Osseointegration at Implants Installed in Composite Bone: A Randomized Clinical Trial on Sinus Floor Elevation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Population
2.3. Devices and Biomaterials
2.4. Sample Size
2.5. Study Design and Allocation Concealment
2.6. Clinical Procedures
2.7. Histological Preparation of the Biopsies
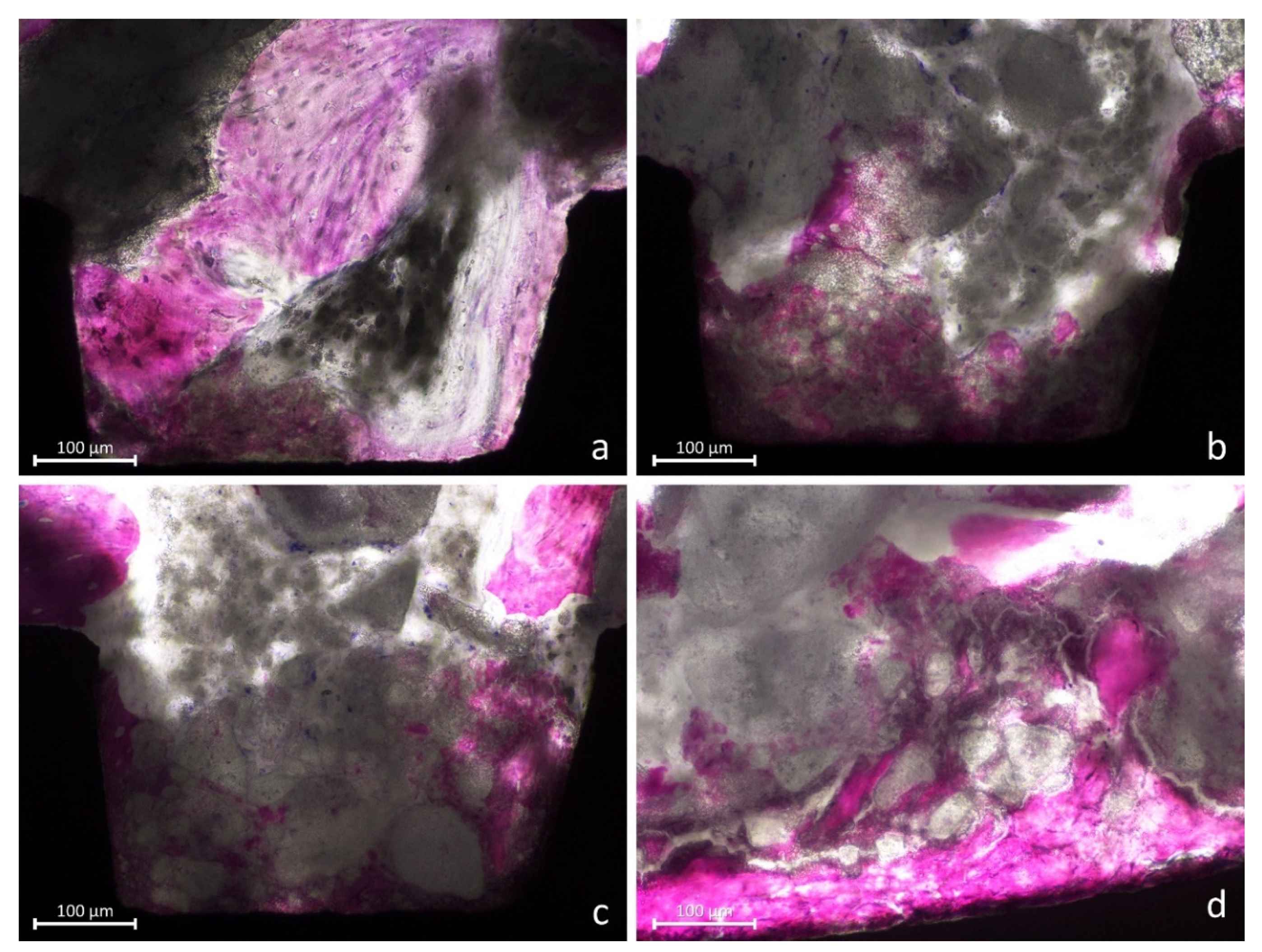
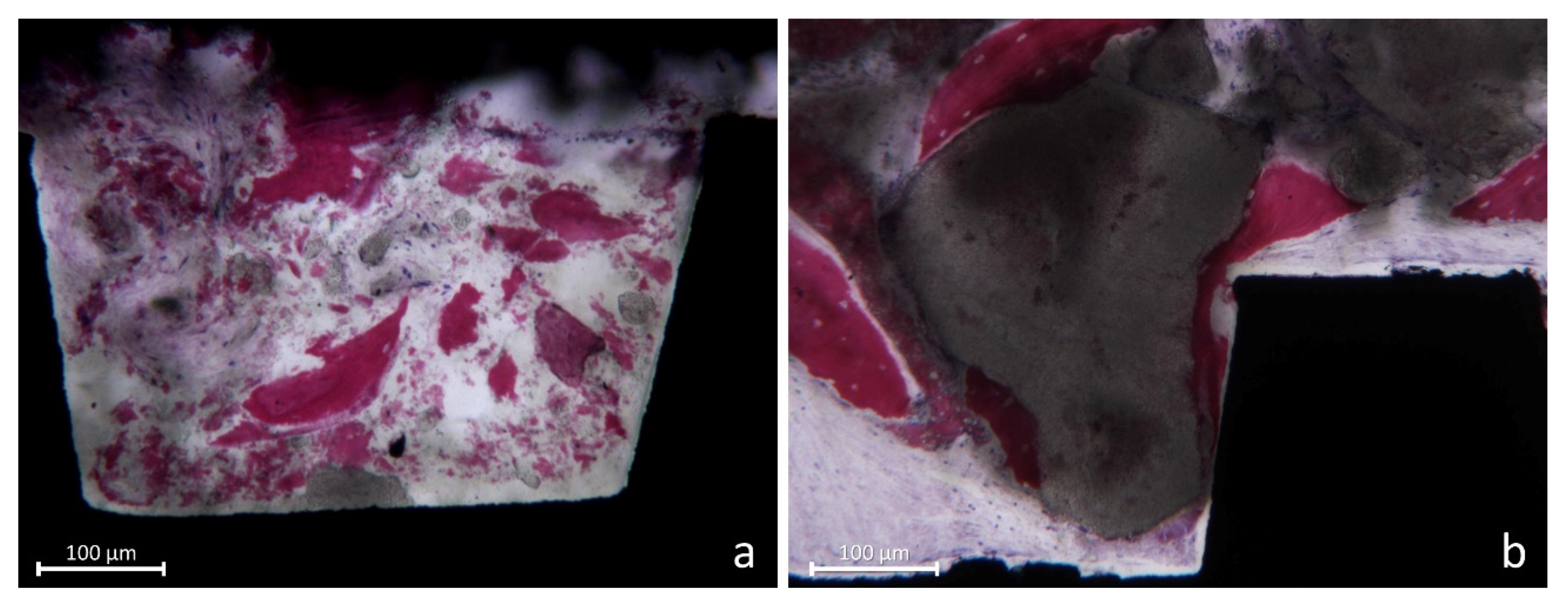
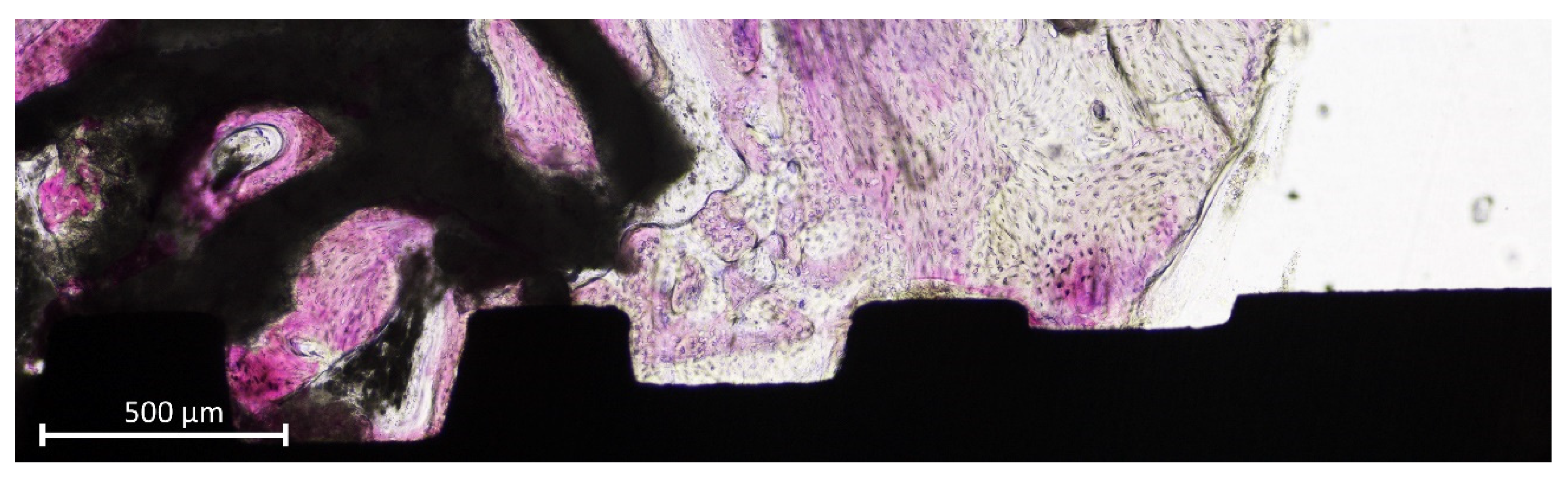
2.8. Histomorphometric Evaluation
2.9. Data Analysis
3. Results
3.1. Clinical Outcomes
3.2. Histometric Evaluations—Tissues in Contact with the Implant Surface
3.3. Morphometric Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 216–240. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Lang, N.P.; Iida, T.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the position of the antrostomy in sinus floor elevation assessed with cone-beam computed tomography: A randomized clinical trial. J. Investig. Clin. Dent. 2018, 9, e12362. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Lang, N.P.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the height of the antrostomy in sinus floor elevation assessed by cone beam computed tomography- a randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2019, 34, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Cricchio, G.; Palma, V.C.; Faria, P.E.; de Olivera, J.A.; Lundgren, S.; Sennerby, L.; Salata, L.A. Histological outcomes on the development of new space-making devices for maxillary sinus floor augmentation. Clin. Implant. Dent. Relat. Res. 2011, 13, 224–230. [Google Scholar] [CrossRef]
- Schweikert, M.; Botticelli, D.; de Oliveira, J.A.; Scala, A.; Salata, L.A.; Lang, N.P. Use of a titanium device in lateral sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 100–105. [Google Scholar] [CrossRef]
- Johansson, L.Å.; Isaksson, S.; Adolfsson, E.; Lindh, C.; Sennerby, L. Bone regeneration using a hollow hydroxyapatite space-maintaining device for maxillary sinus floor augmentation—A clinical pilot study. Clin. Implant. Dent. Relat. Res. 2012, 14, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Botticelli, D.; Ferri, M.; Delgado-Ruiz, R.; Ferreira Balan, V.; Porfirio Xavier, S. Argon Bioactivation of Implants Installed Simultaneously to Maxillary Sinus Lifting without Graft. An Experimental Study in Rabbits. Dent. J. 2021, 9, 105. [Google Scholar] [CrossRef]
- Ye, M.; Liu, W.; Cheng, S.; Yan, L. Outcomes of implants placed after osteotome sinus floor elevation without bone grafts: A systematic review and meta-analysis of single-arm studies. Int. J. Implant. Dent. 2021, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Ekhlasmandkermani, M.; Amid, R.; Kadkhodazadeh, M.; Hajizadeh, F.; Abed, P.F.; Kheiri, L.; Kheiri, A. Sinus floor elevation and simultaneous implant placement in fresh extraction sockets: A systematic review of clinical data. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 411–426. [Google Scholar] [CrossRef]
- Jensen, T.; Schou, S.; Svendsen, P.A.; Forman, J.L.; Gundersen, H.J.; Terheyden, H.; Holmstrup, P. Volumetric changes of the graft after maxillary sinus floor augmentation with Bio-Oss and autogenous bone in different ratios: A radiographic study in minipigs. Clin. Oral Implant. Res. 2012, 23, 902–910. [Google Scholar] [CrossRef]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral Implant. Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef]
- Scala, A.; Botticelli, D.; Faeda, R.S.; Garcia Rangel, I., Jr.; Américo de Oliveira, J.; Lang, N.P. Lack of influence of the Schneiderian membrane in forming new bone apical to implants simultaneously installed with sinus floor elevation: An experimental study in monkeys. Clin. Oral Implant. Res. 2012, 23, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Caneva, M.; Lang, N.P.; Garcia Rangel, I.J.; Ferreira, S.; Caneva, M.; De Santis, E.; Botticelli, D. Sinus mucosa elevation using Bio-Oss(®) or Gingistat(®) collagen sponge: An experimental study in rabbits. Clin. Oral Implant. Res. 2017, 28, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Carneiro Martins Neto, E.; Botticelli, D.; Apaza Alccayhuaman, K.A.; Lang, N.P.; Xavier, S.P. Influence of a collagen membrane positioned subjacent the sinus mucosa following the elevation of the maxillary sinus. A histomorphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 1567–1576. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, H.; Arai, Y. Complications of postoperative swelling of the maxillary sinus membrane after sinus floor augmentation. J. Oral Sci. Rehabil. 2015, 1, 26–33. [Google Scholar]
- Ohayon, L.; Taschieri, S.; Friedmann, A.; Del Fabbro, M. Bone Graft Displacement after Maxillary Sinus Floor Aug-mentation With or Without Covering Barrier Membrane: A Retrospective Computed Tomographic Image Evaluation. Int. J. Oral Maxillofac. Implant. 2019, 34, 681–691. [Google Scholar] [CrossRef]
- Suárez-López Del Amo, F.; Ortega-Oller, I.; Catena, A.; Monje, A.; Khoshkam, V.; Torrecillas-Martínez, L.; Wang, H.L.; Galindo-Moreno, P. Effect of barrier membranes on the outcomes of maxillary sinus floor augmentation: A meta-analysis of histomorphometric outcomes. Int. J. Oral Maxillofac. Implant. 2015, 30, 607–618. [Google Scholar] [CrossRef]
- Caroprese, M.; Lang, N.P.; Baffone, G.M.; Ricci, S.; Caneva, M.; Botticelli, D. Histomorphometric analysis of bone healing at implants with turned or rough surfaces: An experimental study in the dog. J. Oral Sci. Rehabil. 2016, 2, 74–79. [Google Scholar]
- Wennerberg, A.; Albrektsson, T.; Chrcanovic, B. Long-term clinical outcome of implants with different surface modifications. Eur. J. Oral Implantol. 2018, 11, S123–S136. [Google Scholar]
- Garaicoa-Pazmino, C.; Lin, G.H.; Alkandery, A.; Parra-Carrasquer, C.; Suárez-López Del Amo, F. Influence of implant surface characteristics on the initiation, progression and treatment outcomes of peri-implantitis: A systematic review and meta-analysis based on animal model studies. Int. J. Oral Implant. 2021, 14, 367–382. [Google Scholar]
- Saulacic, N.; Schaller, B. Prevalence of Peri-Implantitis in Implants with Turned and Rough Surfaces: A Systematic Review. J. Oral Maxillofac. Res. 2019, 10, e1. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, A.; Bertl, K.; Winning, L.; Polyzois, I. What is the influence of implant surface characteristics and/or implant material on the incidence and progression of peri-implantitis? A systematic literature review. Clin. Oral Implant. Res. 2021, 32 (Suppl. 21), 203–229. [Google Scholar] [CrossRef] [PubMed]
- Gallego, L.; Sicilia, A.; Sicilia, P.; Mallo, C.; Cuesta, S.; Sanz, M. A retrospective study on the crestal bone loss as-sociated with different implant surfaces in chronic periodontitis patients under maintenance. Clin. Oral Implant. Res. 2018, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Sanz-Sánchez, I.; Serrano, K.; Montero, E.; Sanz, M. One-year outcomes of dental implants with a hybrid surface macro-design placed in patients with history of periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2022, 49, 90–100. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; Moreno-Riestra, I.; Avila, G.; Fernández-Barbero, J.E.; Mesa, F.; Aguilar, M.; Wang, H.L.; O’Valle, F. Histomorphometric comparison of maxillary pristine bone and composite bone graft biopsies obtained after sinus augmentation. Clin. Oral Implant. Res. 2010, 21, 122–128. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Lindhe, J. The influence of a biomaterial on the closure of a marginal hard tissue defect adjacent to implants. An experimental study in the dog. Clin. Oral Implant. Res. 2004, 15, 285–292. [Google Scholar] [CrossRef]
- Hirota, A.; Iezzi, G.; Piattelli, A.; Ferri, M.; Tanaka, K.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the position of the antrostomy in sinus floor elevation on the healing of mini-implants: A randomized clinical trial. Oral Maxillofac. Surg. 2020, 24, 299–308. [Google Scholar] [CrossRef]
- Imai, H.; Iezzi, G.; Piattelli, A.; Ferri, M.; Apaza Alccayhuaman, K.A.; Botticelli, D. Influence of the Dimensions of the Antrostomy on Osseointegration of Mini-implants Placed in the Grafted Region after Sinus Floor Elevation: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implants 2020, 35, 591–598. [Google Scholar] [CrossRef]
- Sakuma, S.; Piattelli, A.; Baldi, N.; Ferri, M.; Iezzi, G.; Botticelli, D. Bone Healing at Implants Placed in Sites Prepared Either with a Sonic Device or Drills: A Split-Mouth Histomorphometric Randomized Controlled Trial. Int. J. Oral Maxillofac. Implant. 2020, 35, 187–195. [Google Scholar] [CrossRef]
- Imai, H.; Lang, N.P.; Ferri, M.; Hirota, A.; Apaza Alccayhuaman, K.A.; Botticelli, D. Tomographic Assessment on the In-fluence of the Use of a Collagen Membrane on Dimensional Variations to Protect the Antrostomy After Maxillary Sinus Floor Augmentation: A Randomized Clinical Trial. Int. J. Oral Maxillofac. Implant. 2020, 35, 350–356. [Google Scholar] [CrossRef]
- Caneva, M.; Lang, N.P.; Calvo Guirado, J.L.; Spriano, S.; Iezzi, G.; Botticelli, D. Bone healing at bicortically installed implants with different surface configurations. An experimental study in rabbits. Clin. Oral Implant. Res. 2015, 26, 293–299. [Google Scholar] [CrossRef]
- Ferri, M.; Lang, N.P.; Angarita Alfonso, E.E.; Bedoya Quintero, I.D.; Burgos, E.M.; Botticelli, D. Use of sonic in-struments for implant biopsy retrieval. Clin. Oral Implant. Res. 2015, 26, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.E.; Münzel-Pedrazzoli, S. Correlated morphometric and biochemical analysis of gingival tissue. Mor-phometric model, tissue sampling and test of stereologic procedures. J. Microsc. 1973, 99, 301–329. [Google Scholar] [CrossRef]
- Riachi, F.; Naaman, N.; Tabarani, C.; Aboelsaad, N.; Aboushelib, M.N.; Berberi, A.; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int. J. Dent. 2012, 2012, 737262. [Google Scholar] [CrossRef]
- Mahesh, L.; Mascarenhas, G.; Bhasin, M.T.; Guirado, C.; Juneja, S. Histological evaluation of two different anorganic bovine bone matrixes in lateral wall sinus elevation procedure: A retrospective study. Natl. J. Maxillofac. Surg. 2020, 11, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Zahedpasha, A.; Ghassemi, A.; Bijani, A.; Haghanifar, S.; Majidi, M.S.; Ghorbani, Z.M. Comparison of Bone Formation After Sinus Membrane Lifting without Graft or Using Bone Substitute “Histologic and Radiographic Evaluation”. J. Oral Maxillofac. Surg. 2021, 79, 1246–1254. [Google Scholar] [CrossRef]
- Tawil, G.; Barbeck, M.; Unger, R.; Tawil, P.; Witte, F. Sinus Floor Elevation Using the Lateral Approach and Window Repositioning and a Xenogeneic Bone Substitute as a Grafting Material: A Histologic, Histomorphometric, and Radio-graphic Analysis. Int. J. Oral Maxillofac. Implant. 2018, 33, 1089–1096. [Google Scholar] [CrossRef]
- Perić Kačarević, Z.; Kavehei, F.; Houshmand, A.; Franke, J.; Smeets, R.; Rimashevskiy, D.; Wenisch, S.; Schnettler, R.; Jung, O.; Barbeck, M. Purification processes of xenogeneic bone substitutes and their impact on tissue reactions and regeneration. Int. J. Artif. Organs 2018, 41, 789–800. [Google Scholar] [CrossRef]
- Laschke, M.W.; Witt, K.; Pohlemann, T.; Menger, M.D. Injectable nanocrystalline hydroxyapatite paste for bone substitution: In vivo analysis of biocompatibility and vascularization. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Huber, F.X.; Berger, I.; McArthur, N.; Huber, C.; Kock, H.P.; Hillmeier, J.; Meeder, P.J. Evaluation of a novel nanocrystalline hydroxyapatite paste and a solid hydroxyapatite ceramic for the treatment of critical size bone defects (CSD) in rabbits. J. Mater. Sci. Mater. Med. 2008, 19, 33–38. [Google Scholar] [CrossRef]
- Catros, S.; Sandgren, R.; Pippenger, B.E.; Fricain, J.C.; Herber, V.; El Chaar, E. A Novel Xenograft Bone Substitute Supports Stable Bone Formation in Circumferential Defects around Dental Implants in Minipigs. Int. J. Oral Maxillofac. Implant. 2020, 35, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Shakir, M.; Jolly, R.; Khan, A.A.; Ahmed, S.S.; Alam, S.; Rauf, M.A.; Owais, M.; Farooqi, M.A. Resol based chi-tosan/nano-hydroxyapatite nanoensemble for effective bone tissue engineering. Carbohydr. Polym. 2018, 179, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Lang, N.P.; De Santis, E.; Morelli, F.; Favero, G.; Botticelli, D. Bone-healing pattern at the surface of titanium implants: An experimental study in the dog. Clin. Oral Implant. Res. 2014, 25, 124–131. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Persson, L.G.; Lindhe, J. Bone regeneration at implants with turned or rough surfaces in self-contained defects. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Becker, W.; Persson, R.; Baker, D.A.; Rohrer, M.D.; O’Neal, R.B. Evaluation of titanium implants placed into simulated extraction sockets: A study in dogs. Int. J. Oral Maxillofac. Implant. 1999, 14, 351–360. [Google Scholar]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. Appositional bone formation in marginal defects at implants. Clin. Oral Implant. Res. 2003, 14, 1–9. [Google Scholar] [CrossRef]
- Rossi, F.; Botticelli, D.; Pantani, F.; Pereira, F.P.; Salata, L.A.; Lang, N.P. Bone healing pattern in surgically created cir-cumferential defects around submerged implants: An experimental study in dog. Clin. Oral Implant. Res. 2012, 23, 41–48. [Google Scholar] [CrossRef]
- Botticelli, D.; Berglundh, T.; Buser, D.; Lindhe, J. The jumping distance revisited: An experimental study in the dog. Clin. Oral Implant. Res. 2003, 14, 35–42. [Google Scholar] [CrossRef]
- Carmagnola, D.; Berglundh, T.; Lindhe, J. The effect of a fibrin glue on the integration of Bio-Oss with bone tissue. An experimental study in Labrador dogs. J. Clin. Periodontol. 2002, 29, 377–383. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Araújo, M.; Lindhe, J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J. Clin. Periodontol. 2003, 30, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Lindhe, J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J. Clin. Periodontol. 2005, 32, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Lang, N.P.; Schweikert, M.T.; de Oliveira, J.A.; Rangel-Garcia, I., Jr.; Botticelli, D. Sequential healing of open extraction sockets. An experimental study in monkeys. Clin. Oral Implant. Res. 2014, 25, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Silva, E.R.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P. Histologic and Micro-CT Analyses at Implants Placed Immediately after Maxillary Sinus Elevation Using Large or Small Xenograft Granules: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 2020, 35, 739–748. [Google Scholar] [CrossRef]
- De Santis, E.; Lang, N.P.; Ferreira, S.; Rangel Garcia, I., Jr.; Caneva, M.; Botticelli, D. Healing at implants installed con-currently to maxillary sinus floor elevation with Bio-Oss® or autologous bone grafts. A histo-morphometric study in rabbits. Clin. Oral Implant. Res. 2017, 28, 503–511. [Google Scholar] [CrossRef]
- Tanaka, K.; Botticelli, D.; Canullo, L.; Baba, S.; Xavier, S.P. New bone ingrowth into β-TCP/HA graft activated with argon plasma: A histomorphometric study on sinus lifting in rabbits. Int. J. Implant. Dent. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Perini, A.; Ferrante, G.; Sivolella, S.; Velez, J.U.; Bengazi, F.; Botticelli, D. Bone plate repositioned over the antrostomy after sinus floor elevation: An experimental study in sheep. Int. J. Implant. Dent. 2020, 6, 11. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Wallace, S.S.; Froum, S.J.; Rohrer, M.D.; Cho, S.C. Histologic and clinical comparison of bilateral sinus floor elevations with and without barrier membrane placement in 12 patients: Part 3 of an ongoing prospective study. Int. J. Periodontics Restor. Dent. 2000, 20, 117–125. [Google Scholar]
- Wallace, S.S.; Froum, S.J.; Cho, S.C.; Elian, N.; Monteiro, D.; Kim, B.S.; Tarnow, D.P. Sinus augmentation utilizing anor-ganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: Histo-morphometric and clinical analyses. Int. J. Periodontics Restor. Dent. 2005, 25, 551–559. [Google Scholar]
- Choi, K.S.; Kan, J.Y.; Boyne, P.J.; Goodacre, C.J.; Lozada, J.L.; Rungcharassaeng, K. The effects of resorbable membrane on human maxillary sinus graft: A pilot study. Int. J. Oral Maxillofac. Implant. 2009, 24, 73–80. [Google Scholar]
- Barone, A.; Ricci, M.; Covani, U.; Nannmark, U.; Azarmehr, I.; Calvo-Guirado, J.L. Maxillary sinus augmentation using prehydrated corticocancellous porcine bone: Hystomorphometric evaluation after 6 months. Clin. Implant. Dent. Relat. Res. 2013, 14, 373–379. [Google Scholar] [CrossRef]
- Tanaka, K.; Iezzi, G.; Piattelli, A.; Ferri, M.; Mesa, N.F.; Apaza Alccayhuaman, K.A.; Botticelli, D. Sinus Floor Elevation and Antrostomy Healing: A Histomorphometric Clinical Study in Humans. Implant. Dent. 2019, 28, 537–542. [Google Scholar] [CrossRef]
- Perini, A.; Viña-Almunia, J.; Carda, C.; Martín de Llano, J.J.; Botticelli, D.; Peñarrocha-Diago, M. Influence of the Use of a Collagen Membrane Placed on the Bone Window after Sinus Floor Augmentation-An Experimental Study in Rabbits. Dent. J. 2021, 9, 131. [Google Scholar] [CrossRef]
- Zahid, M.Z.; Rahman, S.A.; Alam, M.K.; Pohchi, A.; Jinno, M.; Sugita, Y.; Maeda, H. Prospective 3D Assessment of CORAGRAF and Bio-Oss as Bone Substitutes in Maxillary Sinus Augmentation for Implant Placement. J. Hard Tissue Biol. 2015, 24, 43–48. [Google Scholar] [CrossRef][Green Version]
- Yousaf Athar, Siti Lailatul Akmar Zainuddin, Zurairah Berahim, Akram Hassan, Aamina Sagheer, Mohammad Khursheed Alam Bovine Pericardium: A Highly Versatile Graft Material. Int. Med. J. 2014, 21, 321–324.
- Al-Zoubi, I.A.; Patil, S.R.; Kato, I.; Sugita, Y.; Maeda, H.; Alam, M.K. 3D CBCT Assessment of Incidental Maxillary Sinus Abnormalities in a Saudi Arabian Population. J. Hard Tissue Biol. 2017, 26, 369–372. [Google Scholar] [CrossRef]
- Athar, Y.; Zainuddin, S.L.A.; Berahim, Z.; Hassan, A.; Sagheer, A.; Alam, M.K. Bovine Pericardium Membrane and Periodontal Guided Tissue Regeneration: A SEM Study. Int. Med. J. 2014, 21, 325–327. [Google Scholar]





| Number | Age | Smokers | Mb | No-Mb | |
|---|---|---|---|---|---|
| Females | 10 | 53.1 ± 9.3 | 10 No | 5 | 5 |
| Males | 4 | 59.0 ± 12.8 | 4 No | 3 | 1 |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 28.9 ± 14.5 25.2 (24.3; 34.1) | 13.5 ± 8.0 14.6 (7.7; 20.1) | 42.4 ± 17.7 35.5 (30.3; 48.1) | 1.6 ± 3.8 0.0 (0.0; 0.0) | 25.2 ± 15.2 24.0 (15.7; 29.7) | 30.8 ± 17.3 37.3 (15.9; 43.3) |
| TURNED | Mean ± SD Median (25%; 75%) | 11.0 ± 5.7 12.9 (8.0; 14.2) | 16.6 ± 15.1 11.2 (5.7; 27.6) | 27.6 ± 14.5 23.1 (17.0; 33.3) | 1.2 ± 2.1 0.0 (0.0; 1.7) | 27.2 ± 17.7 29.7 (15.7; 38.3) | 43.9 ± 26.0 38.4 (34.2; 59.6) |
| p-value ZirTi vs. Turned | 0.030 | 0.750 | 0.258 | >0.999 | 0.828 | 0.305 | |
| p-value Mb vs. No-Mb ZirTi | 0.852 | 0.108 | 0.612 | 0.469 | 0.579 | 0.507 | |
| p-value Mb vs. No-Mb Turned | 0.636 | 0.308 | 0.103 | 0.618 | 0.755 | 0.282 | |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 30.5 ± 14.9 27.1 (19.1; 34.7) | 7.0 ± 8.1 3.8 (1.6; 9.0) | 37.5 ± 17.3 32.4 (26.3; 43.5) | 3.0 ± 3.6 1.3 (0.0; 5.5) | 30.6 ± 20.6 35.6 (12.1; 46.0) | 28.9 ± 12.6 27.9 (23.0; 30.1) |
| TURNED | Mean ± SD Median (25%; 75%) | 9.2 ± 7.3 6.5 (5.1; 13.1) | 6.1 ± 6.1 5.8 (0.5; 9.7) | 15.3 ± 8.1 13.9 (8.7; 21.7) | 2.4 ± 4.2 0.3 (0.0; 3.1) | 23.4 ± 24.3 14.4 (10.6; 28.2) | 58.9 ± 23.4 70.6 (47.9; 75.4) |
| p-value ZirTi vs. Turned | 0.008 | 0.672 | 0.001 | 0.625 | 0.461 | 0.016 | |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 29.8 ± 14.2 26.0 (20.9; 34.9) | 9.8 ± 8.4 5.9 (2.4; 16.7) | 39.6 ± 17.0 32.9 (28.9; 48.1) | 2.4 ± 3.7 0.0 (0.0; 4.2) | 28.3 ± 18.1 28.5 (13.4; 43.9) | 29.7 ± 14.2 29.0 (19.4; 38.4) |
| TURNED | Mean ± SD Median (25%; 75%) | 10.0 ± 6.5 9.2 (5.4; 14.2) | 10.6 ± 11.7 9.3 (2.3; 11.5) | 20.6 ± 12.5 17.1 (12.5; 27.0) | 1.9 ± 3.4 0.0 (0.0; 2.7) | 25.0 ± 21.0 17.5 (12.0; 38.3) | 52.5 ± 24.8 58.7 (37.6; 74.5) |
| p-value ZirTi vs. Turned | 0.000 | 0.594 | 0.003 | 0.813 | 0.580 | 0.004 | |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 21.8 ± 4.8 22.9 (18.1; 23.7) | 7.4 ± 4.0 6.6 (5.0; 8.5) | 29.2 ± 7.0 27.3 (25.2; 28.2) | 0.1 ± 0.2 0.0 (0.0; 0.0) | 46.2 ± 4.6 45.3 (42.9; 50.1) | 24.5 ± 7.5 26.9 (21.2; 30.4) |
| TURNED | Mean ± SD Median (25%; 75%) | 19.9 ± 8.9 21.2 (18.5; 22.0) | 11.4 ± 9.0 8.4 (6.6; 11.2) | 31.3 ± 6.2 31.6 (28.9; 33.7) | 3.2 ± 4.5 0.7 (0.0; 5.5) | 36.6 ± 11.3 39.5 (34.5; 43.5) | 28.9 ± 5.4 31.0 (24.8; 32.7) |
| p-value ZirTi vs. Turned | 0.552 | 0.438 | 0.563 | 0.250 | 0.048 | 0.282 | |
| p-value Mb vs. No-Mb ZirTi | 0.662 | 0.878 | >0.9999 | 0.021 | 0.342 | 0.883 | |
| p-value Mb vs. No-Mb Turned | 0.573 | 0.282 | 0.534 | 0.505 | 0.308 | 0.037 | |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 23.7 ± 10.3 20.3 (17.1; 29.3) | 7.1 ± 3.9 6.7 (3.4; 11.0) | 30.7 ± 10.0 28.1 (22.7; 37.5) | 4.9 ± 6.3 2.7 (0.4; 6.7) | 39.1 ± 19.4 44.3 (25.7; 51.4) | 25.4 ± 13.6 27.5 (12.5; 31.4) |
| TURNED | Mean ± SD Median (25%; 75%) | 22.6 ± 8.2 22.1 (19.3; 25.9) | 6.3 ± 3.2 6.2 (5.4; 7.4) | 28.9 ± 7.6 31.3 (25.3; 33.6) | 3.2 ± 3.7 2.2 (0.7; 3.9) | 28.9 ± 15.7 26.2 (19.7; 32.7) | 39.0 ± 10.3 41.1 (36.3; 44.9) |
| p-value ZirTi vs. Turned | 0.771 | 0.558 | 0.550 | 0.375 | 0.318 | 0.121 | |
| New Bone | IBN | Total Bone | Old Bone | Graft | Soft Tissues | ||
|---|---|---|---|---|---|---|---|
| ZIRTI | Mean ± SD Median (25%; 75%) | 22.9 ± 8.1 22.5 (17.0; 27.4) | 7.2 ± 3.8 6.7 (3.9; 10.4) | 30.1 ± 8.6 27.6 (24.4; 34.1) | 2.8 ± 5.2 0.2 (0.0; 3.4) | 42.1 ± 15.0 45.3 (41.2; 51.2) | 25.0 ±11.0 27.5 (14.8; 30.6) |
| TURNED | Mean ± SD Median (25%; 75%) | 21.5 ± 8.3 21.3 (18.5; 23.9) | 8.5 ± 6.6 6.5 (5.8; 9.6) | 29.9 ± 6.9 31.3 (27.8; 33.7) | 3.2 ± 3.9 1.4 (0.1; 4.9) | 32.2 ± 14.1 32.2 (21.1; 39.6) | 34.7 ± 9.8 33.6 (27.1; 41.6) |
| p-value ZirTi vs. Turned | 0.547 | 0.726 | 0.987 | 0.846 | 0.092 | 0.061 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsu, M.; Apaza Alccayhuaman, K.A.; Ferri, M.; Iezzi, G.; Piattelli, A.; Fortich Mesa, N.; Botticelli, D. Osseointegration at Implants Installed in Composite Bone: A Randomized Clinical Trial on Sinus Floor Elevation. J. Funct. Biomater. 2022, 13, 22. https://doi.org/10.3390/jfb13010022
Kotsu M, Apaza Alccayhuaman KA, Ferri M, Iezzi G, Piattelli A, Fortich Mesa N, Botticelli D. Osseointegration at Implants Installed in Composite Bone: A Randomized Clinical Trial on Sinus Floor Elevation. Journal of Functional Biomaterials. 2022; 13(1):22. https://doi.org/10.3390/jfb13010022
Chicago/Turabian StyleKotsu, Mitsuo, Karol Alí Apaza Alccayhuaman, Mauro Ferri, Giovanna Iezzi, Adriano Piattelli, Natalia Fortich Mesa, and Daniele Botticelli. 2022. "Osseointegration at Implants Installed in Composite Bone: A Randomized Clinical Trial on Sinus Floor Elevation" Journal of Functional Biomaterials 13, no. 1: 22. https://doi.org/10.3390/jfb13010022
APA StyleKotsu, M., Apaza Alccayhuaman, K. A., Ferri, M., Iezzi, G., Piattelli, A., Fortich Mesa, N., & Botticelli, D. (2022). Osseointegration at Implants Installed in Composite Bone: A Randomized Clinical Trial on Sinus Floor Elevation. Journal of Functional Biomaterials, 13(1), 22. https://doi.org/10.3390/jfb13010022










