Microbial Adhesion to Dental Polymers for Conventional, Computer-Aided Subtractive and Additive Manufacturing: A Comparative In Vitro Study
Abstract
:1. Introduction
- H01: There are no differences in the values of the adhesion index of normal microbiota to the materials of the studied groups;
- H02: There are no differences in the values of the adhesion index of periodontopathogenic microbiota to the materials of the studied groups;
- H03: There are no differences in the values of the adhesion index of fungal microbiota to the materials of the studied groups.
2. Materials and Methods
2.1. Study Design
2.1.1. General Information
2.1.2. Sample Size
2.2. Sample Making
2.2.1. Computer-Aided Additive Manufacturing
2.2.2. Computer-Aided Subtractive Manufacturing
2.2.3. Conventional Samples
2.3. Microbiological Techniques
2.4. Statistical Analysis
3. Results
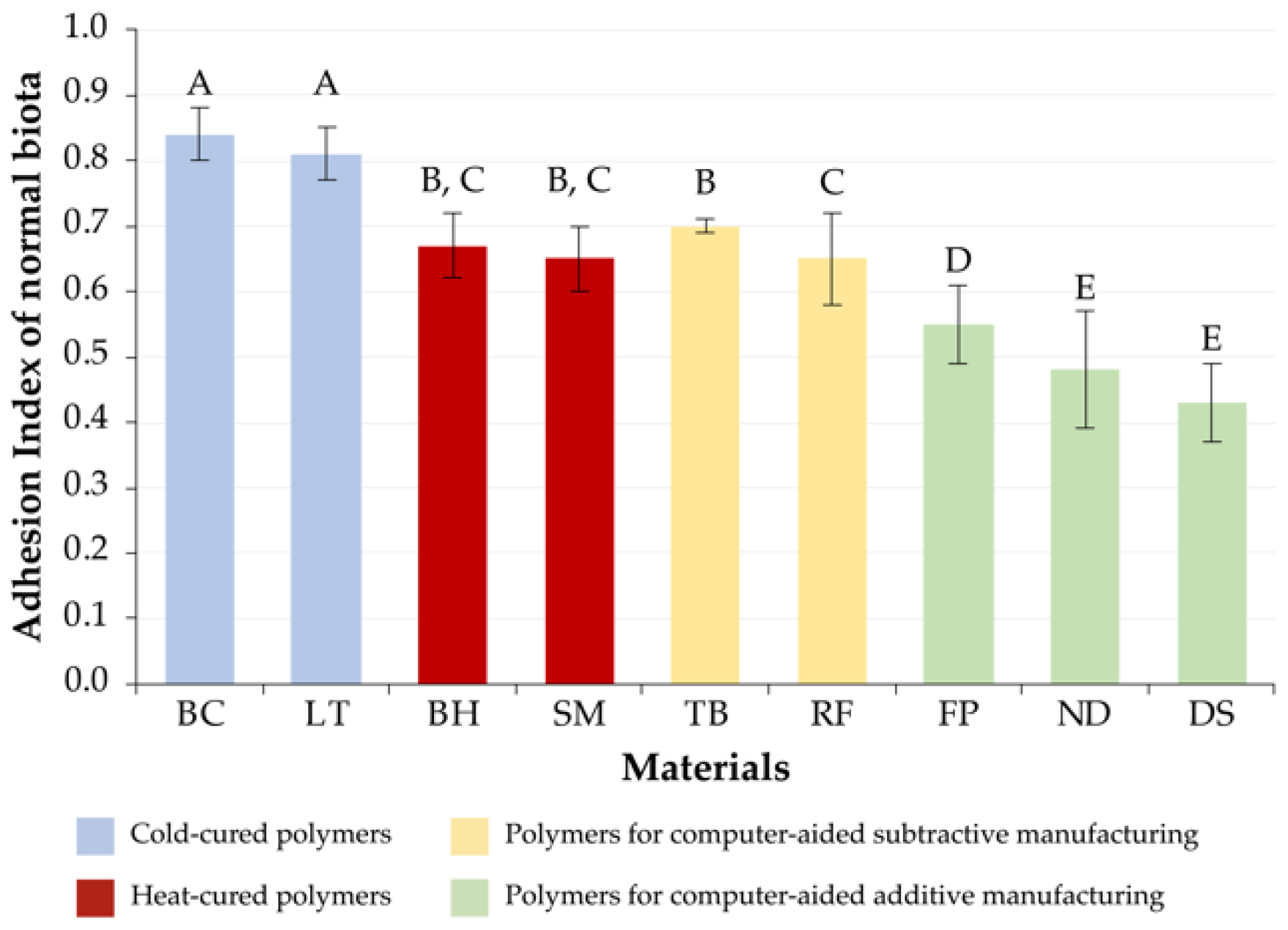
3.1. Normal Microbiota
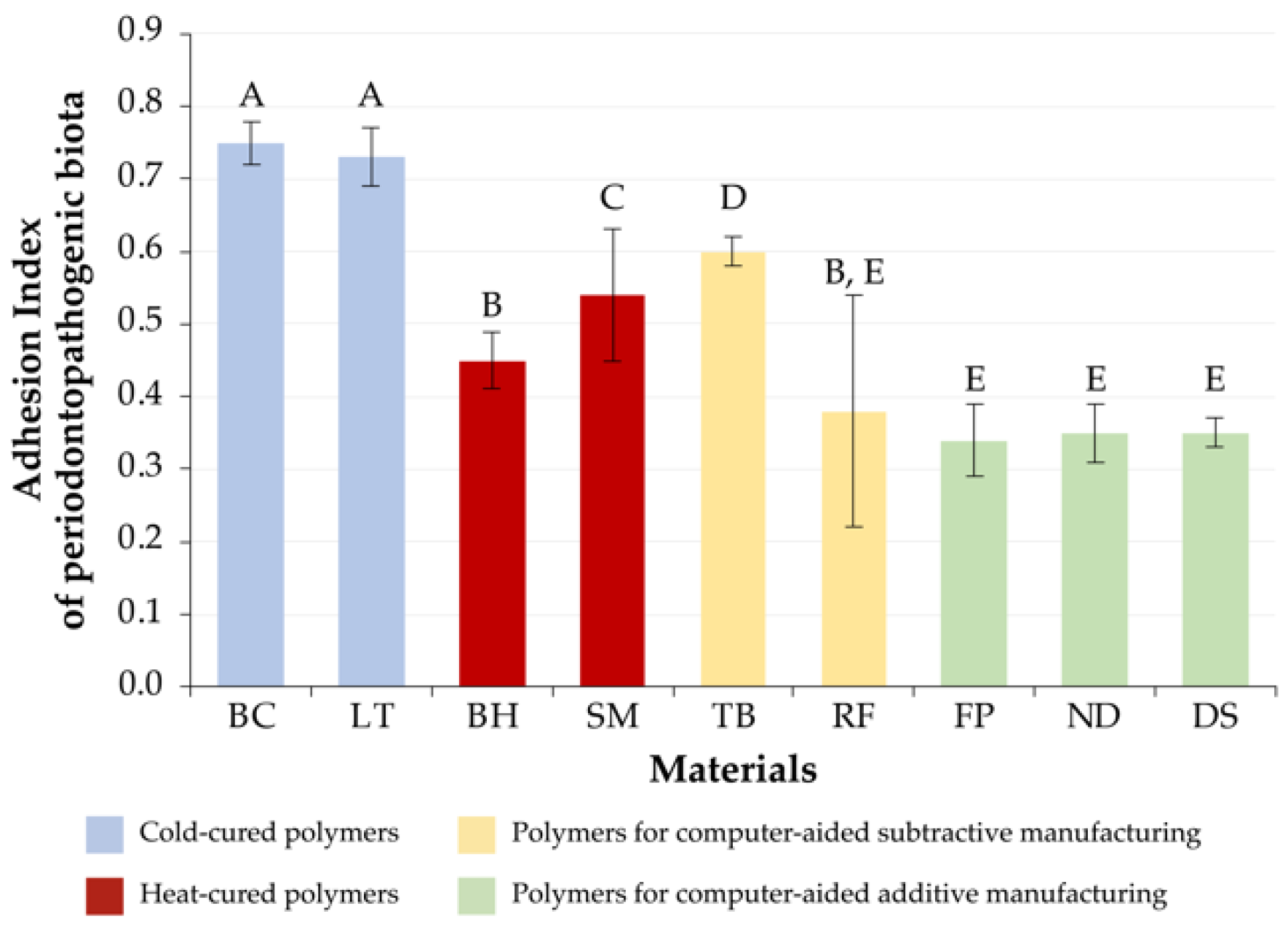
3.2. Periodontopathogenic Microbiota
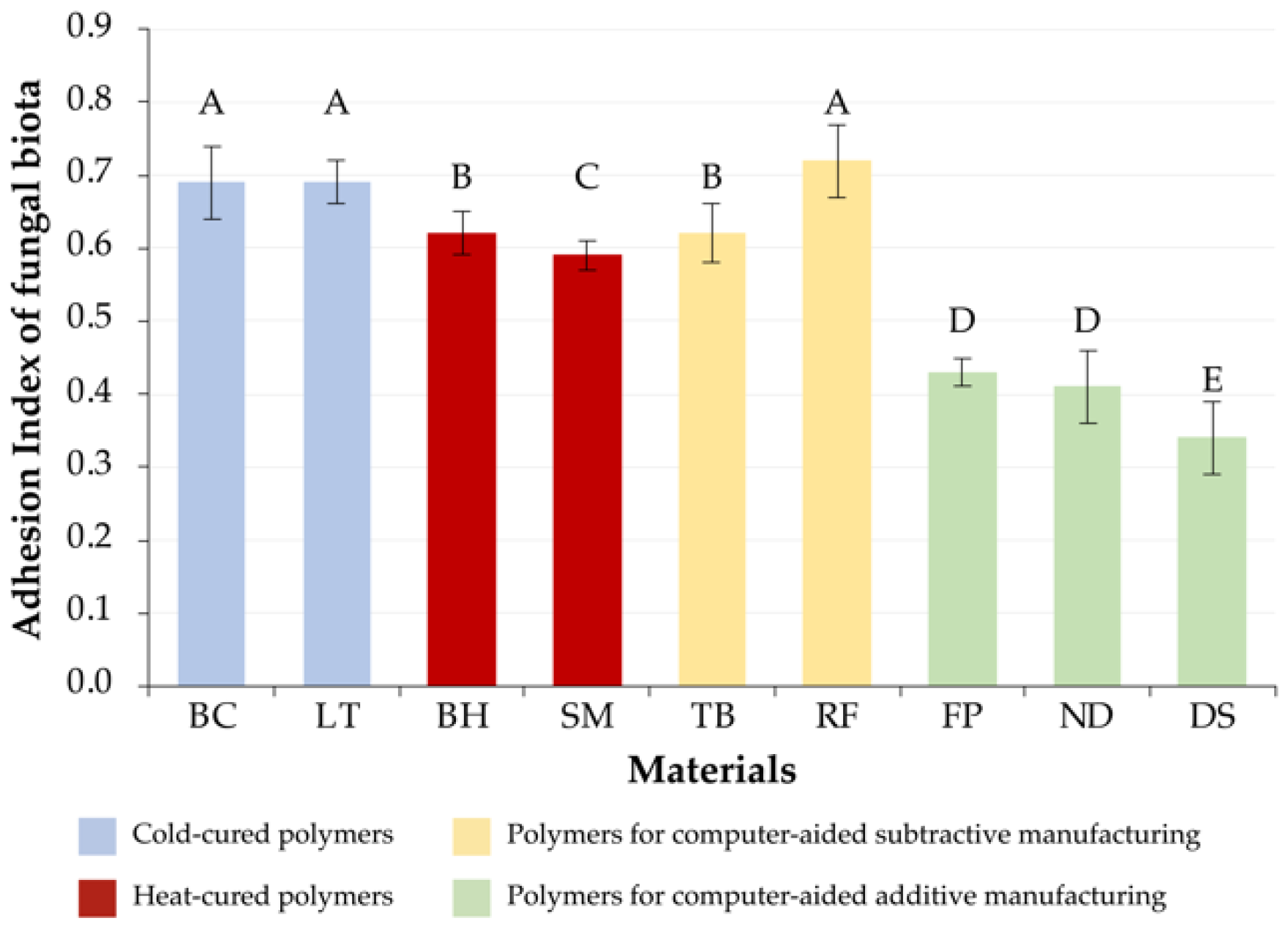
3.3. Fungal Microbiota
3.4. Summary Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galindo, D.; Soltys, J.L.; Graser, G.N. Long-term reinforced fixed provisional restorations. J. Prosthet. Dent. 1998, 79, 698–701. [Google Scholar] [CrossRef]
- Burns, D.R.; Beck, D.A.; Nelson, S.K. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: Report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J. Prosthet. Dent. 2003, 90, 474–497. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Campanelli de Morais, D.; Melo de Matos, J.D.; Lopes, G.d.R.S.; Dal Piva, A.M.d.O.; Souto Borges, A.L.; Bottino, M.A.; Lanzotti, A.; Martorelli, M.; Ausiello, P. Influence of Framework Material and Posterior Implant Angulation in Full-Arch All-on-4 Implant-Supported Prosthesis Stress Concentration. Dent. J. 2022, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Penteado, M.M.; Tribst, J.P.M.; Dal Piva, A.M.; Ausiello, P.; Zarone, F.; Garcia-Godoy, F.; Borges, A.L. Mechanical behavior of conceptual posterior dental crowns with functional elasticity gradient. Am. J. Dent. 2019, 32, 165–168. [Google Scholar] [PubMed]
- Takeuchi, Y.; Nakajo, K.; Sato, T.; Koyama, S.; Sasaki, K.; Takahashi, N. Quantification and identification of bacteria in acrylic resin dentures and dento-maxillary obturator-prostheses. Am. J. Dent. 2012, 25, 171–175. [Google Scholar]
- Xu, X.; He, L.; Zhu, B.; Lib, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- An, S.; Evans, J.L.; Hamlet, S.; Love, R.M. Incorporation of antimicrobial agents in denture base resin: A systematic review. J. Prosthet. Dent. 2021, 126, 188–195. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Zanocco, M.; Honma, T.; Zhu, W.; Pezzotti, G. Explorative study on the antibacterial effects of 3D-printed PMMA/nitrides composites. Mater. Des. 2021, 206, 109788. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [Green Version]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.-J.; Kim, M.-J.; Oh, S.-H.; Kwon, J.-S. Novel Dental Poly (Methyl Methacrylate) Containing Phytoncide for Antifungal Effect and Inhibition of Oral Multispecies Biofilm. Materials 2020, 13, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulthwaite, L.; Verran, J. Potential pathogenic aspects of denture plaque. Br. J. Biomed. Sci. 2007, 64, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Rylev, M.; Kilian, M. Prevalence and distribution of principal periodontal pathogens worldwide. J. Clin. Periodontol. 2008, 35, 346–361. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Gómez-Gaviria, M.; Mora-Montes, H.M. Current Aspects in the Biology, Pathogeny, and Treatment of Candida krusei, a Neglected Fungal Pathogen. Infect. Drug. Resist. 2020, 13, 1673–1689. [Google Scholar] [CrossRef]
- Kinkela Devcic, M.; Simonic-Kocijan, S.; Prpic, J.; Paskovic, I.; Cabov, T.; Kovac, Z.; Glazar, I. Oral Candidal Colonization in Patients with Different Prosthetic Appliances. J. Fungi 2021, 7, 662. [Google Scholar] [CrossRef]
- Lionakis, M.S. New insights into innate immune control of systemic candidiasis. Med. Mycol. 2014, 52, 555–564. [Google Scholar] [CrossRef] [Green Version]
- McFarland, J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA 1907, 49, 1176–1178. [Google Scholar] [CrossRef] [Green Version]
- Busscher, H.J.; Cowan, M.M.; van der Mei, H.C. On the relative importance of specific and non-specific approaches to oral microbial adhesion. FEMS Microbiol. Rev. 1992, 8, 199–209. [Google Scholar] [CrossRef]
- Giti, R.; Dabiri, S.; Motamedifar, M.; Derafshi, R. Surface roughness, plaque accumulation, and cytotoxicity of provisional restorative materials fabricated by different methods. PLoS ONE 2021, 16, e0249551. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Marechal, M.; Busscher, H.J.; Weerkamp, A.H.; Darius, P.L.; Steenberghe, D. The influence of surface free energy and surface roughness on early plaque formation. An in vivo study in man. J. Clin. Periodontol. 1990, 17, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A review on chemical composition, mechanical properties, and manufacturing work flow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gantz, L.; Fauxpoint, G.; Arntz, Y.; Pelletier, H.; Etienne, O. In vitro comparison of the surface roughness of polymethyl methacrylate and bis-acrylic resins for interim restorations before and after polishing. J. Prosthet. Dent. 2021, 125, 833.e1–833.e10. [Google Scholar] [CrossRef] [PubMed]
- Paolone, G.; Moratti, E.; Goracci, C.; Gherlone, E.; Vichi, A. Effect of Finishing Systems on Surface Roughness and Gloss of Full-Body Bulk-Fill Resin Composites. Materials 2020, 13, 5657. [Google Scholar] [CrossRef]
- Arnold, C.; Monsees, D.; Hey, J.; Schweyen, R. Surface Quality of 3D-Printed Models as a Function of Various Printing Parameters. Materials 2019, 12, 1970. [Google Scholar] [CrossRef] [Green Version]
- Favero, C.S.; English, J.D.; Cozad, B.E.; Wirthlin, J.O.; Short, M.M.; Kasper, F.K. Effect of print layer height and printer type on the accuracy of 3-dimensional printed orthodontic models. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 557–565. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.S.; Kim, J.E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Li, P.L.; Chu, F.T.; Shen, G. Influence of the three-dimensional printing technique and printing layer thickness on model accuracy. J. Orofac. Orthop. 2019, 80, 194–204. [Google Scholar] [CrossRef]
- Taylor, R.L.; Verran, J.; Lees, G.C.; Ward, A.J. The influence of substratum topography on bacterial adhesion to polymethyl methacrylate. J. Mater. Sci. Mater. Med. 1998, 9, 17–22. [Google Scholar] [CrossRef]
- Benli, M.; Eker Gümüş, B.; Kahraman, Y.; Gökçen-Rohlig, B.; Evlioğlu, G.; Huck, O.; Özcan, M. Surface roughness and wear behavior of occlusal splint materials made of contemporary and high-performance polymers. Odontology 2020, 108, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Bollenl, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Kerr, A.; Cowling, M.J. The effects of surface topography on the accumulation of biofouling. Philos. Mag. 2003, 83, 2779–2795. [Google Scholar] [CrossRef]
- Mondelli, R.F.; Garrido, L.M.; Soares, A.F.; Rodriguez-Medina, A.D.; Mondelli, J.; de Lucena, F.S.; Furuse, A.Y. Effect of simulated brushing on surface roughness and wear of bis-acryl-based materials submitted to different polishing protocols. J. Clin. Exp. Dent. 2022, 14, e168–e176. [Google Scholar] [CrossRef]
- Revilla-León, M.; Morillo, J.A.; Att, W.; Özcan, M. Chemical Composition, Knoop Hardness, Surface Roughness, and Adhesion Aspects of Additively Manufactured Dental Interim Materials. J. Prosthodont. 2021, 30, 698–705. [Google Scholar] [CrossRef]
- Di Fiore, A.; Meneghello, R.; Brun, P.; Rosso, S.; Gattazzo, A.; Stellini, E.; Yilmaz, B. Comparison of the flexural and surface properties of milled, 3D-printed, and heat polymerized PMMA resins for denture bases: An in vitro study. J. Prosthodont. Res. 2021. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A Comparison of the Flexural and Impact Strengths and Flexural Modulus of CAD/CAM and Conventional Heat-Cured Polymethyl Methacrylate (PMMA). J. Prosthodont. 2020, 29, 341–349. [Google Scholar] [CrossRef]
- Marigo, L.; Nocca, G.; Fiorenzano, G.; Callà, C.; Castagnola, R.; Cordaro, M.; Paolone, G.; Sauro, S. Influences of Different Air-Inhibition Coatings on Monomer Release, Microhardness, and Color Stability of Two Composite Materials. BioMed Res. Int. 2019, 2019, 4240264. [Google Scholar] [CrossRef] [Green Version]
- Kraigsley, A.M.; Tang, K.; Lippa, K.A.; Howarter, J.A.; Lin-Gibson, S.; Lin, N.J. Effect of Polymer Degree of Conversion on Streptococcus mutans Biofilms. Macromol. Biosci. 2012, 12, 1706–1713. [Google Scholar] [CrossRef]
- da Silva Barboza, A.; Fang, L.K.; Ribeiro, J.S.; Cuevas-Suárez, C.E.; Moraes, R.R.; Lund, R.G. Physicomechanical, optical, and antifungal properties of polymethyl methacrylate modified with metal methacrylate monomers. J. Prosthet. Dent. 2021, 125, 706.e1–706.e6. [Google Scholar] [CrossRef]
- Al-Fouzan, A.F.; Al-Mejrad, L.A.; Albarrag, A.M. Adherence of Candida to complete denture surfaces in vitro: A comparison of conventional and CAD/CAM complete dentures. J. Adv. Prosthodont. 2017, 9, 402–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murat, S.; Alp, G.; Alatali, C.; Uzun, M. In Vitro Evaluation of Adhesion of Candida albicans on CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2019, 28, e873–e879. [Google Scholar] [CrossRef] [PubMed]
- Meirowitz, A.; Rahmanov, A.; Shlomo, E.; Zelikman, H.; Dolev, E.; Sterer, N. Effect of Denture Base Fabrication Technique on Candida albicans Adhesion In Vitro. Materials 2021, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Meurman, J.H.; Kari, K.; Rautemaa, R.; Samaranayake, L.P. In vitro adhesion of Candida species to denture base materials. Mycoses 2006, 49, 80–84. [Google Scholar] [CrossRef]
| Material | Code | Manufacturer | Composition | Manufacturing Technology [Code] |
|---|---|---|---|---|
| Belakril-M HO Tempo, A2 | BC | LTD “TD Vladmiva”, Belgorod, Russia | PMMA a | Conventional cold-cured polymer [C] |
| Luxatemp Automix Plus, A2 | LT | DMG Chemisch-Pharmazeutische, Fabrik GmbH, Hamburg, Germany | Glass filler in a matrix of multifunctional methacrylates; catalysts, stabilizersand additives. Free of methyl methacrylate and peroxides. Total filler volume: 44 w.% = 24 vol.% (0.02 to 2.5 μm) a | |
| Belakril-M GO Tempo A2 | BH | LTD “TD Vladmiva”, Belgorod, Russia | PMMA a | Conventional heat-cured polymer [H] |
| Sinma-M, A2 | SM | AO “Stoma”, Kharkiv, Ukraine | Acrylic fluorine-containing heat-polymerized resin of powder-liquid type a | |
| Temp Basic, A2–B2 | TB | ZirkonZahn GmbH, Gais, Italy | PMMA, 1% pigments b | Computer-aided subtractive manufacturing [S] |
| Re-Fine Acrylic, A2 | RF | Yamahachi Dental MFG., Co., Gamagori, Japan | PMMA with crosslinker and pigments b | |
| FreePrint Temp 385, A2 | FP | DETAX GmbH & Co. KG, Ettlingen, Germany | Liquid, light-curing (meth)acrylate-based onecomponent material b | Computer-aided additive manufacturing [A] |
| NextDent C&B MFH, N2 | ND | NextDent B.V., Soesterberg, Netherlands | Dimethacrylate-based resins with photo-initiator, and pigments b | |
| Dental Sand, A1–A2 | DS | Harz labs, Moscow, Russia | (Meth)acrylated oligomers, (meth)acrylated monomers, photo-initiator a |
| Microbiota | Clinical Isolates | Number of Samples (n = 774) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | LT | BH | SM | TB | RF | FP | ND | DS | ||
| Normal | Streptococcus sanguinis | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Streptococcus intermedius | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Staphylococcus aureus | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Staphylococcus warnery | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Corynebacterium xerosis | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |
| Periodontopathogenic | Porphyromonas gingivalis | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Prevotella intermedia | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
| Fungal | Candida albicans | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Candida krusei | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
| Manufacturing | Material | ||
|---|---|---|---|
| FP | ND | DS | |
| Slicing software | Asiga Composer v. 1.1.7 (Asiga, Alexandria, Australia) | PreForm v. 3.23.0 (Formlabs, Somerville, MA, USA) | Chitubox PRO v. 1.1.0 (ChiTuBox, Shenzhen, China) |
| Device | MAX UV (Asiga, Alexandria, Australia) | Form 2 (Formlabs, Somerville, USA) | Mono X (Shenzhen Anycubic Technology Co., Ltd., Shenzhen, China) |
| Tech. | Digital light processing printing technology (DLP) | Stereolithography printing technology (SLA) | Digital light processing printing technology (DLP) |
| Specifications | Manufacturer’s recommendations | Lift speed: 100 mm/min Retract speed: 1.7 mm/s Wait before print: 4 s | |
| Layer thickness | 50 µm | 50 µm | 50 µm |
| Post-processing | Anycubic Wash & Cure 2.0 (IPA 70%, wash 3 min, UV 30 min) | ||
| Rank | Microbiota | ||
|---|---|---|---|
| Normal | Periodontopathogenic | Fungal | |
| 1 | DS[A] (0.43 ± 0.06) ND[A] (0.48 ± 0.09) | FP[A] (0.34 ± 0.05) DS[A] (0.35 ± 0.02) ND[A] (0.35 ± 0.04) | DS[A] (0.34 ± 0.05) |
| 2 | FP[A] (0.55 ± 0.06) | RF[S] (0.38 ± 0.16) BH[H] (0.45 ± 0.04) | ND[A] (0.41 ± 0.05) FP[A] (0.43 ± 0.02) |
| 3 | SM[H] (0.65 ± 0.05) RF[S] (0.65 ± 0.07) BH[H] (0.67 ± 0.05) TB[S] (0.70 ± 0.01) | SM[H] (0.54 ± 0.09) | SM[H] (0.59 ± 0.02) |
| 4 | LT[C] (0.81 ± 0.04) BC[C] (0.84 ± 0.04) | TB[S] (0.60 ± 0.02) | BH[H] (0.62 ± 0.03) TB[S] (0.62 ± 0.04) |
| 5 | - | LT[C] (0.73 ± 0.04) BC[C] (0.75 ± 0.03) | LT[C] (0.69 ± 0.03) BC[C] (0.69 ± 0.05) RF[S] (0.72 ± 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arutyunov, S.; Kirakosyan, L.; Dubova, L.; Kharakh, Y.; Malginov, N.; Akhmedov, G.; Tsarev, V. Microbial Adhesion to Dental Polymers for Conventional, Computer-Aided Subtractive and Additive Manufacturing: A Comparative In Vitro Study. J. Funct. Biomater. 2022, 13, 42. https://doi.org/10.3390/jfb13020042
Arutyunov S, Kirakosyan L, Dubova L, Kharakh Y, Malginov N, Akhmedov G, Tsarev V. Microbial Adhesion to Dental Polymers for Conventional, Computer-Aided Subtractive and Additive Manufacturing: A Comparative In Vitro Study. Journal of Functional Biomaterials. 2022; 13(2):42. https://doi.org/10.3390/jfb13020042
Chicago/Turabian StyleArutyunov, Sergey, Levon Kirakosyan, Lubov Dubova, Yaser Kharakh, Nikolay Malginov, Gadzhi Akhmedov, and Viktor Tsarev. 2022. "Microbial Adhesion to Dental Polymers for Conventional, Computer-Aided Subtractive and Additive Manufacturing: A Comparative In Vitro Study" Journal of Functional Biomaterials 13, no. 2: 42. https://doi.org/10.3390/jfb13020042
APA StyleArutyunov, S., Kirakosyan, L., Dubova, L., Kharakh, Y., Malginov, N., Akhmedov, G., & Tsarev, V. (2022). Microbial Adhesion to Dental Polymers for Conventional, Computer-Aided Subtractive and Additive Manufacturing: A Comparative In Vitro Study. Journal of Functional Biomaterials, 13(2), 42. https://doi.org/10.3390/jfb13020042






