, T.V.; van Noort, R.; Misdorp, W. Liposome-Encapsulated Muramyl Tripeptide Phosphatidyl-Ethanolamine (L-MTP-PE): A Randomized Clinical Trial in Dogs with Mammary Carcinoma. Anticancer. Res. 1998, 18, 1015–1020. [Google Scholar] [PubMed]
- MacEwen, E.G.; Kurzman, I.D.; Vail, D.M.; Dubielzig, R.R.; Everlith, K.; Madewell, B.R.; Rodriguez Jr, C.O.; Phillips, B.; Zwahlen, C.H.; Obradovich, J. Adjuvant Therapy for Melanoma in Dogs: Results of Randomized Clinical Trials Using Surgery, Liposome-Encapsulated Muramyl Tripeptide, and Granulocyte Macrophage Colony-Stimulating Factor. Clin. Cancer Res. 1999, 5, 4249–4258. [Google Scholar] [PubMed]
- Frampton, J.E. Mifamurtide. Pediatr. Drugs 2010, 12, 141–153. [Google Scholar] [CrossRef]
- Hafeman, S.; London, C.; Elmslie, R.; Dow, S. Evaluation of Liposomal Clodronate for Treatment of Malignant Histiocytosis in Dogs. Cancer Immunol. Immunother. 2010, 59, 441–452. [Google Scholar] [CrossRef]
- Withers, S.S.; York, D.; Johnson, E.; Al-Nadaf, S.; Skorupski, K.A.; Rodriguez Jr, C.O.; Burton, J.H.; Guerrero, T.; Sein, K.; Wittenburg, L. In Vitro and in Vivo Activity of Liposome-encapsulated Curcumin for Naturally Occurring Canine Cancers. Vet. Comp. Oncol. 2018, 16, 571–579. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Helson, L.; Bolger, G.; Majeed, M.; Vcelar, B.; Pucaj, K.; Matabudul, D. Infusion Pharmacokinetics of LipocurcTM (Liposomal Curcumin) and Its Metabolite Tetrahydrocurcumin in Beagle Dogs. Anticancer Res. 2012, 32, 4365–4370. [Google Scholar] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Feeney, O.M.; Crum, M.F.; McEvoy, C.L.; Trevaskis, N.L.; Williams, H.D.; Pouton, C.W.; Charman, W.N.; Bergström, C.A.S.; Porter, C.J.H. 50 Years of Oral Lipid-Based Formulations: Provenance, Progress and Future Perspectives. Adv. Drug Deliv. Rev. 2016, 101, 167–194. [Google Scholar] [CrossRef]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle SiRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-L.; Tang, W.-H.; Li, S.-D. Cancer Theranostic Applications of Lipid-Based Nanoparticles. Drug Discov. Today 2018, 23, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; Von Euler, H.; Rusk, A.W.; Barber, L.; Clifford, C.; Elmslie, R.; Fulton, L.; Hirschberger, J.; Klein, M.; London, C. A Randomized Trial Investigating the Efficacy and Safety of Water Soluble Micellar Paclitaxel (Paccal Vet) for Treatment of Nonresectable Grade 2 or 3 Mast Cell Tumors in Dogs. J. Vet. Intern. Med. 2012, 26, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Von Euler, H.; Rivera, P.; Nyman, H.; Häggström, J.; Borgå, O. A Dose-finding Study with a Novel Water-soluble Formulation of Paclitaxel for the Treatment of Malignant High-grade Solid Tumours in Dogs. Vet. Comp. Oncol. 2013, 11, 243–255. [Google Scholar] [CrossRef]
- Peterson, C.; Vitols, S.; Rudling, M.; Blomgren, H.; Edsmyr, F.; Skoog, L. Hypocholesterolemia in Cancer Patients May Be Caused by Elevated LDL Receptor Activities in Malignant Cells. Med. Oncol. Tumor Pharmacother. 1985, 2, 143–147. [Google Scholar] [CrossRef]
- Alhadad, L.J.; Harisa, G.I.; Alanazi, F.K. Design and Encapsulation of Anticancer Dual HSP27 and HER2 Inhibitor into Low Density Lipoprotein to Target Ovarian Cancer Cells. Saudi Pharm. J. 2020, 28, 387–396. [Google Scholar] [CrossRef]
- Lucas, S.R.R.; Maranhão, R.C.; Guerra, J.L.; Coelho, B.M.P.; Barboza, R.; Pozzi, D.H.B. Pilot Clinical Study of Carmustine Associated with a Lipid Nanoemulsion in Combination with Vincristine and Prednisone for the Treatment of Canine Lymphoma. Vet. Comp. Oncol. 2015, 13, 184–193. [Google Scholar] [CrossRef]
- Lu, Z.; Yeh, T.-K.; Tsai, M.; Au, J.L.-S.; Wientjes, M.G. Paclitaxel-Loaded Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. Clin. Cancer Res. 2004, 10, 7677–7684. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.; Nduom, E.; Kent, M.; Freeman, C.; Machaidze, R.; Kaluzova, M.; Wang, L.; Mao, H.; Hadjipanayis, C.G. Canine Model of Convection-Enhanced Delivery of Cetuximab-Conjugated Iron-Oxide Nanoparticles Monitored with Magnetic Resonance Imaging. Neurosurgery 2012, 59, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Venable, R.O.; Worley, D.R.; Gustafson, D.L.; Hansen, R.J.; Ehrhart, E.J.; Cai, S.; Cohen, M.S.; Forrest, M.L. Effects of Intratumoral Administration of a Hyaluronan-Cisplatin Nanoconjugate to Five Dogs with Soft Tissue Sarcomas. Am. J. Vet. Res. 2012, 73, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yeh, T.-K.; Wang, J.; Chen, L.; Lyness, G.; Xin, Y.; Wientjes, M.G.; Bergdall, V.; Couto, G.; Alvarez-Berger, F. Paclitaxel Gelatin Nanoparticles for Intravesical Bladder Cancer Therapy. J. Urol. 2011, 185, 1478–1483. [Google Scholar] [CrossRef]
- Zhang, T.; Cai, S.; Groer, C.; Forrest, W.C.; Yang, Q.; Mohr, E.; Douglas, J.; Aires, D.; Axiak-Bechtel, S.M.; Selting, K.A. Hyaluronan-Lysine Cisplatin Drug Carrier for Treatment of Localized Cancers: Pharmacokinetics, Tolerability, and Efficacy in Rodents and Canines. J. Pharm. Sci. 2016, 105, 1891–1900. [Google Scholar] [CrossRef][Green Version]
- Lin, T.; Zhang, H.; Luo, J.; Li, Y.; Gao, T.; Lara Jr, P.N.; de Vere White, R.; Lam, K.S.; Pan, C.-X. Multifunctional Targeting Micelle Nanocarriers with Both Imaging and Therapeutic Potential for Bladder Cancer. Int. J. Nanomed. 2012, 7, 2793. [Google Scholar] [CrossRef]
- Tang, L.; Tong, R.; Coyle, V.J.; Yin, Q.; Pondenis, H.; Borst, L.B.; Cheng, J.; Fan, T.M. Targeting Tumor Vasculature with Aptamer-Functionalized Doxorubicin–Polylactide Nanoconjugates for Enhanced Cancer Therapy. ACS Nano 2015, 9, 5072–5081. [Google Scholar] [CrossRef]
- Feldhaeusser, B.; Platt, S.R.; Marrache, S.; Kolishetti, N.; Pathak, R.K.; Montgomery, D.J.; Reno, L.R.; Howerth, E.; Dhar, S. Evaluation of Nanoparticle Delivered Cisplatin in Beagles. Nanoscale 2015, 7, 13822–13830. [Google Scholar] [CrossRef]
- Lin, J.; Cai, Q.; Tang, Y.; Xu, Y.; Wang, Q.; Li, T.; Xu, H.; Wang, S.; Fan, K.; Liu, Z. PEGylated Lipid Bilayer Coated Mesoporous Silica Nanoparticles for Co-Delivery of Paclitaxel and Curcumin: Design, Characterization and Its Cytotoxic Effect. Int. J. Pharm. 2018, 536, 272–282. [Google Scholar] [CrossRef]
- Danmaigoro, A.; Selvarajah, G.T.; Mohd Noor, M.H.; Mahmud, R.; Abu Bakar, M.Z. Toxicity and Safety Evaluation of Doxorubicin-Loaded Cockleshell-Derived Calcium Carbonate Nanoparticle in Dogs. Adv. Pharmacol. Sci. 2018, 2018, 4848602. [Google Scholar] [CrossRef]
- Małek, A.; Taciak, B.; Sobczak, K.; Grzelak, A.; Wójcik, M.; Mieczkowski, J.; Lechowski, R.; Zabielska-Koczywąs, K.A. Enhanced Cytotoxic Effect of Doxorubicin Conjugated to Glutathione-Stabilized Gold Nanoparticles in Canine Osteosarcoma—In Vitro Studies. Molecules 2021, 26, 3487. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, T.; Forrest, W.C.; Yang, Q.; Groer, C.; Mohr, E.; Aires, D.J.; Axiak-Bechtel, S.M.; Flesner, B.K.; Henry, C.J. Phase I-II Clinical Trial of Hyaluronan-Cisplatin Nanoconjugate in Dogs with Naturally Occurring Malignant Tumors. Am. J. Vet. Res. 2016, 77, 1005–1016. [Google Scholar] [CrossRef]
- Gagliardi, A.; Voci, S.; Paolino, D.; Fresta, M.; Cosco, D. Influence of Various Model Compounds on the Rheological Properties of Zein-Based Gels. Molecules 2020, 25, 3174. [Google Scholar] [CrossRef]
- Sarcan, E.T.; Silindir-Gunay, M.; Ozer, A.Y. Theranostic Polymeric Nanoparticles for NIR Imaging and Photodynamic Therapy. Int. J. Pharm. 2018, 551, 329–338. [Google Scholar] [CrossRef] [PubMed]
- George, B.; Suchithra, T. V Plant-Derived Bioadhesives for Wound Dressing and Drug Delivery System. Fitoterapia 2019, 137, 104241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, H.; Bu, X.; Lei, C.; Fang, Q.; Hu, Z. Quantitative Proteomic Analysis of Cellular Resistance to the Nanoparticle Abraxane. ACS Nano 2015, 9, 10099–10112. [Google Scholar] [CrossRef]
- Cai, S.; Zhang, T.; Groer, C.; Forrest, M.; Aires, D.; Otte, V.; Barchman, S.; Faerber, A.; Forrest, M.L. Injectable Chemotherapy Downstaged Oral Squamous Cell Carcinoma from Nonresectable to Resectable in a Rescue Dog: Diagnosis, Treatment, and Outcome. Case Rep. Vet. Med. 2018, 2018, 9078537. [Google Scholar] [CrossRef]
- Cai, S.; Xie, Y.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Pharmacokinetics and Disposition of a Localized Lymphatic Polymeric Hyaluronan Conjugate of Cisplatin in Rodents. J. Pharm. Sci. 2010, 99, 2664–2671. [Google Scholar] [CrossRef]
- Yin, Q.; Tang, L.; Cai, K.; Tong, R.; Sternberg, R.; Yang, X.; Dobrucki, L.W.; Borst, L.B.; Kamstock, D.; Song, Z. Pamidronate Functionalized Nanoconjugates for Targeted Therapy of Focal Skeletal Malignant Osteolysis. Proc. Natl. Acad. Sci. USA 2016, 113, 4601–4609. [Google Scholar] [CrossRef]
- Ottaviani, G.; Jaffe, N. The Epidemiology of Osteosarcoma. Pediatr. Adolesc. Osteosarcoma 2009, 152, 3–13. [Google Scholar]
- Rankin, K.S.; Starkey, M.; Lunec, J.; Gerrand, C.H.; Murphy, S.; Biswas, S. Of Dogs and Men: Comparative Biology as a Tool for the Discovery of Novel Biomarkers and Drug Development Targets in Osteosarcoma. Pediatr. Blood Cancer 2012, 58, 327–333. [Google Scholar] [CrossRef]
- Young, J.S.; Bernal, G.; Polster, S.P.; Nunez, L.; Larsen, G.F.; Mansour, N.; Podell, M.; Yamini, B. Convection-Enhanced Delivery of Polymeric Nanoparticles Encapsulating Chemotherapy in Canines with Spontaneous Supratentorial Tumors. World Neurosurg. 2018, 117, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-Enhanced Delivery in Glioblastoma: A Review of Preclinical and Clinical Studies. J. Neurosurg. 2017, 126, 191–200. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic Iron Oxide Nanoparticles for Drug Delivery: Applications and Characteristics. Expert Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, P.J.; Wagner, R.J.; Duval, K.; Kang, K.; Gladstone, D.J.; Moodie, K.L.; Crary-Burney, M.; Ariaspulido, H.; Veliz, F.A.; Steinmetz, N.F. Treatment of Canine Oral Melanoma with Nanotechnology-Based Immunotherapy and Radiation. Mol. Pharm. 2018, 15, 3717–3722. [Google Scholar] [CrossRef] [PubMed]
- Nkanga, C.I.; Steinmetz, N.F. The Pharmacology of Plant Virus Nanoparticles. Virology 2021, 556, 39–61. [Google Scholar] [CrossRef]
- Axiak-Bechtel, S.M.; Upendran, A.; Lattimer, J.C.; Kelsey, J.; Cutler, C.S.; Selting, K.A.; Bryan, J.N.; Henry, C.J.; Boote, E.; Tate, D.J. Gum Arabic-Coated Radioactive Gold Nanoparticles Cause No Short-Term Local or Systemic Toxicity in the Clinically Relevant Canine Model of Prostate Cancer. Int. J. Nanomed. 2014, 9, 5001. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Hassani, A.; Azarian, M.M.S.; Ibrahim, W.N.; Hussain, S.A. Preparation, Characterization and Therapeutic Properties of Gum Arabic-Stabilized Gallic Acid Nanoparticles. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Gum Arabic as an Environmentally Sustainable Polymeric Anticorrosive Material: Recent Progresses and Future Opportunities. Int. J. Biol. Macromol. 2021, 184, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, M.; Gao, X.; Chen, Y.; Liu, T. Nanotechnology in Cancer Diagnosis: Progress, Challenges and Opportunities. J. Hematol. Oncol. 2019, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Drug Delivery System | Study Type | Obtained Results | Reference |
|---|---|---|---|
| Liposomes containing SN-38 | Safety | Dogs were treated with three different dosages of liposomal SN-38 (0.4, 0.8 and 1.2 mg/kg of drug). The last one was considered the MTD 1, because emesis, decrease of hematopoiesis and neutropenia were registered in dogs treated with 1.2 mg/kg of SN-38. The other two dosages were well tolerated. | [37] |
| Low temperature sensitive liposomes containing doxorubicin | Safety and pharmacokinetic | Administration of liposomal formulation, followed by over 30 min of local tumor hyperthermia, was well tolerated by most subjects. The MTD 1 established was 0.93 mg/kg IV. Pharmacokinetic values resemble those of the free drug, except for clearance which was ~17-fold lower for the liposomal formulation. Doxorubicin’s intratumor concentrations were variable, probably as a consequence of the different tumor vascularization | [38] |
| Liposomes encapsulating topotecan using transmembrane NH4EDTA gradient | Pharmacokinetic | The encapsulation of topotecan within liposomes dramatically increases the plasmatic levels and decreases the plasmatic clearance. NH4EDTA-L’s AUC0 was 30-fold that of the free drug. Unexpectedly, NH4EDTA did not increase topotecan’s intraliposomal retention. | [39] |
| Liposomes containing paclitaxel | Pharmacokinetic | The liposomal formulation showed similar Cmax, a 2-fold lower AUC and half-time, and a 2-fold higher clearance and volume of distribution compared with the free form of paclitaxel after IV administration. Moreover, the concentration of liposomal paclitaxel was found to be higher in the lungs than in other organs | [40] |
| PEGylated liposomes containing topotecan | Safety | No skin toxicity was observed in healthy dogs after IV administration even when high concentrations of the drug were used. | [41] |
| Liposomes containing Aluminum-Chloride-Phthalocyanine | In vitro efficacy | Aluminum-Chloride-Phthalocyanine encapsulated within liposomes associated with LED light irradiation showed antineoplastic activity on canine mammary gland complex carcinoma cells. | [42] |
| Vincristine sulfate-loaded liposomes (Marqibo) | Pharmacokinetic | Marqibo significantly increases the AUC0 and Cmax of the drug and drastically decreases its volume of distribution and clearance with respect to the free form of vincristine sulfate. | [43] |
| Liposomal paclitaxel (Lipusu) | Pharmacokinetic | Liposomal paclitaxel was quickly localized in various organs after IV administration, especially in the spleen and liver, but it was slowly eliminated. | [44] |
| Liposomal vincristine sulfate | Pharmacokinetic | Liposomal vincristine is characterized by an increased AUC0 and half-time and a decreased volume of distribution after IV administration in healthy beagles compared with free vincristine | [45] |
| Liposomes containing SN-38 | Pharmacokinetic | The concentration of liposomal SN-38 quickly decreases after IV administration. The elimination profile is independent of the injected dose. | [46] |
| Multivesicular liposomes containing cytarabine | Pharmacokinetic | Liposomal cytarabine (LC) reaches a tmax 4-fold higher than free drug (FC) after subcutaneous administration. Cytarabine-loaded multivesicular liposomes did not reach the cytotoxic plasma concentration with respect to its free form after s.c. administration. Only 20–30% of the injected liposomes were absorbed. The elimination profiles of the two forms of the active compound were similar. | [47] |
| Temperature-sensitive liposomes containing doxorubicin | Biodistribution and safety | Temperature-sensitive liposomal doxorubicin increased the localization of the active compound in the brain when combined with 15–30 min local hyperthermia after IV administration. Only a weak toxicity was observed in healthy tissues. | [48] |
| Non phospholipid-based nanoparticles | |||
| Paccal Vet | In vitro efficacy | Paccal Vet (paclitaxel-loaded micelles) decreased the viability of canine hemangiosarcoma cells. | [49] |
| Lipid nanocapsules functionalized with the NFL 2- peptide | In vitro efficacy | The NFL-peptide promoted a better uptake and cytotoxicity of lipid nanocapsules in J3T canine glioblastoma cells | [50] |
| Lipid based nanoparticles containing miR-124 | In vivo safety | The formulation was demonstrated to be safe when IV administered in healthy beagles. | [51] |
| Drug Delivery Systems | Cancer Types | Reference |
|---|---|---|
| Liposomal doxorubicin | Multiple myeloma | [52] |
| Doxil | Mycosis fungoides; anal gland adenocarcinoma; non-Hodgkin’s lymphoma; Malignant melanoma; mammary gland carcinoma; hemangiosarcoma; squamous cell carcinoma; thymoma; mast cell tumor; anaplastic sarcoma; malignant histiocytoma; fibrosarcoma; transitional cell carcinoma; thyroid carcinoma; mesenchymoma; neurofibrosarcoma; pulmonary adenocarcinoma; sweat gland adenocarcinoma; multiple myeloma | [53] |
| Doxil | Splenic hemangiosarcoma | [54] |
| Doxil | Splenic hemangiosarcoma. | [55] |
| Doxil | non-Hodgkin’s lymphoma | [57] |
| Liposomes containing cisplatin | Osteosarcoma | [58] |
| Liposomes containing untargeted tumor RNA | Malignant glioma | [60] |
| LDC-containing canine endostatin | Cutaneous soft tissue sarcomas | [61] |
| Liposomal muramyl tripeptide-phosphatidylethanolamine (L-MTP-PE) | Hemangiosarcoma; osteosarcoma. | [62] |
| L-MTP-PE | Osteosarcoma | [63] |
| L-MTP-PE | Hemangiosarcoma | [64] |
| L-MTP-PE | Mammary carcinoma | [65] |
| L-MTP-PE | Oral melanoma | [66] |
| Phosphatidylcholine-based liposomes containing clodronate | Malignant histiocytosis; lung and adrenal glands metastasis | [68] |
| Lipocurc | Primary or metastatic pulmonary neoplasia | [69] |
| Non phospholipids-based nanoparticles | ||
| Paccal Vet | Advanced stage mast cell tumor | [76] |
| Paccal Vet | Mast cell tumor; mammary tumor; lymphoma; squamous cell carcinoma; anal sac carcinoma; bladder transitional cell carcinoma; fibrosarcoma; hemangiosarcoma; histiocytoma; malignant melanoma; mediastinal mass; osteosarcoma; synovial cell sarcoma | [77] |
| Lipid nanoemulsions containing carmustine | Lymphoma | [80] |
| Drug Delivery Systems | Study Type | Results | Reference |
|---|---|---|---|
| Paclitaxel-loaded gelatin nanoparticles | Pharmacokinetics | Gelatin nanoparticles promoted a three-fold greater concentration of paclitaxel in bladder tissues with respect to the free form of the drug. | [81] |
| Convention-enhanced delivery of cetuximab conjugated to iron-oxide nanoparticles | Pharmacokinetics and safety | Distribution volume of cetuximab-free and cetuximab-conjugated to iron-oxide nanoparticles (IONPs) was similar after CED 1 administration in healthy beagles; a slower infusion showed a more uniform diffusion. Both formulations were safe. | [82] |
| Hyaluronan-cisplatin nanoconjugate | Pharmacokinetics | Hyaluronan-cisplatin nanoconjugate intratumorally injected in five tumor-bearing dogs, dramatically increased the concentration of the active compound inside the tumor masses compared with the free form of the drug. In addition, a significant localization of cisplatin within sentinel lymph nodes was obtained. | [83] |
| Paclitaxel-loaded gelatin nanoparticles | Pharmacokinetics | Paclitaxel-loaded nanoparticles (PNP) intra-vesically injected once a week in healthy and tumor bearing dogs favoured (i) a constant concentration of the drug in urine, (ii) a systemic distribution of only 1% of the injected dosage, (iii) a localization in the bladder tissue four times higher compared with free paclitaxel. | [84] |
| Hyaluronan-cisplatin nanoconjugates | Pharmacokinetics and safety | Hyaluronan-cisplatin nanoconjugates linked by N-Ac-Lys residue promoted an increased AUC of the drug in treated dogs and determined a Tmax of 6 h, much higher than that of the free form of the active compound. These in vivo features decreased the toxicity of cisplatin. | [85] |
| PZ4-decorated micelles made up of polyethylene glycol and cholic acid containing imaging agents, daunorubicin or paclitaxel | In vitro efficacy | PZ4-decorated micelles selectively targeted canine bladder cancer cells but not normal urothelial cells. PLZ4 increased the cytotoxicity of daunorubicin and the cellular uptake of micelles. | [86] |
| Aptamer-functionalized doxorubicin-Polylactide nanoconjugates | In vitro efficacy | Aptamer-functionalized doxorubicin- polylactide nanoconjugates incubated with canine hemangiosarcoma cells increased the intracellular localization of the drug and its toxicity with respect to the aptamer-free formulation | [87] |
| Poly(lactic-co-glycolic acid) (PLGA)-block(b)-PEG functionalized with triphenylphosphonium (TPP) cation nanoparticles containing cisplatin prodrug | In vitro efficacy and pharmacokinetics | The targeting of the mitochondria by PLGA-(b)-PEG-TPP-based nanoparticles containing the cisplatin prodrug (T-platin-M-NPs). The nanosystems significantly increased the toxicity of carboplatin and cisplatin on canine glioma and glioblastoma cells. In vivo studies demonstrated that T-platin-M-NPs are able to overcome the BBB 2 and reach the brain. T-platin-M-NPs were shown to be safe, and no severe adverse effects occurred on organs | [88] |
| Paclitaxel and curcumin encapsulated into PEG-coated mesoporous silica nanoparticles | In vitro efficacy | Paclitaxel and curcumin co-encapsulated into PEG-lipid-coated silica nanoparticles increased their cytotoxicity on canine breast cells | [89] |
| Cockleshell derived CaCO3 nanoparticles containing doxorubicin | Safety | Cockleshell derived CaCO3 nanoparticles promoted a decreased cardio- and nephrotoxicity of doxorubicin after injection in healthy dogs | [90] |
| Doxorubicin conjugated to glutathione-stabilized gold nanoparticles | In vitro efficacy | Doxorubicin conjugated to glutathione-stabilized gold nanoparticles showed a higher cytotoxicity of the drug on canine osteosarcoma cell lines with respect to the free form of the active compound. | [91] |
| Drug Delivery Systems | Cancer Types | Reference |
|---|---|---|
| Polymeric hyaluronan cisplatin-nanoconjugate | Oral squamous cell carcinomas; nasal cancers; sarcoma; anal sac adenocarcinoma | [92] |
| HylaPlat | Oral squamous cell carcinomas; regional lymph node metastasis | [97] |
| Pam-Doxo-NPs | Osteosarcoma | [99] |
| PEG-PLA-PCL based nanoparticles, containing temozolomide-loaded superparamagnetic iron oxide | Glioblastoma; Anaplastic Astrocytoma; Cystic meningioma; High-grade astrocytoma | [102] |
| Iron oxide nanoparticles and/or virus plant nanoparticles | Oral melanoma | [106] |
| GA-AuNPs | Prostatic carcinoma; regional lymph nodes metastasis | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrosio, N.; Voci, S.; Gagliardi, A.; Palma, E.; Fresta, M.; Cosco, D. Application of Biocompatible Drug Delivery Nanosystems for the Treatment of Naturally Occurring Cancer in Dogs. J. Funct. Biomater. 2022, 13, 116. https://doi.org/10.3390/jfb13030116
Ambrosio N, Voci S, Gagliardi A, Palma E, Fresta M, Cosco D. Application of Biocompatible Drug Delivery Nanosystems for the Treatment of Naturally Occurring Cancer in Dogs. Journal of Functional Biomaterials. 2022; 13(3):116. https://doi.org/10.3390/jfb13030116
Chicago/Turabian StyleAmbrosio, Nicola, Silvia Voci, Agnese Gagliardi, Ernesto Palma, Massimo Fresta, and Donato Cosco. 2022. "Application of Biocompatible Drug Delivery Nanosystems for the Treatment of Naturally Occurring Cancer in Dogs" Journal of Functional Biomaterials 13, no. 3: 116. https://doi.org/10.3390/jfb13030116
APA StyleAmbrosio, N., Voci, S., Gagliardi, A., Palma, E., Fresta, M., & Cosco, D. (2022). Application of Biocompatible Drug Delivery Nanosystems for the Treatment of Naturally Occurring Cancer in Dogs. Journal of Functional Biomaterials, 13(3), 116. https://doi.org/10.3390/jfb13030116