Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Atomic Layer Deposition
2.3. Samples Characterization
2.4. In Vitro Assessment of the Cellular Response
2.4.1. Cell Culture
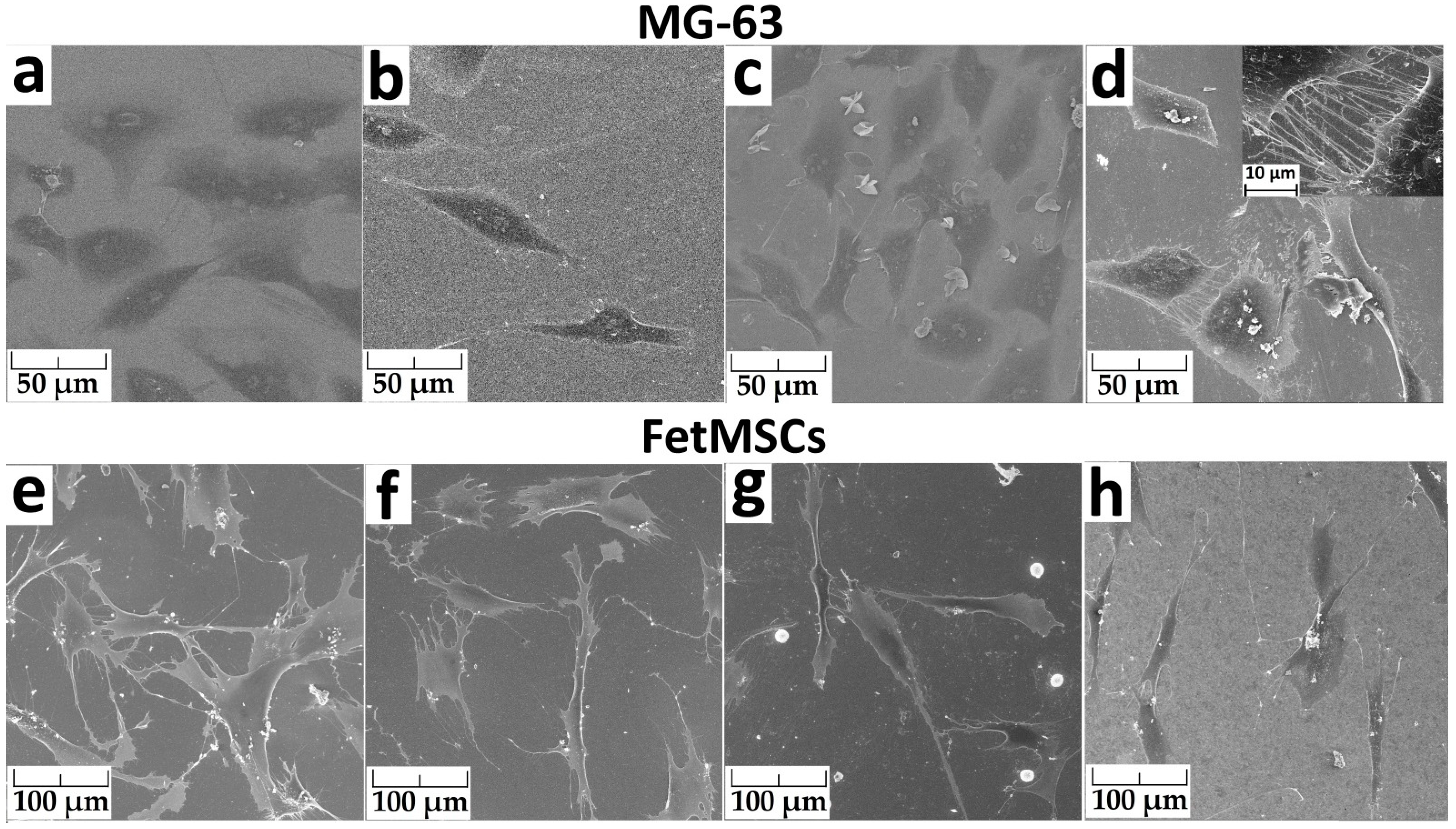
2.4.2. Cell Morphology
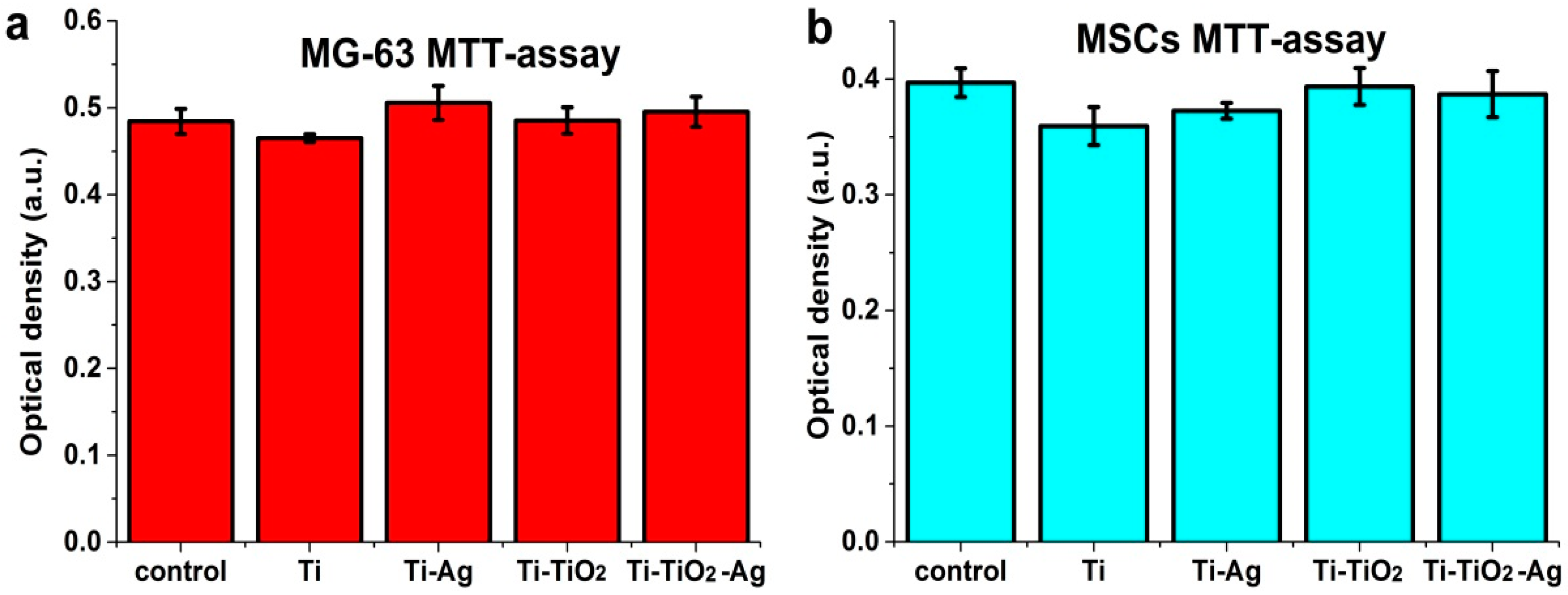
2.4.3. Cell Viability
2.4.4. Cells Osteogenic Differentiation Analysis
2.4.5. Statistical Analysis
2.5. Antibacterial Activity
3. Results
3.1. Search for Optimal Ag ALD Conditions
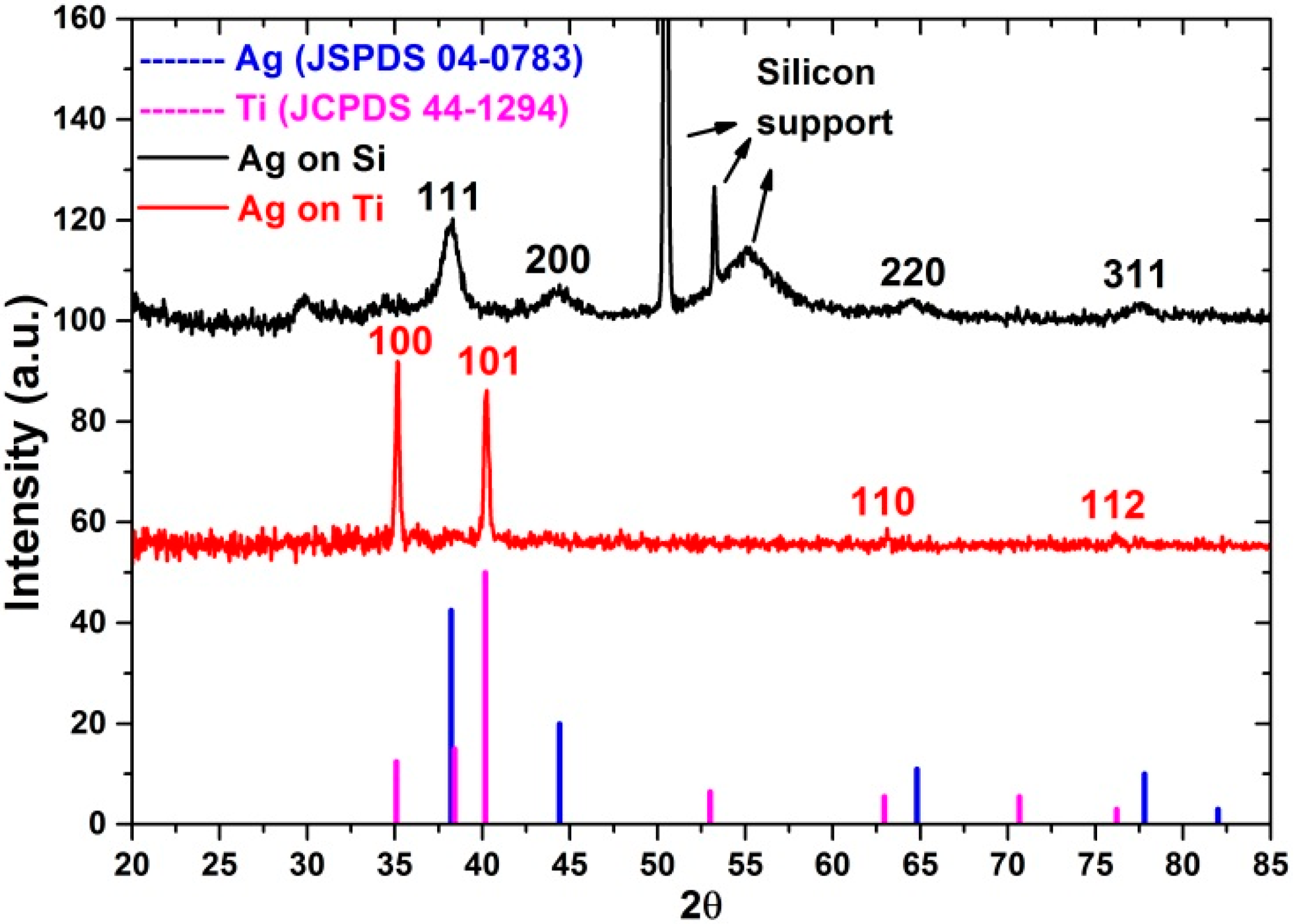
3.2. Study of Composition, Morphology and Structure of ALD Ag Nanoparticles
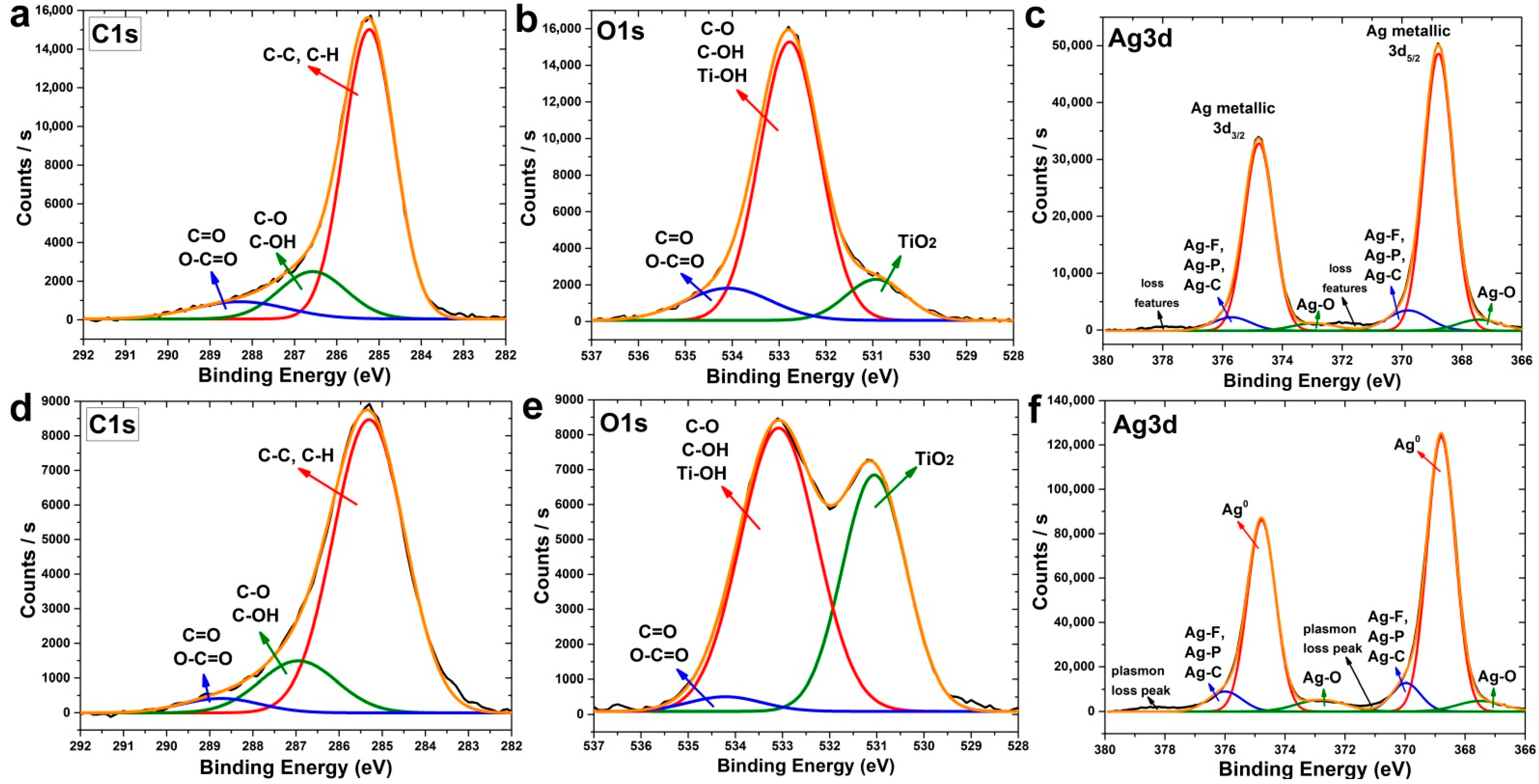
3.3. Study of Composition, Morphology and Structure of Samples Deposited for Antibacterial and In Vitro Study
- (1)
- Polished titanium (Ti);
- (2)
- Titanium with ALD silver NPs (Ti-Ag);
- (3)
- Titanium with ALD titanium oxide nanocoatings (Ti-TiO2);
- (4)
- Titanium with ALD titanium oxide nanocoatings and silver NPs (Ti-TiO2-Ag).
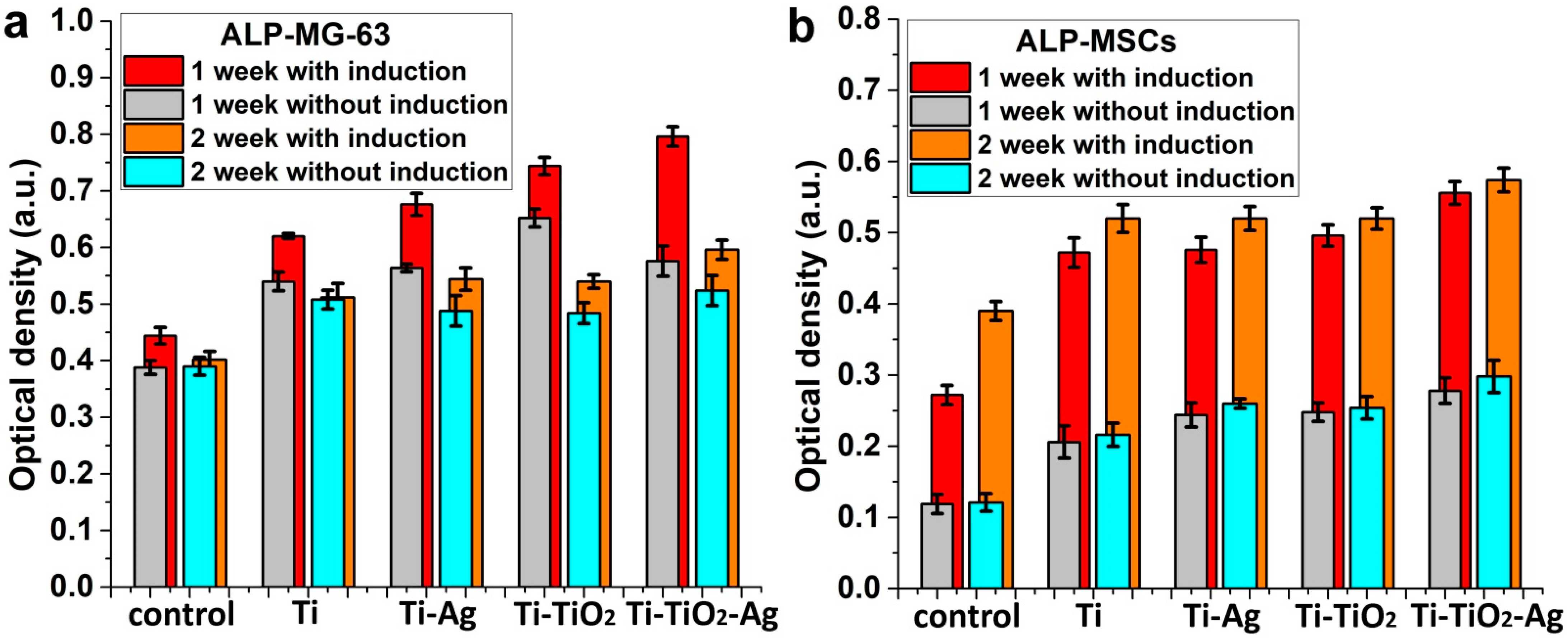
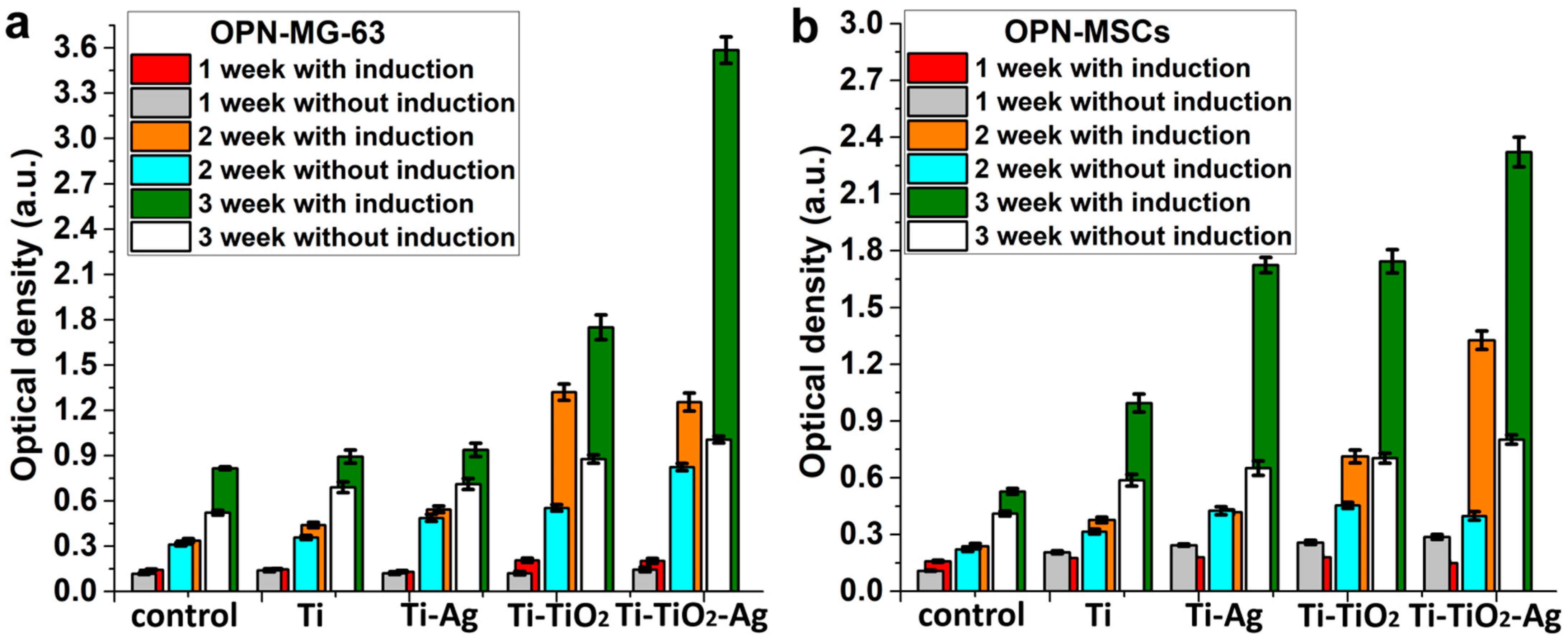
3.4. Study of Antibacterial and Cytological Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Valiev, R.Z.; Prokofiev, E.A.; Kazarinov, N.A.; Raab, G.I.; Minasov, T.B.; Strasky, J. Developing Nanostructured Ti Alloys for Innovative Implantable Medical Devices. Materals 2020, 13, 967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.; Akhavan, B.; Wise, S.G.; Bilek, M.M.M. A review of biomimetic surface functionalization for boneintegrating orthopedic implants: Mechanisms, current approaches, and future directions. Prog. Mater. Sci. 2019, 106, 100588. [Google Scholar] [CrossRef]
- Wen, C.E.; Xu, W.; Hu, W.Y.; Hodgson, P.D. Hydroxyapatite/titania sol-gel coatings on titanium-zirconium alloy for biomedical applications. Acta Biomater. 2007, 3, 403–410. [Google Scholar] [CrossRef]
- Hertz, A.; Bruce, I.J. Inorganic materials for bone repair or replacement applications. Nanomedicine 2007, 2, 899–918. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Grigal, I.P.; Markeev, A.M.; Gudkova, S.A.; Chernikova, A.G.; Mityaev, A.S.; Alekhin, A.P. Correlation between bioactivity and structural properties of titanium dioxide coatings grown by atomic layer deposition. Appl. Surf. Sci. 2012, 258, 3415–3419. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Ji, W.; Shi, B. The risk factors of early implant failure: A retrospective study of 6113 implants. Clin. Implant Dent. Relat. Res. 2021, 23, 280–288. [Google Scholar] [CrossRef]
- Hamilton, H.; Jamieson, J. Deep infection in total hip arthroplasty. Can. J. Surg. 2008, 51, 111–117. [Google Scholar]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Zheng, Y.; Wang, Y.; Du, T.; Li, C.; Wang, X.; Jiang, H. Versatile roles of silver in Ag-based nanoalloys for antibacterial applications. Coord. Chem. Rev. 2021, 449, 214218. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Miikkulainen, V.; Leskelä, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301. [Google Scholar] [CrossRef]
- Gupta, B.; Hossain, M.A.; Riaz, A.; Sharma, A.; Zhang, D.; Tan, H.H.; Jagadish, C.; Catchpole, K.; Hoex, B.; Karuturi, S. Recent Advances in Materials Design Using Atomic Layer Deposition for Energy Applications. Adv. Funct. Mater. 2021, 32, 2109105. [Google Scholar] [CrossRef]
- Kariniemi, M.; Niinisto, J.; Hatanpaa, T.; Kemell, M.; Sajavaara, T.; Ritala, M.; Leskela, M. Plasma-Enhanced Atomic Layer Deposition of Silver Thin Films. Chem. Mater. 2011, 23, 2901–2907. [Google Scholar] [CrossRef]
- Wack, S.; Lunca Popa, P.; Adjeroud, N.; Guillot, J.; Pistillo, B.R.; Leturcq, R. Large-Scale Deposition and Growth Mechanism of Silver Nanoparticles by Plasma-Enhanced Atomic Layer Deposition. J. Phys. Chem. C 2019, 123, 27196–27206. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Zemtsova, E.G.; Solokhin, A.Y.; Valiev, R.Z.; Smirnov, V.M. Modification of the Surface Topography and Composition of Ultrafine and Coarse Grained Titanium by Chemical Etching. Nanomaterials 2017, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, D.V.; Smirnov, V.M.; Zemtsova, E.G.; Yudintceva, N.M.; Shevtsov, M.A.; Valiev, R.Z. Enhanced Osseointegrative Properties of Ultra-Fine-Grained Titanium Implants Modified by Chemical Etching and Atomic Layer Deposition. ACS Biomater. Sci. Eng. 2018, 4, 3268–3281. [Google Scholar] [CrossRef]
- Nazarov, D.; Zemtsova, E.; Smirnov, V.; Mitrofanov, I.; Maximov, M.; Yudintceva, N.; Shevtsov, M. The Effects of Chemical Etching and Ultra-Fine Grain Structure of Titanium on MG-63 Cells Response. Metals 2021, 11, 510. [Google Scholar] [CrossRef]
- Nazarov, D.; Rudakova, A.; Borisov, E.; Popovich, A. Surface Modification of Additively Manufactured Nitinol by Wet Chemical Etching. Materials 2021, 14, 7683. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Chatakun, P.; Nunez-Toldra, R.; Diaz Lopez, E.J.; Gil-Recio, C.; Martinez-Sarra, E.; Hernandez-Alfaro, F.; Ferres-Padro, E.; Giner-Tarrida, L.; Atari, M. The effect of five proteins on stem cells used for osteoblast differentiation and proliferation: A current review of the literature. Cell. Mol. Life Sci. 2014, 71, 113–142. [Google Scholar] [CrossRef] [PubMed]
- Minjauw, M.M.; Solano, E.; Sree, S.P.; Asapu, R.; Van Daele, M.; Ramachandran, R.K.; Heremans, G.; Verbruggen, S.W.; Lenaerts, S.; Martens, J.A.; et al. Plasma-Enhanced Atomic Layer Deposition of Silver Using Ag(fod)(PEt3) and NH3-Plasma. Chem. Mater. 2017, 29, 7114–7121. [Google Scholar] [CrossRef]
- Amusan, A.A.; Kalkofen, B.; Gargouri, H.; Wandel, K.; Pinnow, C.; Lisker, M.; Burte, E.P. Ag films grown by remote plasma enhanced atomic layer deposition on different substrates. J. Vac. Sci. Technol. A 2016, 34, 01A126. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Yudintceva, N.; Mitrofanov, I.; Shevtsov, M.; Rudakova, A.; Maximov, M. MG-63 and FetMSC Cell Response on Atomic Layer Deposited TiO2 Nanolayers Prepared Using Titanium Tetrachloride and Tetraisopropoxide. Coatings 2022, 12, 668. [Google Scholar] [CrossRef]
- Bhat, B.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Yudintceva, N.M.; Bogolyubova, I.O.; Muraviov, A.N.; Sheykhov, M.G.; Vinogradova, T.I.; Sokolovich, E.G.; Samusenko, I.A.; Shevtsov, M.A. Application of the allogenic mesenchymal stem cells in the therapy of the bladder tuberculosis. J. Tissue Eng. Regen. Med. 2018, 12, e1580–e1593. [Google Scholar] [CrossRef]
- Motola, M.; Capek, J.; Zazpe, R.; Bacova, J.; Hromadko, L.; Bruckova, L.; Ng, S.; Handl, J.; Spotz, Z.; Knotek, P.; et al. Thin TiO2 Coatings by ALD Enhance the Cell Growth on TiO2 Nanotubular and Flat Substrates. ACS Appl. Bio Mater. 2020, 3, 6447–6456. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Wang, L.; Feng, K.-C.; Subramanian, A.; Chang, C.-C.; Simon, M.; Nam, C.-Y.; Rafailovich, M. The Role of Titania Surface Coating by Atomic Layer Deposition in Improving Osteogenic Differentiation and Hard Tissue Formation of Dental Pulp Stem Cells. Adv. Eng. Mater. 2021, 23, 2100097. [Google Scholar] [CrossRef]
- Benhabbour, S.R.; Sheardown, H.; Adronov, A. Cell adhesion and proliferation on hydrophilic dendritically modified surfaces. Biomaterials 2008, 29, 4177–4186. [Google Scholar] [CrossRef] [PubMed]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Jedrzejewski, T.; Kozak, W.; Sadowska, B.; Wieckowska-Szakiel, M.; Talik, E.; Mäkelä, M.; Leskelä, M.; Piszczek, P. Optimization of the Silver Nanoparticles PEALD Process on the Surface of 1-D Titania Coatings. Nanmaterials 2017, 7, 193. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, V.; Kadian, S.; Detwiler, D.A.; Zareei, A.; Woodhouse, I.; Qi, Z.; Peana, S.; Alcaraz, A.M.; Wang, H.; Rahimi, R. Laser-Assisted Nanotexturing and Silver Immobilization on Titanium Implant Surfaces to Enhance Bone Cell Mineralization and Antimicrobial Properties. Langmuir 2022, 38, 4014–4027. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.K.; Lappalainen, P. Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 446–454. [Google Scholar] [CrossRef]
- Sengstock, C.; Diendorf, J.; Epple, M.; Schildhauer, T.A.; Koller, M. Effect of silver nanoparticles on human mesenchymal stem cell differentiation. Beilstein J. Nanotechnol. 2014, 5, 2058–2069. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Zhu, Y.; Lu, D.Z.; Dong, W.; Bi, W.J.; Feng, X.J.; Wen, L.M.; Sun, H.; Qi, M.C. Evaluation of osteogenic and antibacterial properties of strontium/silver-containing porous TiO2 coatings prepared by micro-arc oxidation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 505–516. [Google Scholar] [CrossRef]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, A.; Kapoor, U.; Jothinarayanan, N.; Lenka, N.; Pattanayak, D.K. Effect of Silver-Containing Titania Layers for Bioactivity, Antibacterial Activity, and Osteogenic Differentiation of Human Mesenchymal Stem Cells on Ti Metal. ACS Appl. Bio Mater. 2019, 2, 3808–3819. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Fu, J.; Zhao, L.; Chu, P.K.; Huo, K. Biofunctional Elements Incorporated Nano/Microstructured Coatings on Titanium Implants with Enhanced Osteogenic and Antibacterial Performance. Adv. Healthc. Mater. 2020, 9, e2000681. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chang, R.; Webster, T.J. Atomic Layer Deposition Coating of TiO2 Nano-Thin Films on Magnesium-Zinc Alloys to Enhance Cytocompatibility for Bioresorbable Vascular Stents. Int. J. Nanomed. 2019, 14, 9955–9970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111505. [Google Scholar] [CrossRef] [PubMed]
- Ylivaara, O.M.E.; Langner, A.; Liu, X.; Schneider, D.; Julin, J.; Arstila, K.; Sintonen, S.; Ali, S.; Lipsanen, H.; Sajavaara, T.; et al. Mechanical and optical properties of as-grown and thermally annealed titanium dioxide from titanium tetrachloride and water by atomic layer deposition. Thin Solid Film. 2021, 732, 138758. [Google Scholar] [CrossRef]
- Kilpi, L.; Ylivaara, O.M.E.; Vaajoki, A.; Malm, J.; Sintonen, S.; Tuominen, M.; Puurunen, R.L.; Ronkainen, H. Microscratch testing method for systematic evaluation of the adhesion of atomic layer deposited thin films on silicon. J. Vac. Sci. Technol. A Vac. Surf. Film. 2016, 34, 01A124. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Markeev, A.M.; Tetyukhin, D.V.; Kozlov, E.N.; Molchanov, S.A. Applications of atomic layer deposition in implant dentistry. Eur. Cells Mater. 2014, 27, 17. [Google Scholar]
- Picosun Strengthens Its Presence in the Healthcare Industries. Available online: https://markets.businessinsider.com/news/stocks/picosun-strengthens-its-presence-in-the-healthcare-industries-1028237785 (accessed on 3 May 2022).





| Time of Sputtering, s | C, % | O, % | Ag, % | Ti, % | F, % | P, % |
|---|---|---|---|---|---|---|
| 0 | 59.46 | 24.76 | 10.45 | 2.74 | 1.89 | 0.70 |
| 30 | 42.92 | 21.21 | 25.34 | 7.29 | 2.04 | 1.20 |
| 60 | 2.73 | 25.56 | 3.43 | 68.28 | 0 | 0 |
| Sample | 107 CFU/mL | 106 CFU/mL |
|---|---|---|
| Ti | ~105 | ~104 |
| Ti-TiO2 | ~280 | ~150 |
| Ti-Ag | ~140 | ~80 |
| Ti-TiO2-Ag | ~85 | 42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, D.; Ezhov, I.; Yudintceva, N.; Shevtsov, M.; Rudakova, A.; Kalganov, V.; Tolmachev, V.; Zharova, Y.; Lutakov, O.; Kraeva, L.; et al. Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition. J. Funct. Biomater. 2022, 13, 62. https://doi.org/10.3390/jfb13020062
Nazarov D, Ezhov I, Yudintceva N, Shevtsov M, Rudakova A, Kalganov V, Tolmachev V, Zharova Y, Lutakov O, Kraeva L, et al. Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition. Journal of Functional Biomaterials. 2022; 13(2):62. https://doi.org/10.3390/jfb13020062
Chicago/Turabian StyleNazarov, Denis, Ilya Ezhov, Natalia Yudintceva, Maxim Shevtsov, Aida Rudakova, Vladimir Kalganov, Vladimir Tolmachev, Yuliya Zharova, Oleksiy Lutakov, Ludmila Kraeva, and et al. 2022. "Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition" Journal of Functional Biomaterials 13, no. 2: 62. https://doi.org/10.3390/jfb13020062
APA StyleNazarov, D., Ezhov, I., Yudintceva, N., Shevtsov, M., Rudakova, A., Kalganov, V., Tolmachev, V., Zharova, Y., Lutakov, O., Kraeva, L., Rogacheva, E., & Maximov, M. (2022). Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition. Journal of Functional Biomaterials, 13(2), 62. https://doi.org/10.3390/jfb13020062










