Uncovering Novel Pre-Treatment Molecular Biomarkers for Anti-TNF Therapeutic Response in Patients with Crohn’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Datasets
2.2. Biological Function Analysis
2.3. Microenvironmental Feature Evaluation
2.4. Statistical Analyses
3. Results
3.1. Identification of the Target Gene Signatures Attributed to Anti-TNF Therapy Resistance
3.2. Functional Enrichment Analysis of the Top DEGs Associated with Anti-TNF Therapy Resistance
3.3. Tissue-Microenvironment Landscape Associated with Anti-TNF Therapy Resistance
3.4. Development of Biomarkers for Anti-TNF Therapy Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares-Pineda, T.M.; Cañadas-Garre, M.; Sánchez-Pozo, A.; Calleja-Hernández, M. Pharmacogenetic biomarkers of response in Crohn’s disease. Pharmacogenomics J. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.M.; Dave, M.; Loftus, E.V., Jr. Understanding the cautions and contraindications of immunomodulator and biologic therapies for use in inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Bernstein, C.N.; Iliopoulos, D.; Macpherson, A.; Neurath, M.F.; Ali, R.A.R.; Vavricka, S.R.; Fiocchi, C. Environmental triggers in IBD: A review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 39–49. [Google Scholar] [CrossRef]
- Aardoom, M.A.; Veereman, G.; de Ridder, L. A review on the use of anti-TNF in children and adolescents with inflammatory bowel disease. Int. J. Mol. Sci. 2019, 20, 2529. [Google Scholar] [CrossRef] [Green Version]
- Lopetuso, L.R.; Gerardi, V.; Papa, V.; Scaldaferri, F.; Rapaccini, G.L.; Gasbarrini, A.; Papa, A. Can we predict the efficacy of anti-TNF-α agents? Int. J. Mol. Sci. 2017, 18, 1973. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, B.L.; Mao, R.; Zhang, S.H.; He, Y.; Zeng, Z.R.; Ben-Horin, S.; Chen, M.H. Systematic review with meta-analysis: Loss of response and requirement of anti-TNFα dose intensification in Crohn’s disease. J. Gastroenterol. 2017, 52, 535–554. [Google Scholar] [CrossRef]
- Cui, G.; Fan, Q.; Li, Z.; Goll, R.; Florholmen, J. Evaluation of anti-TNF therapeutic response in patients with inflammatory bowel disease: Current and novel biomarkers. EBioMedicine 2021, 66, 103329. [Google Scholar] [CrossRef]
- Aubert, J.; Bar-Hen, A.; Daudin, J.J.; Robin, S. Determination of the differentially expressed genes in microarray experiments using local FDR. BMC Bioinform. 2004, 5, 125. [Google Scholar] [CrossRef] [Green Version]
- Tokar, T.; Pastrello, C.; Jurisica, I. GSOAP: A tool for visualization of gene set over-representation analysis. Bioinformatics 2020, 36, 2923–2925. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari Fuchs, S.; Lieder, I.; Stelzer, G.; Mazor, Y.; Buzhor, E.; Kaplan, S.; Bogoch, Y.; Plaschkes, I.; Shitrit, A.; Rappaport, N.; et al. GeneAnalytics: An integrative gene set analysis tool for next generation sequencing, RNAseq and microarray data. Omics 2016, 20, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asplund, A.; Edqvist, P.H.; Schwenk, J.M.; Pontén, F. Antibodies for profiling the human proteome—The Human Protein Atlas as a resource for cancer research. Proteomics 2012, 12, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Karlsson, M.J.; Zhong, W.; Tebani, A.; Pou, C.; Mikes, J.; Lakshmikanth, T.; Forsström, B.; Edfors, F.; Odeberg, J.; et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science 2019, 366, eaax9198. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Karlsson, M.J.; Hober, A.; Svensson, A.S.; Scheffel, J.; Kotol, D.; Zhong, W.; Tebani, A.; Strandberg, L.; Edfors, F.; et al. The human secretome. Sci. Signal. 2019, 12, eaaz0274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becht, E.; Giraldo, N.A.; Lacroix, L.; Buttard, B.; Elarouci, N.; Petitprez, F.; Selves, J.; Laurent-Puig, P.; Sautès-Fridman, C.; Fridman, W.H.; et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016, 17, 218. [Google Scholar] [CrossRef]
- Vos, A.C.; Wildenberg, M.E.; Duijvestein, M.; Verhaar, A.P.; van den Brink, G.R.; Hommes, D.W. Anti-tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology 2011, 140, 221–230. [Google Scholar] [CrossRef]
- Agnholt, J.; Kelsen, J.; Brandsborg, B.; Jakobsen, N.O.; Dahlerup, J.F. Increased production of granulocyte-macrophage colony-stimulating factor in Crohn’s disease—A possible target for infliximab treatment. Eur. J. Gastroenterol. Hepatol. 2004, 16, 649–655. [Google Scholar] [CrossRef]
- Zeissig, S.; Bojarski, C.; Buergel, N.; Mankertz, J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut 2004, 53, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Di Sabatino, A.; Pender, S.L.; Jackson, C.L.; Prothero, J.D.; Gordon, J.N.; Picariello, L.; Rovedatti, L.; Docena, G.; Monteleone, G.; Rampton, D.S.; et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 2007, 133, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Kaymakcalan, Z.; Sakorafas, P.; Bose, S.; Scesney, S.; Xiong, L.; Hanzatian, D.K.; Salfeld, J.; Sasso, E.H. Comparisons of affinities, avidities, and complement activation of adalimumab, infliximab, and etanercept in binding to soluble and membrane tumor necrosis factor. Clin. Immunol. 2009, 131, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Borra, J.; López-Larrea, C.; González, S.; Fuentes, D.; Dieguez, A.; Deschamps, E.M.; Pérez-Pariente, J.M.; López-Vázquez, A.; de Francisco, R.; Rodrigo, L. High serum tumor necrosis factor-alpha levels are associated with lack of response to infliximab in fistulizing Crohn’s disease. Am. J. Gastroenterol. 2002, 97, 2350–2356. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.D.; Plevy, S.E.; Yang, H.; Landers, C.J.; Barry, M.J.; Rotter, J.I.; Targan, S.R. ANCA pattern and LTA haplotype relationship to clinical responses to anti-TNF antibody treatment in Crohn’s disease. Gastroenterology 2001, 120, 1347–1355. [Google Scholar] [CrossRef]
- Ferrante, M.; Vermeire, S.; Katsanos, K.H.; Noman, M.; Van Assche, G.; Schnitzler, F.; Arijs, I.; De Hertogh, G.; Hoffman, I.; Geboes, J.K.; et al. Predictors of early response to infliximab in patients with ulcerative colitis. Inflamm. Bowel Dis. 2007, 13, 123–128. [Google Scholar] [CrossRef]
- Pękala, A.; Filip, R.; Aebisher, D. Anti-drug antibodies in patients with inflammatory bowel diseases treated with biosimilar infliximab: A prospective cohort study. J. Clin. Med. 2021, 10, 2653. [Google Scholar] [CrossRef]
- Salvador-Martín, S.; Kaczmarczyk, B.; Álvarez, R.; Navas-López, V.M.; Gallego-Fernández, C.; Moreno-Álvarez, A.; Solar-Boga, A.; Sánchez, C.; Tolin, M.; Velasco, M.; et al. Whole transcription profile of responders to anti-TNF drugs in pediatric inflammatory bowel disease. Pharmaceutics 2021, 13, 77. [Google Scholar] [CrossRef]
- Toonen, E.J.; Gilissen, C.; Franke, B.; Kievit, W.; Eijsbouts, A.M.; den Broeder, A.A.; van Reijmersdal, S.V.; Veltman, J.A.; Scheffer, H.; Radstake, T.R.; et al. Validation study of existing gene expression signatures for anti-TNF treatment in patients with rheumatoid arthritis. PLoS ONE 2012, 7, e33199. [Google Scholar] [CrossRef] [Green Version]
- Verstockt, B.; Verstockt, S.; Dehairs, J.; Ballet, V.; Blevi, H.; Wollants, W.-J.; Breynaert, C.; Van Assche, G.; Vermeire, S.; Ferrante, M. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine 2019, 40, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Fauny, M.; Moulin, D.; D’Amico, F.; Netter, P.; Petitpain, N.; Arnone, D.; Jouzeau, J.-Y.; Loeuille, D.; Peyrin-Biroulet, L. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann. Rheum. Dis. 2020, 79, 1132–1138. [Google Scholar] [CrossRef]
- Ouyang, W.; Kolls, J.K.; Zheng, Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity 2008, 28, 454–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gálvez, J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta-Rodriguez, E.V.; Napolitani, G.; Lanzavecchia, A.; Sallusto, F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 2007, 8, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Wedebye Schmidt, E.G.; Larsen, H.L.; Kristensen, N.N.; Poulsen, S.S.; Lynge Pedersen, A.M.; Claesson, M.H.; Pedersen, A.E. TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Ngo, V.L.; Geem, D.; Harusato, A.; Hirota, S.A.; Parkos, C.A.; Lukacs, N.W.; Nusrat, A.; Gaboriau-Routhiau, V.; Cerf-Bensussan, N.; et al. IL-17A-mediated neutrophil recruitment limits expansion of segmented filamentous bacteria. Mucosal Immunol. 2017, 10, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Meddens, C.A.; van der List, A.C.J.; Nieuwenhuis, E.E.S.; Mokry, M. Non-coding DNA in IBD: From sequence variation in DNA regulatory elements to novel therapeutic potential. Gut 2019, 68, 928–941. [Google Scholar] [CrossRef] [Green Version]
- Mesko, B.; Poliska, S.; Váncsa, A.; Szekanecz, Z.; Palatka, K.; Hollo, Z.; Horvath, A.; Steiner, L.; Zahuczky, G.; Podani, J.; et al. Peripheral blood derived gene panels predict response to infliximab in rheumatoid arthritis and Crohn’s disease. Genome Med. 2013, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Gorenjak, M.; Zupin, M.; Jezernik, G.; Skok, P.; Potočnik, U. Omics data integration identifies ELOVL7 and MMD gene regions as novel loci for adalimumab response in patients with Crohn’s disease. Sci. Rep. 2021, 11, 5449. [Google Scholar] [CrossRef]
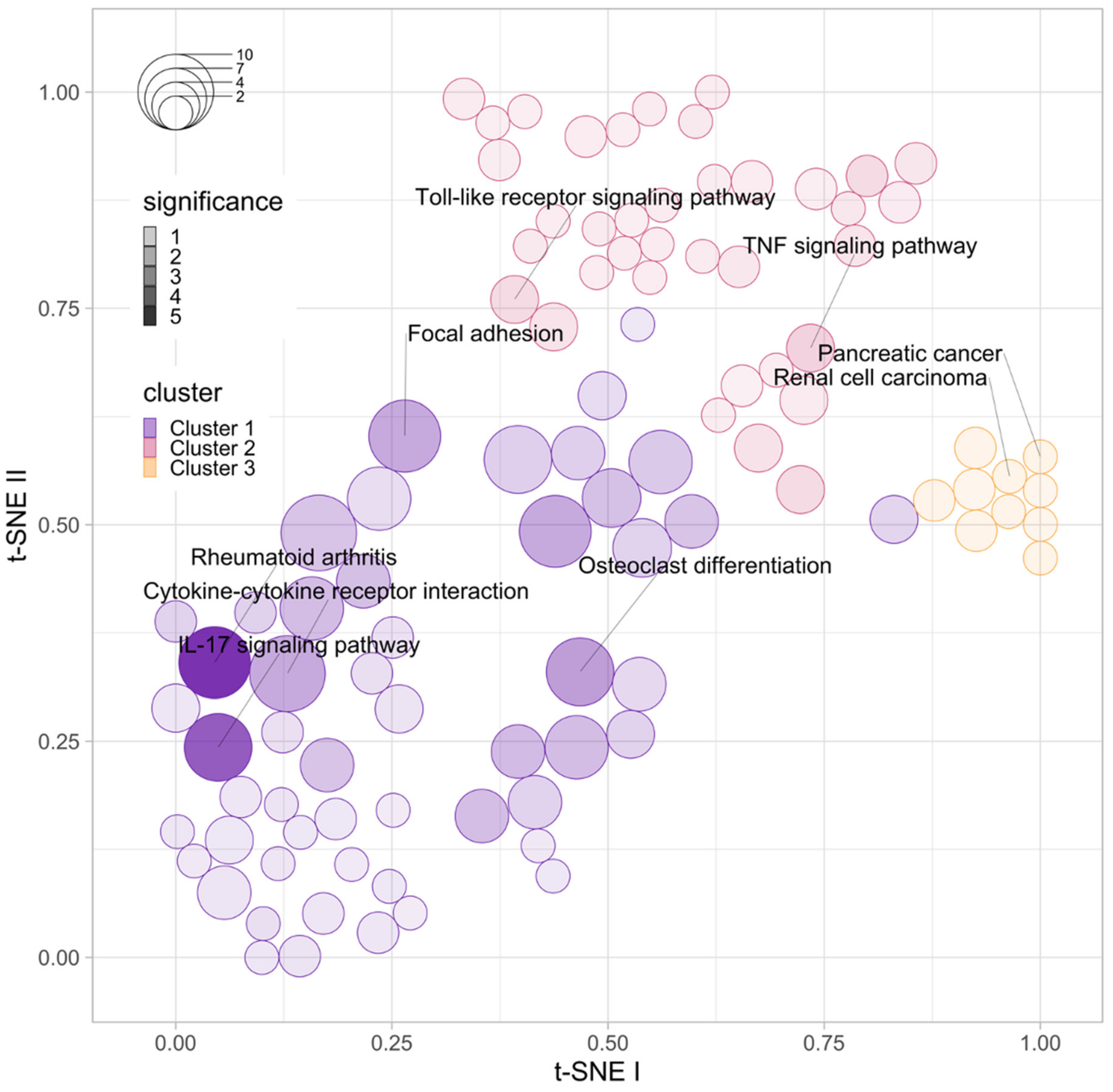
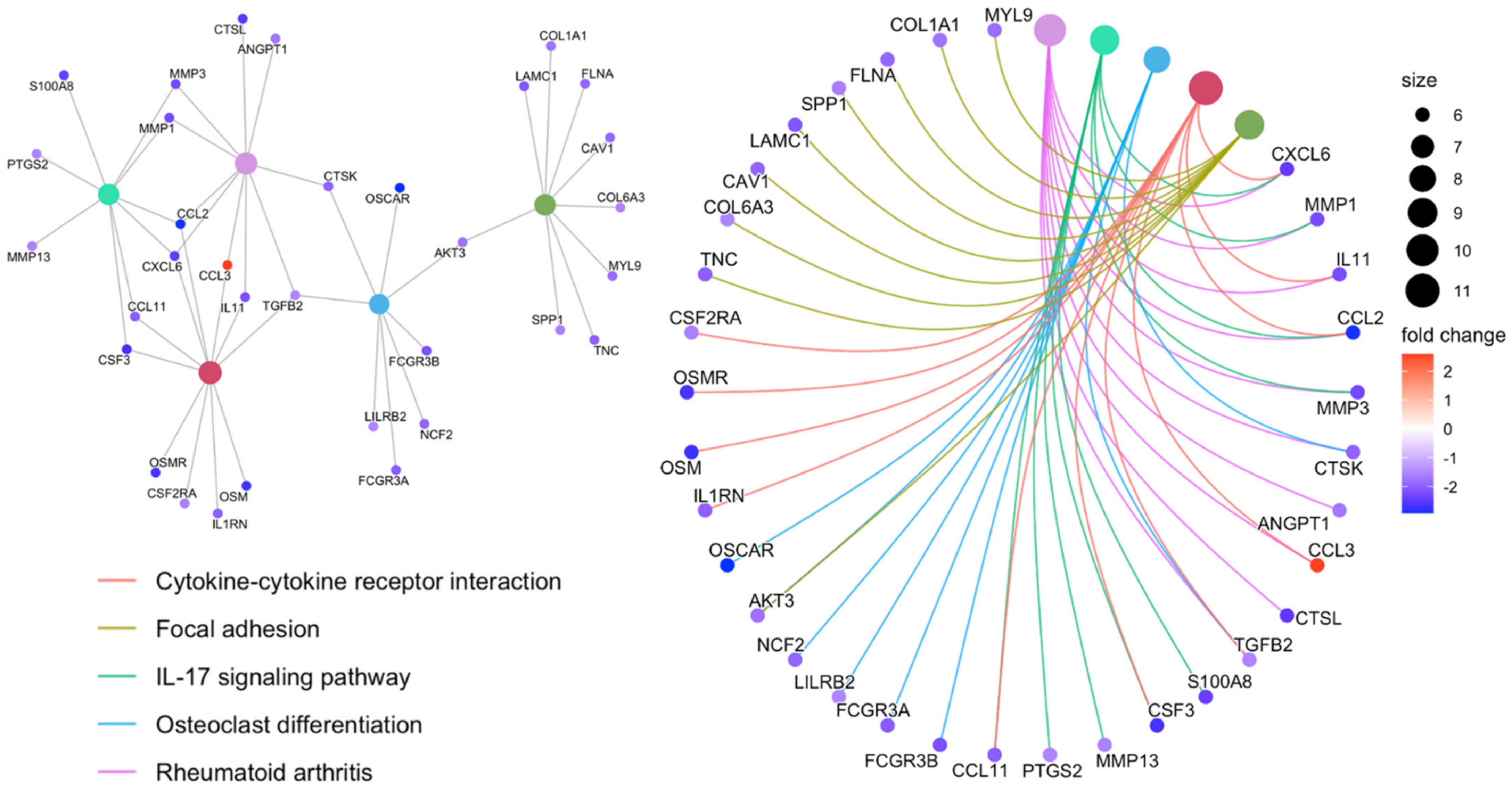


| Immune Marker | Log FC | Average Expression | t-Statistic | p-Value | Adjusted p-Value | B-Statistic |
|---|---|---|---|---|---|---|
| Fibroblast | −1.233 | 9.062 | −4.284 | <0.001 | 0.001 | 1.170 |
| Neutrophil | −0.758 | 7.115 | −4.052 | <0.001 | 0.001 | 0.486 |
| Endothelial cell | −0.568 | 5.94 | −3.452 | 0.001 | 0.005 | −1.196 |
| Monocyte | −0.594 | 7.101 | −3.045 | 0.004 | 0.009 | −2.252 |
| Macrophage/Monocyte | −0.594 | 7.101 | −3.045 | 0.004 | 0.009 | −2.252 |
| B cell | −0.476 | 7.407 | −1.434 | 0.159 | 0.291 | −5.432 |
| NK cell | 0.218 | 4.652 | 1.299 | 0.201 | 0.315 | −5.608 |
| Myeloid dendritic cell | −0.227 | 5.085 | −1.196 | 0.238 | 0.327 | −5.731 |
| cytotoxicity score | −0.220 | 4.861 | −0.996 | 0.325 | 0.397 | −5.945 |
| T cell CD8+ | −0.181 | 0.922 | −0.89 | 0.378 | 0.416 | −6.042 |
| T cell | −0.061 | 5.295 | −0.349 | 0.729 | 0.729 | −6.373 |
| Gene | Log FC | Average Expression | t-Statistic | p-Value | Adjusted p-Value | B-Statistic | GeneHancer Identifiers | Activity |
|---|---|---|---|---|---|---|---|---|
| CBR4 | 0.284 | 7.105 | 4.778 | <0.0001 | 0.236 | 2.232 | - | - |
| ACAD10 | 0.215 | 7.08 | 3.981 | <0.0001 | 0.246 | 0.228 | GH12J112025 | Enhancer active in: |
| CD4+ CD25− IL17− PMA Th | ||||||||
| primary cells | ||||||||
| BAIAP3 | −0.264 | 6.6 | −4.538 | <0.0001 | 0.246 | 1.618 | - | - |
| BMP6 | −0.702 | 7.506 | −4.298 | <0.0001 | 0.246 | 1.013 | - | - |
| DDX11L2 | −0.767 | 8.115 | −4.104 | <0.0001 | 0.246 | 0.532 | GH02J113885 | Enhancer active in: |
| CD4+ CD25− IL17− PMA Th | ||||||||
| primary cells | ||||||||
| DYNLL2 | −0.317 | 8.359 | −4.292 | <0.0001 | 0.246 | 0.999 | GH17J058328 | Enhancer active in: |
| CD4+ CD25− IL17− PMA Th | ||||||||
| primary cells, CD4+ CD25− | ||||||||
| IL17+ PMA Th17 primary cells | ||||||||
| KAT2B | −0.587 | 9.99 | −3.945 | <0.0001 | 0.246 | 0.142 | GH03J020038 | Enhancer active in: |
| GH03J020037 | CD4+ CD25− IL17+ PMA Th17 | |||||||
| GH03J020048 | primary cells | |||||||
| GH03J020047 | ||||||||
| GH03J020060 | ||||||||
| GH03J020053 | ||||||||
| GH03J020070 | ||||||||
| KLF9 | −0.29 | 7.425 | −3.859 | <0.0001 | 0.246 | −0.068 | GH09J070402 | Enhancer active in: |
| GH09J070395 | CD4+ CD25− IL17- PMA Th | |||||||
| GH09J070389 | primary cells, CD4+ CD25− | |||||||
| GH09J070400 | IL17+ PMA Th17 primary cells | |||||||
| GH09J070394 | ||||||||
| SPTSSB | −0.465 | 5.504 | −4.024 | <0.0001 | 0.246 | 0.335 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwak, M.S.; Cha, J.M.; Jeon, J.W.; Yoon, J.Y.; Park, S.B. Uncovering Novel Pre-Treatment Molecular Biomarkers for Anti-TNF Therapeutic Response in Patients with Crohn’s Disease. J. Funct. Biomater. 2022, 13, 36. https://doi.org/10.3390/jfb13020036
Kwak MS, Cha JM, Jeon JW, Yoon JY, Park SB. Uncovering Novel Pre-Treatment Molecular Biomarkers for Anti-TNF Therapeutic Response in Patients with Crohn’s Disease. Journal of Functional Biomaterials. 2022; 13(2):36. https://doi.org/10.3390/jfb13020036
Chicago/Turabian StyleKwak, Min Seob, Jae Myung Cha, Jung Won Jeon, Jin Young Yoon, and Su Bee Park. 2022. "Uncovering Novel Pre-Treatment Molecular Biomarkers for Anti-TNF Therapeutic Response in Patients with Crohn’s Disease" Journal of Functional Biomaterials 13, no. 2: 36. https://doi.org/10.3390/jfb13020036
APA StyleKwak, M. S., Cha, J. M., Jeon, J. W., Yoon, J. Y., & Park, S. B. (2022). Uncovering Novel Pre-Treatment Molecular Biomarkers for Anti-TNF Therapeutic Response in Patients with Crohn’s Disease. Journal of Functional Biomaterials, 13(2), 36. https://doi.org/10.3390/jfb13020036







