Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook
Abstract
:1. Introduction
2. Biological and Structural Requirements for Engineered Replacements
2.1. Biological Requirements
2.2. Structural Requirements
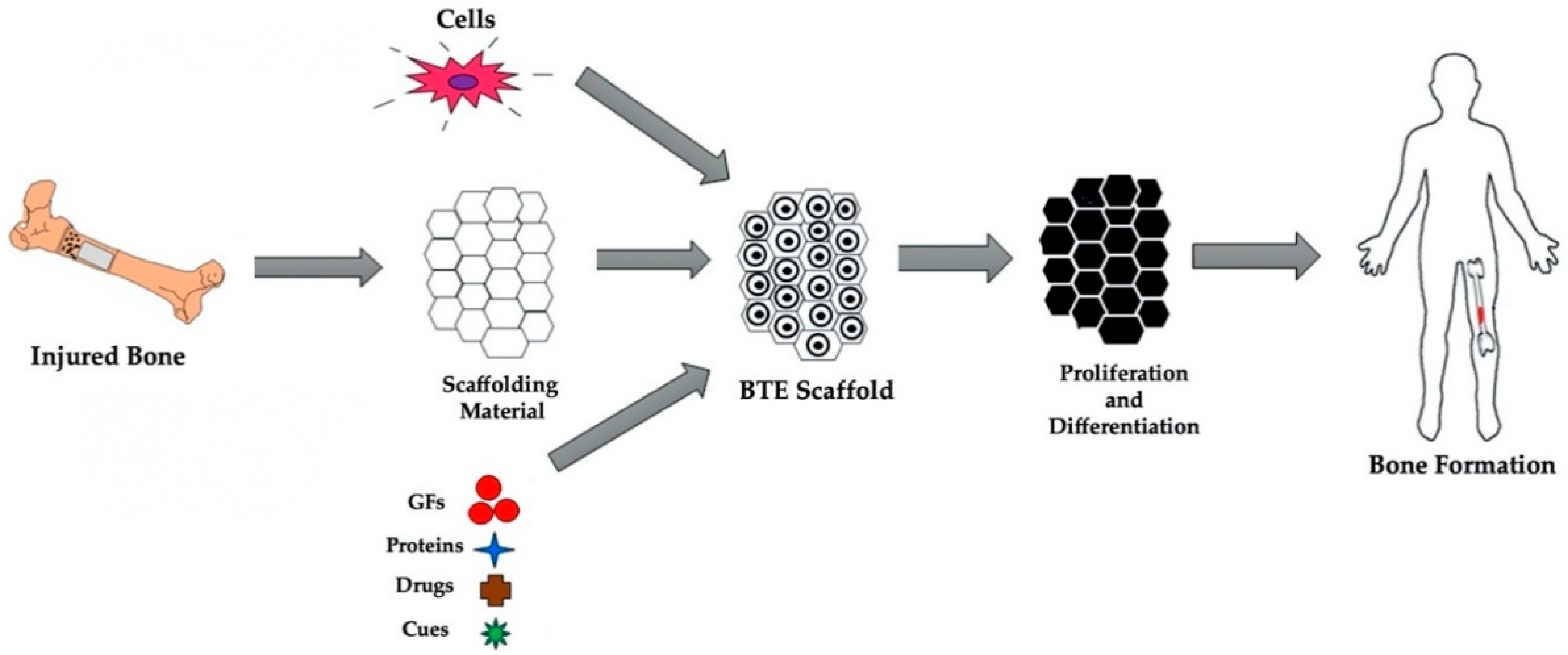
3. Bone Tissue Engineering: Cells, Materials and Cues
3.1. Cells
3.2. Materials
3.2.1. Polymers
3.2.2. Ceramics
3.2.3. Metals
3.2.4. Composites
3.3. Biophysical and Biochemical Cues
4. Piezoelectric Effect in Bone
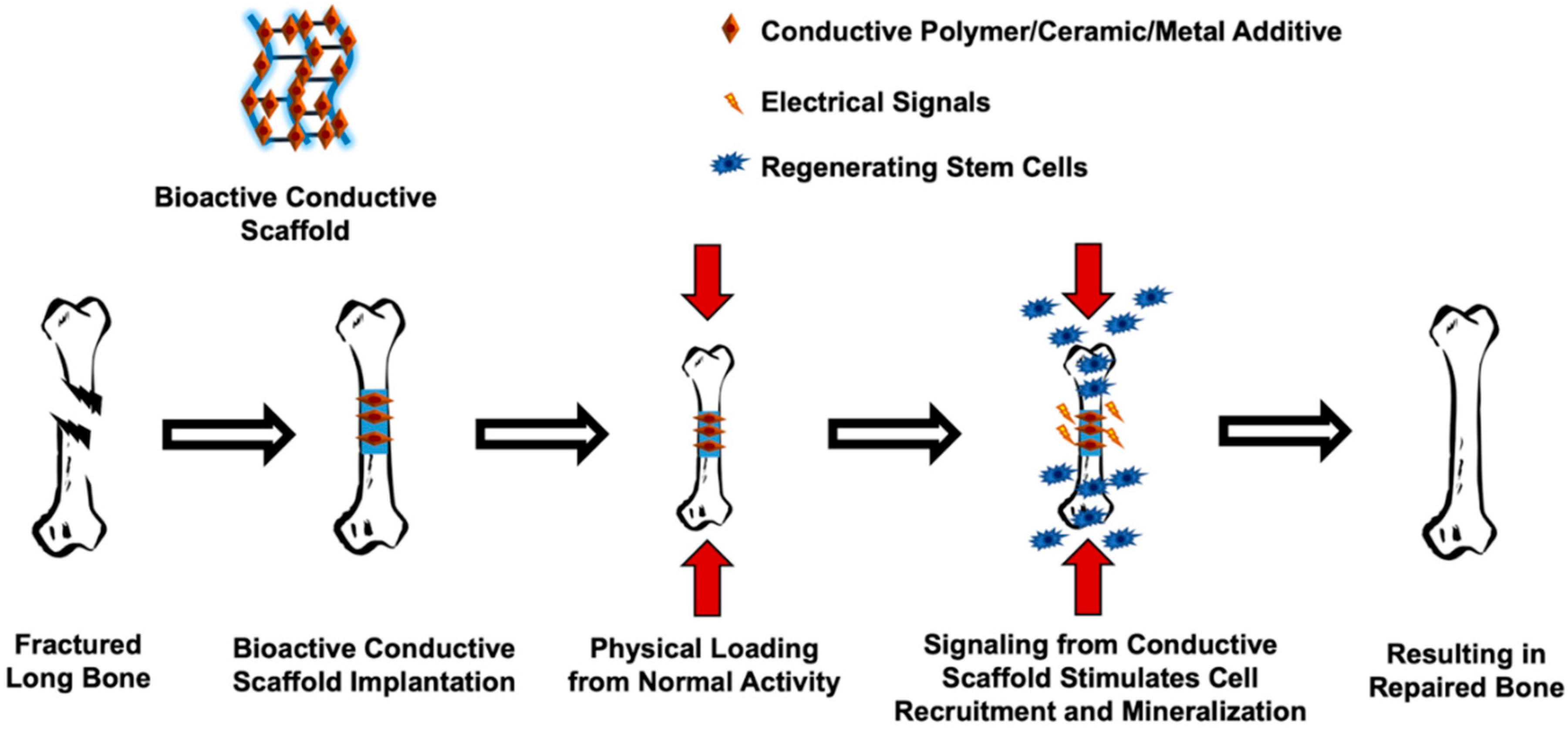
5. Conductive Materials and Strategies for Induced Bone Regeneration
5.1. Application of Conductive Materials
5.2. Strategies for Induced Bone Regeneration: Electrical and Mechanical Stimulation
5.3. Advantages and Disadvantages
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padilla Colon, C.J.; Molina-Vicenty, I.L.; Frontera-Rodriguez, M.; Garcia-Ferre, A.; Rivera, B.P.; Cintron-Velez, G.; Frontera-Rodriguez, S. Muscle and Bone Mass Loss in the Elderly Population: Advances in diagnosis and treatment. J. Biomed. 2018, 3, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.; Nakamura, M.; Miclau, T.; Marcucio, R. Effects of Aging on Fracture Healing. Curr. Osteoporos Rep. 2017, 15, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., 3rd. Trends in fracture incidence: A population-based study over 20 years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.; Atluri, K.; Geary, S.M.; Hong, L.; Elangovan, S.; Salem, A.K. Bone Regeneration Using Gene-Activated Matrices. Aaps. J. 2016, 19, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Manivasagam, G.; Sen, D. Progress of Regenerative Therapy in Orthopedics. Curr. Osteoporos Rep. 2018, 16, 169–181. [Google Scholar] [CrossRef]
- Chiarello, E.; Cadossi, M.; Tedesco, G.; Capra, P.; Calamelli, C.; Shehu, A.; Giannini, S. Autograft, allograft and bone substitutes in reconstructive orthopedic surgery. Aging Clin. Exp. Res. 2013, 25 (Suppl. 1), S101–S103. [Google Scholar] [CrossRef]
- Roffi, A.; Krishnakumar, G.S.; Gostynska, N.; Kon, E.; Candrian, C.; Filardo, G. The Role of Three-Dimensional Scaffolds in Treating Long Bone Defects: Evidence from Preclinical and Clinical Literature-A Systematic Review. Biomed. Res. Int. 2017, 2017, 8074178. [Google Scholar] [CrossRef]
- Horner, E.A.; Kirkham, J.; Wood, D.; Curran, S.; Smith, M.; Thomson, B.; Yang, X.B. Long bone defect models for tissue engineering applications: Criteria for choice. Tissue Eng. Part. B Rev. 2010, 16, 263–271. [Google Scholar] [CrossRef]
- Molina, C.S.; Stinner, D.J.; Obremskey, W.T. Treatment of Traumatic Segmental Long-Bone Defects: A Critical Analysis Review. JBJS Rev. 2014, 2, e1. [Google Scholar] [CrossRef]
- Tajbakhsh, S.; Hajiali, F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 70, 897–912. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [Green Version]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2014, 43, 641–656. [Google Scholar] [CrossRef] [Green Version]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Das, R.; Curry, E.J.; Le, T.T.; Awale, G.; Liu, Y.; Li, S.Y.; Contreras, J.; Bednarz, C.; Millender, J.; Xin, X.N.; et al. Biodegradable nanofiber bone-tissue scaffold as remotely-controlled and self-powering electrical stimulator. Nano Energy 2020, 76, 105028. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 6307. [Google Scholar] [CrossRef]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. Biotechniques 2016, 60, 95–98. [Google Scholar] [CrossRef] [Green Version]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Bassett, C.A.; Becker, R.O. Generation of electric potentials by bone in response to mechanical stress. Science 1962, 137, 1063–1064. [Google Scholar] [CrossRef]
- Shahini, A.; Yazdimamaghani, M.; Walker, K.J.; Eastman, M.A.; Hatami-Marbini, H.; Smith, B.J.; Ricci, J.L.; Madihally, S.V.; Vashaee, D.; Tayebi, L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014, 9, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Eischen-Loges, M.; Oliveira, K.M.C.; Bhavsar, M.B.; Barker, J.H.; Leppik, L. Pretreating mesenchymal stem cells with electrical stimulation causes sustained long-lasting pro-osteogenic effects. PeerJ 2018, 6, e4959. [Google Scholar] [CrossRef]
- Qu, H.W.; Fu, H.Y.; Han, Z.Y.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.P.; Sethi, B.; Cao, X.D. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7, 2041731416648810. [Google Scholar] [CrossRef] [Green Version]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Yusop, A.H.; Bakir, A.A.; Shaharom, N.A.; Abdul Kadir, M.R.; Hermawan, H. Porous biodegradable metals for hard tissue scaffolds: A review. Int. J. Biomater. 2012, 2012, 641430. [Google Scholar] [CrossRef] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Zhang, Y.; Gandhi, A.A.; Zeglinski, J.; Gregor, M.; Tofail, S.A.M. A Complementary Contribution to Piezoelectricity from Bone Constituents. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1151–1157. [Google Scholar] [CrossRef]
- Confavreux, C.B. Bone: From a reservoir of minerals to a regulator of energy metabolism. Kidney Int. 2011, 79 (Suppl. 121), S14–S19. [Google Scholar] [CrossRef] [Green Version]
- Barth, H.D.; Zimmermann, E.A.; Schaible, E.; Tang, S.Y.; Alliston, T.; Ritchie, R.O. Characterization of the effects of X-ray irradiation on the hierarchical structure and mechanical properties of human cortical bone. Biomaterials 2011, 32, 8892–8904. [Google Scholar] [CrossRef] [Green Version]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.I.; Wang, X.Y.; Wu, T.J.; Lee, Y.T.; Kim, S.; Miguez, P.; Ko, C.C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofac. Res. 2019, 22, 127–133. [Google Scholar] [CrossRef]
- Zhao, H.X.; Li, L.H.; Ding, S.; Liu, C.X.; Ai, J.Y. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold design for bone regeneration. J. Nanosci. Nanotechnol. 2014, 14, 15–56. [Google Scholar] [CrossRef] [Green Version]
- Bouet, G.; Marchat, D.; Cruel, M.; Malaval, L.; Vico, L. In vitro three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng. Part B Rev. 2015, 21, 133–156. [Google Scholar] [CrossRef] [PubMed]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef]
- Orciani, M.; Fini, M.; Di Primio, R.; Mattioli-Belmonte, M. Biofabrication and Bone Tissue Regeneration: Cell Source, Approaches, and Challenges. Front. Bioeng. Biotechnol. 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, A.; Gronthos, S. Clinical Application of Bone Marrow Mesenchymal Stem/Stromal Cells to Repair Skeletal Tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Piatetzky, S., II; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol Exp. Morphol 1966, 16, 381–390. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Hutton, D.L.; Grayson, W.L. Stem cell-based approaches to engineering vascularized bone. Curr. Opin. Chem. Eng. 2014, 3, 75–82. [Google Scholar] [CrossRef]
- Wada, N.; Gronthos, S.; Bartold, P.M. Immunomodulatory effects of stem cells. Periodontology 2013, 63, 198–216. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuffi, S.; Zonefrati, R.; Brandi, M.L. Adipose stem cells for bone tissue repair. Clin. Cases Miner. Bone Metab. 2017, 14, 217–226. [Google Scholar] [CrossRef]
- Storti, G.; Scioli, M.G.; Kim, B.S.; Orlandi, A.; Cervelli, V. Adipose-Derived Stem Cells in Bone Tissue Engineering: Useful Tools with New Applications. Stem Cells Int. 2019, 2019, 3673857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Y.; Zhao, J.; Nie, F.; Qin, Z.; Xue, H.; Wang, G.; Li, D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci. Rep. 2019, 9, 12861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratushnyy, A.; Ezdakova, M.; Yakubets, D.; Buravkova, L. Angiogenic Activity of Human Adipose-Derived Mesenchymal Stem Cells Under Simulated Microgravity. Stem Cells Dev. 2018, 27, 831–837. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Y.; Qin, H.; Tong, S.; Sun, Q.; Wang, T.; Zhang, H.; Cui, M.; Guo, S. Osteogenically-induced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank 2021, 22, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Gotherstrom, C. Human Foetal Mesenchymal Stem Cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Thavornyutikarn, B.; Chantarapanich, N.; Sitthiseripratip, K.; Thouas, G.A.; Chen, Q. Bone tissue engineering scaffolding: Computer-aided scaffolding techniques. Prog. Biomater. 2014, 3, 61–102. [Google Scholar] [CrossRef] [Green Version]
- Hum, J.; Boccaccini, A.R. Collagen as Coating Material for 45S5 Bioactive Glass-Based Scaffolds for Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1807. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, B.N.; Aprile, P.; Mendonca, R.H.; Kelly, D.J.; Thire, R. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.; You, M.; Li, J.; Li, Z. Emerging bone tissue engineering via Polyhydroxyalkanoate (PHA)-based scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 917–929. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ye, H.; Li, Y.; Fang, J.; Mei, Q.; Lu, X.; Ren, F. Cancellous-Bone-like Porous Iron Scaffold Coated with Strontium Incorporated Octacalcium Phosphate Nanowhiskers for Bone Regeneration. ACS Biomater. Sci. Eng. 2019, 5, 509–518. [Google Scholar] [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.R.; Nasseri, B.A.; Vacanti, J.P. Tissue engineering: A 21st century solution to surgical reconstruction. Ann. Thorac. Surg. 2001, 72, 577–591. [Google Scholar] [CrossRef]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Khorramnezhad, M.; Akbari, B.; Akbari, M.; Kharaziha, M. Effect of surface modification on physical and cellular properties of PCL thin film. Colloids Surf. B Biointerfaces 2021, 200, 111582. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A tailored polylactic acid/polycaprolactone biodegradable and bioactive 3D porous scaffold containing gelatin nanofibers and Taurine for bone regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.N.; Lee, S.J.; Song, J.E.; Kwon, S.Y.; Chung, J.W.; Lee, D.; Khang, G. Effect of pore sizes of PLGA scaffolds on mechanical properties and cell behaviour for nucleus pulposus regeneration in vivo. J. Tissue Eng. Regen Med. 2017, 11, 44–57. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Biltz, R.M.; Pellegrino, E.D. The chemical anatomy of bone. I. A comparative study of bone composition in sixteen vertebrates. J. Bone Jt. Surg. Am. 1969, 51, 456–466. [Google Scholar] [CrossRef]
- Keogh, M.B.; O’Brien, F.J.; Daly, J.S. A novel collagen scaffold supports human osteogenesis-applications for bone tissue engineering. Cell Tissue Res. 2010, 340, 169–177. [Google Scholar] [CrossRef]
- Karp, J.M.; Sarraf, F.; Shoichet, M.S.; Davies, J.E. Fibrin-filled scaffolds for bone-tissue engineering: An in vivo study. J. Biomed. Mater. Res. A 2004, 71, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40. [Google Scholar] [CrossRef] [Green Version]
- Soundarya, S.P.; Menon, A.H.; Chandran, S.V.; Selvamurugan, N. Bone tissue engineering: Scaffold preparation using chitosan and other biomaterials with different design and fabrication techniques. Int. J. Biol. Macromol. 2018, 119, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Kundu, B.; Naskar, D.; Kim, H.W.; Maiti, T.K.; Bhattacharya, D.; Kundu, S.C. Silk scaffolds in bone tissue engineering: An overview. Acta Biomater. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Li, J.F.; Liu, X.; Crook, J.M.; Wallace, G.G. Electrical stimulation-induced osteogenesis of human adipose derived stem cells using a conductive graphene-cellulose scaffold. Mat. Sci. Eng. C-Mater. 2020, 107, 110312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginebra, M.P.; Espanol, M.; Maazouz, Y.; Bergez, V.; Pastorino, D. Bioceramics and bone healing. EFORT Open Rev. 2018, 3, 173–183. [Google Scholar] [CrossRef]
- Salinas, A.J.; Vallet-Regi, M. Bioactive ceramics: From bone grafts to tissue engineering. RSC Adv. 2013, 3, 11116–11131. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [Green Version]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable Materials for Bone Repair and Tissue Engineering Applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef]
- Gu, X.; Lin, W.; Li, D.; Guo, H.; Li, P.; Fan, Y. Degradation and biocompatibility of a series of strontium substituted hydroxyapatite coatings on magnesium alloys. RSC Adv. 2019, 9, 15013–15021. [Google Scholar] [CrossRef] [Green Version]
- de Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.; Zhong, Q.; Zhou, Y.; Kundu, S.C.; Yao, J.; Cai, Y. Controlled degradation pattern of hydroxyapatite/calcium carbonate composite microspheres. Microsc. Res. Tech. 2016, 79, 518–524. [Google Scholar] [CrossRef]
- Cunha, C.; Sprio, S.; Panseri, S.; Dapporto, M.; Marcacci, M.; Tampieri, A. High biocompatibility and improved osteogenic potential of novel Ca-P/titania composite scaffolds designed for regeneration of load-bearing segmental bone defects. J. Biomed. Mater. Res. A 2013, 101, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Weng, W.; Tam, K.C. Novel highly biodegradable biphasic tricalcium phosphates composed of alpha-tricalcium phosphate and beta-tricalcium phosphate. Acta Biomater. 2007, 3, 251–254. [Google Scholar] [CrossRef]
- Li, X.N.; Yang, X.; Liu, X.J.; He, W.; Huang, Q.L.; Li, S.R.; Feng, Q.L. Calcium carbonate nanoparticles promote osteogenesis compared to adipogenesis in human bone-marrow mesenchymal stem cells. Prog. Nat. Sci.-Mater. 2018, 28, 598–608. [Google Scholar] [CrossRef]
- Echezarreta-Lopez, M.M.; De Miguel, T.; Quintero, F.; Pou, J.; Landin, M. Antibacterial properties of laser spinning glass nanofibers. Int. J. Pharm. 2014, 477, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, C.A.; Lin, K.Y. Complications of rigid internal fixation. Craniomaxillofacial Trauma Reconstr. 2009, 2, 41–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansone, V.; Pagani, D.; Melato, M. The effects on bone cells of metal ions released from orthopaedic implants. A review. Clin. Cases Miner. Bone Metab. 2013, 10, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Li, X.; Zhang, X.; Wang, C.; Meng, X.; Pei, Y.; Fan, X.; Lan, P.; Wang, C.; et al. Improving osteointegration and osteogenesis of three-dimensional porous Ti6Al4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl. Mater. Interfaces 2015, 7, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Sachot, N.; Mateos-Timoneda, M.A.; Planell, J.A.; Velders, A.H.; Lewandowska, M.; Engel, E.; Castano, O. Towards 4th generation biomaterials: A covalent hybrid polymer-ormoglass architecture. Nanoscale 2015, 7, 15349–15361. [Google Scholar] [CrossRef] [Green Version]
- Abarrategi, A.; Moreno-Vicente, C.; Martinez-Vazquez, F.J.; Civantos, A.; Ramos, V.; Sanz-Casado, J.V.; Martinez-Corria, R.; Perera, F.H.; Mulero, F.; Miranda, P.; et al. Biological properties of solid free form designed ceramic scaffolds with BMP-2: In vitro and in vivo evaluation. PLoS ONE 2012, 7, e34117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Jang, L.; Kim, S.; Yang, J.; Yang, K.; Cho, S.W.; Lee, J.Y. Polypyrrole/Alginate Hybrid Hydrogels: Electrically Conductive and Soft Biomaterials for Human Mesenchymal Stem Cell Culture and Potential Neural Tissue Engineering Applications. Macromol. Biosci. 2016, 16, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Zanjanizadeh Ezazi, N.; Shahbazi, M.A.; Shatalin, Y.V.; Nadal, E.; Makila, E.; Salonen, J.; Kemell, M.; Correia, A.; Hirvonen, J.; Santos, H.A. Conductive vancomycin-loaded mesoporous silica polypyrrole-based scaffolds for bone regeneration. Int. J. Pharm. 2018, 536, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, P.; Peng, S.; Shuai, C.; Gao, C.; Yang, W.; Bin, S.; Min, A. In Situ Generation of Hydroxyapatite on Biopolymer Particles for Fabrication of Bone Scaffolds Owning Bioactivity. ACS Appl. Mater. Interfaces 2020, 12, 46743–46755. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2021, 6, 490–502. [Google Scholar] [CrossRef]
- Bejarano, J.; Detsch, R.; Boccaccini, A.R.; Palza, H. PDLLA scaffolds with Cu- and Zn-doped bioactive glasses having multifunctional properties for bone regeneration. J. Biomed. Mater. Res. A 2017, 105, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Fan, J.J.; Li, Z.Q.; Liu, Y.W.; Wu, Y.P.; Liu, J. Effects of Pore Size on the Osteoconductivity and Mechanical Properties of Calcium Phosphate Cement in a Rabbit Model. Artif. Organs 2017, 41, 199–204. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, Y.; Yao, B.; Li, Z.; Song, W.; Wang, Y.; Duan, X.; Yuan, X.; Fu, X.; et al. Biophysical and Biochemical Cues of Biomaterials Guide Mesenchymal Stem Cell Behaviors. Front. Cell Dev. Biol. 2021, 9, 640388. [Google Scholar] [CrossRef]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Pei, X.; Zhou, C.C.; Fan, Y.J.; Jiang, Q.; Ronca, A.; D’Amora, U.; Chen, Y.; Li, H.Y.; Sun, Y.; et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load-bearing bone reconstruction. Mater. Design 2018, 152, 30–39. [Google Scholar] [CrossRef]
- Anitua, E.; Tejero, R.; Zalduendo, M.M.; Orive, G. Plasma rich in growth factors promotes bone tissue regeneration by stimulating proliferation, migration, and autocrine secretion in primary human osteoblasts. J. Periodontol. 2013, 84, 1180–1190. [Google Scholar] [CrossRef]
- Nichols, S.P.; Storm, W.L.; Koh, A.; Schoenfisch, M.H. Local delivery of nitric oxide: Targeted delivery of therapeutics to bone and connective tissues. Adv. Drug Deliv. Rev. 2012, 64, 1177–1188. [Google Scholar] [CrossRef] [Green Version]
- Blackwood, K.A.; Bock, N.; Dargaville, T.R.; Woodruff, M.A. Scaffolds for Growth Factor Delivery as Applied to Bone Tissue Engineering. Int. J. Polym. Sci. 2012, 2012, 174942. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.P.E.; Huang, B.Y.; Helaehil, J.V.; Nalesso, P.R.L.; Bagne, L.; de Oliveira, M.A.; Albiazetti, G.C.C.; Aldalbahi, A.; El-Newehy, M.; Santamaria, M.; et al. In vivo study of conductive 3D printed PCL/MWCNTs scaffolds with electrical stimulation for bone tissue engineering. Bio-Des Manuf. 2021, 4, 190–202. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen 2018, 38, 2. [Google Scholar] [CrossRef] [Green Version]
- Kapat, K.; Shubhra, Q.T.H.; Zhou, M.; Leeuwenburgh, S. Piezoelectric Nano-Biomaterials for Biomedicine and Tissue Regeneration. Adv. Funct. Mater. 2020, 30, 1909045. [Google Scholar] [CrossRef] [Green Version]
- Sutka, A.; Sherrell, P.C.; Shepelin, N.A.; Lapcinskis, L.; Malnieks, K.; Ellis, A.V. Measuring Piezoelectric Output-Fact or Friction? Adv. Mater. 2020, 32, e2002979. [Google Scholar] [CrossRef]
- Wieland, D.C.F.; Krywka, C.; Mick, E.; Willumeit-Romer, R.; Bader, R.; Kluess, D. Investigation of the inverse piezoelectric effect of trabecular bone on a micrometer length scale using synchrotron radiation. Acta Biomater. 2015, 25, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenberg, Z.B.; Brighton, C.T. Bioelectric potentials in bone. J. Bone Jt. Surg. Am. 1966, 48, 915–923. [Google Scholar] [CrossRef]
- Bassett, C.A. Biologic significance of piezoelectricity. Calcif. Tissue Res. 1968, 1, 252–272. [Google Scholar] [CrossRef]
- Piekarski, K.; Munro, M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature 1977, 269, 80–82. [Google Scholar] [CrossRef]
- Johnson, M.W.; Williams, W.S.; Gross, D. Ceramic models for piezoelectricity in dry bone. J. Biomech. 1980, 13, 565–573. [Google Scholar] [CrossRef]
- Pienkowski, D.; Pollack, S.R. The origin of stress-generated potentials in fluid-saturated bone. J. Orthop. Res. 1983, 1, 30–41. [Google Scholar] [CrossRef]
- Weinbaum, S.; Cowin, S.C.; Zeng, Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J. Biomech. 1994, 27, 339–360. [Google Scholar] [CrossRef]
- Cowin, S.C.; Weinbaum, S.; Zeng, Y. A case for bone canaliculi as the anatomical site of strain generated potentials. J. Biomech. 1995, 28, 1281–1297. [Google Scholar] [CrossRef]
- Brighton, C.T.; Wang, W.; Seldes, R.; Zhang, G.; Pollack, S.R. Signal transduction in electrically stimulated bone cells. J. Bone Jt. Surg. Am. 2001, 83, 1514–1523. [Google Scholar] [CrossRef] [Green Version]
- Catterall, W.A. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011, 3, a003947. [Google Scholar] [CrossRef] [PubMed]
- Almagor, L.; Chomsky-Hecht, O.; Ben-Mocha, A.; Hendin-Barak, D.; Dascal, N.; Hirsch, J.A. The role of a voltage-dependent Ca2+ channel intracellular linker: A structure-function analysis. J. Neurosci. 2012, 32, 7602–7613. [Google Scholar] [CrossRef] [Green Version]
- el Haj, A.J.; Walker, L.M.; Preston, M.R.; Publicover, S.J. Mechanotransduction pathways in bone: Calcium fluxes and the role of voltage-operated calcium channels. Med. Biol. Eng. Comput. 1999, 37, 403–409. [Google Scholar] [CrossRef]
- Barradas, A.M.; Fernandes, H.A.; Groen, N.; Chai, Y.C.; Schrooten, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.T.; Pollack, S.R.; Reilly, T.M.; Brighton, C.T. Real-time calcium response of cultured bone cells to fluid flow. Clin. Orthop. Relat. Res. 1995, 1995, 256–269. [Google Scholar]
- Liu, D.; Genetos, D.C.; Shao, Y.; Geist, D.J.; Li, J.; Ke, H.Z.; Turner, C.H.; Duncan, R.L. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone 2008, 42, 644–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, L.M.; Publicover, S.J.; Preston, M.R.; Said Ahmed, M.A.; El Haj, A.J. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J. Cell Biochem. 2000, 79, 648–661. [Google Scholar] [CrossRef]
- You, J.; Reilly, G.C.; Zhen, X.; Yellowley, C.E.; Chen, Q.; Donahue, H.J.; Jacobs, C.R. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J. Biol. Chem. 2001, 276, 13365–13371. [Google Scholar] [CrossRef] [Green Version]
- Hung, C.T.; Allen, F.D.; Pollack, S.R.; Brighton, C.T. Intracellular Ca2+ stores and extracellular Ca2+ are required in the real-time Ca2+ response of bone cells experiencing fluid flow. J. Biomech. 1996, 29, 1411–1417. [Google Scholar] [CrossRef]
- Bassett, C.A.; Pawluk, R.J.; Becker, R.O. Effects of Electric Currents on Bone in Vivo. Nature 1964, 204, 652–654. [Google Scholar] [CrossRef]
- Khorshidi, S.; Karkhaneh, A. Hydrogel/fiber conductive scaffold for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 718–724. [Google Scholar] [CrossRef]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; Macdiarmid, A.G. Electrical-Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Le, T.H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Kumar, D.; Sharma, R.C. Advances in conductive polymers. Eur. Polym. J. 1998, 34, 1053–1060. [Google Scholar] [CrossRef]
- Rahaman, M.; Aldalbahi, A.; Almoiqli, M.; Alzahly, S. Chemical and Electrochemical Synthesis of Polypyrrole Using Carrageenan as a Dopant: Polypyrrole/Multi-Walled Carbon Nanotube Nanocomposites. Polymers 2018, 10, 632. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.A.; Rodriguez, J.; Grande, H.; Mecerreyes, D.; Pomposo, J.A. Chemical oxidative polymerization of pyrrole in the presence of m-hydroxybenzoic acid and m-hydroxycinnamic acid-related compounds. Synth. Met. 2002, 126, 111–116. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Georgevich, A.; Hodgson, A.J.; Liu, L.; Wallace, G.G. Polypyrrole-heparin composites as stimulus-responsive substrates for endothelial cell growth. J. Biomed. Mater. Res. 1999, 44, 121–129. [Google Scholar] [CrossRef]
- Song, H.K.; Toste, B.; Ahmann, K.; Hoffman-Kim, D.; Palmore, G.T. Micropatterns of positive guidance cues anchored to polypyrrole doped with polyglutamic acid: A new platform for characterizing neurite extension in complex environments. Biomaterials 2006, 27, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, N.; Stromme, M.; Fellstrom, B.; Pradhan, S.; Nyholm, L.; Mihranyan, A. In vitro and in vivo toxicity of rinsed and aged nanocellulose-polypyrrole composites. J. Biomed. Mater. Res. A 2012, 100, 2128–2138. [Google Scholar] [CrossRef]
- Borriello, A.; Guarino, V.; Schiavo, L.; Alvarez-Perez, M.A.; Ambrosio, L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011, 22, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, M.P.; Ghasemi-Mobarakeh, L.; Jin, G.; Ramakrishna, S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011, 112, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Baharvand, H.; Kiani, S.; Al-Deyab, S.S.; Ramakrishna, S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen Med. 2011, 5, e17–e35. [Google Scholar] [CrossRef] [PubMed]
- Peramo, A.; Urbanchek, M.G.; Spanninga, S.A.; Povlich, L.K.; Cederna, P.; Martin, D.C. In situ polymerization of a conductive polymer in acellular muscle tissue constructs. Tissue Eng. Part A 2008, 14, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Bartolo, P. 3D Printing of Polycaprolactone-Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Marsudi, M.A.; Ariski, R.T.; Wibowo, A.; Cooper, G.; Barlian, A.; Rachmantyo, R.; Bartolo, P. Conductive Polymeric-Based Electroactive Scaffolds for Tissue Engineering Applications: Current Progress and Challenges from Biomaterials and Manufacturing Perspectives. Int. J. Mol. Sci. 2021, 22, 1543. [Google Scholar] [CrossRef]
- Khan, M.A.; Cantù, E.; Tonello, S.; Serpelloni, M.; Lopomo, N.F.; Sardini, E. A Review on Biomaterials for 3D Conductive Scaffolds for Stimulating and Monitoring Cellular Activities. Appl. Sci. 2019, 9, 961. [Google Scholar] [CrossRef] [Green Version]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.F.; Yu, J.X.; Chang, H.X. Two dimensional nanosheets as conductive, flexible elements in biomaterials. J. Mater. Chem B 2015, 3, 4959–4964. [Google Scholar] [CrossRef]
- Kim, T.G.; Kim, J.M.; Jang, K.S.; Lee, S.J. Dispersibility-tailored conductive epoxy nanocomposites with silica nanoparticle-embedded silver nanowires. Polym. Test. 2021, 96, 107111. [Google Scholar] [CrossRef]
- Naghdi, S.; Rhee, K.Y.; Hui, D.; Park, S.J. A Review of Conductive Metal Nanomaterials as Conductive, Transparent, and Flexible Coatings, Thin Films, and Conductive Fillers: Different Deposition Methods and Applications. Coatings 2018, 8, 278. [Google Scholar] [CrossRef] [Green Version]
- Munir, K.S.; Wen, C.; Li, Y.C. Carbon Nanotubes and Graphene as Nanoreinforcements in Metallic Biomaterials: A Review. Adv. Biosyst 2019, 3, 1800212. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.L.; Zhao, T.S.; Zhou, Y.Q.; Li, S.H.; Li, J.J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Neofytou, E.; Cahill, T.J., 3rd; Beygui, R.E.; Zare, R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.; Li, L.; Zeng, X. A glucose biosensor based on the synergistic action of nanometer-sized TiO2 and polyaniline. Talanta 2015, 131, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthes, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Richards, R.G. The effect of surface roughness on fibroblast adhesion in vitro. Injury 1996, 27 (Suppl. 3), SC38–SC43. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Huang, C.; Wang, Y.; Pei, D. Effect of intracanal dentine wettability on human dental pulp cell attachment. Int. Endod. J. 2012, 45, 346–353. [Google Scholar] [CrossRef]
- Zareidoost, A.; Yousefpour, M.; Ghaseme, B.; Amanzadeh, A. The relationship of surface roughness and cell response of chemical surface modification of titanium. J. Mater. Sci. Mater. Med. 2012, 23, 1479–1488. [Google Scholar] [CrossRef]
- Liang, C.; Luo, Y.; Yang, G.; Xia, D.; Liu, L.; Zhang, X.; Wang, H. Graphene Oxide Hybridized nHAC/PLGA Scaffolds Facilitate the Proliferation of MC3T3-E1 Cells. Nanoscale Res. Lett. 2018, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Vyas, C.; Roberts, I.; Poutrel, Q.A.; Chiang, W.H.; Blaker, J.J.; Huang, Z.; Bartolo, P. Fabrication and characterisation of 3D printed MWCNT composite porous scaffolds for bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 266–278. [Google Scholar] [CrossRef]
- Zhao, F.; Shi, Y.; Pan, L.; Yu, G. Multifunctional Nanostructured Conductive Polymer Gels: Synthesis, Properties, and Applications. Acc. Chem. Res. 2017, 50, 1734–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Zhu, G.; Huang, Y.; Shi, X.; Wang, Y. The stimulation of the differentiation of pheochromocytoma (PC12-L) cells into neuron-like cells by electrically conductive nanofibre mesh. Appl. Mater. Today 2016, 5, 215–222. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Paíno, C.L.; Trillo, M.Á.; Úbeda, A. Electric Stimulation at 448 kHz Promotes Proliferation of Human Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2014, 34, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Oida, H.; Kobayashi, T.; Maruyama, T.; Tanaka, M.; Katayama, T.; Yamaguchi, K.; Segi, E.; Tsuboyama, T.; Matsushita, M.; et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc. Natl. Acad. Sci. USA 2002, 99, 4580–4585. [Google Scholar] [CrossRef] [Green Version]
- Turner, C.H.; Robling, A.G. Exercises for improving bone strength. Br. J. Sports Med. 2005, 39, 188–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lirani-Galvao, A.P.; Bergamaschi, C.T.; Silva, O.L.; Lazaretti-Castro, M. Electrical field stimulation improves bone mineral density in ovariectomized rats. Braz J. Med. Biol. Res. 2006, 39, 1501–1505. [Google Scholar] [CrossRef] [Green Version]
- Florio, C.S. Effectiveness of various isometric exercises at improving bone strength in cortical regions prone to distal tibial stress fractures. Int. J. Numer. Method Biomed. Eng. 2018, 34, e2976. [Google Scholar] [CrossRef]
- Hiltunen, M.; Pelto, J.; Ella, V.; Kellomaki, M. Uniform and electrically conductive biopolymer-doped polypyrrole coating for fibrous PLA. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.R.; Li, K. The electrically conductive scaffold as the skeleton of stem cell niche in regenerative medicine. Mat. Sci. Eng. C-Mater. 2014, 45, 671–681. [Google Scholar] [CrossRef]
- Guex, A.G.; Puetzer, J.L.; Armgarth, A.; Littmann, E.; Stavrinidou, E.; Giannelis, E.P.; Malliaras, G.G.; Stevens, M.M. Highly porous scaffolds of PEDOT:PSS for bone tissue engineering. Acta Biomater. 2017, 62, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Portan, D.V.; Deligianni, D.D.; Papanicolaou, G.C.; Kostopoulos, V.; Psarras, G.C.; Tyllianakis, M. Combined Optimized Effect of a Highly Self-Organized Nanosubstrate and an Electric Field on Osteoblast Bone Cells Activity. Biomed. Res. Int. 2019, 2019, 7574635. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.S.; Edwards, J.R.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boroojeni, F.R.R.; Mashayekhan, S.; Abbaszadeh, H.; Ansarizadeh, M. Conductive Nanofiber Scaffold for Bone Tissue Engineering. In Proceedings of the 2017 24th National and 2nd International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 30 November–1 December 2017; pp. 1–5. [Google Scholar]
- Kuzyk, P.R.; Schemitsch, E.H. The science of electrical stimulation therapy for fracture healing. Indian J. Orthop. 2009, 43, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Aleem, I.S.; Aleem, I.; Evaniew, N.; Busse, J.W.; Yaszemski, M.; Agarwal, A.; Einhorn, T.; Bhandari, M. Efficacy of Electrical Stimulators for Bone Healing: A Meta-Analysis of Randomized Sham-Controlled Trials. Sci. Rep. 2016, 6, 31724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorgensen, N.R.; Geist, S.T.; Civitelli, R.; Steinberg, T.H. ATP- and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J. Cell Biol. 1997, 139, 497–506. [Google Scholar] [CrossRef]
- Cao, J.; Man, Y.H.; Li, L.S. Electrical stimuli improve osteogenic differentiation mediated by aniline pentamer and PLGA nanocomposites. Biomed. Rep. 2013, 1, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Z.P.; Xia, P.; Pan, S.; Zheng, S.; Fu, C.; Chang, Y.X.; Ma, Y.; Wang, J.C.; Yang, X.Y. Combined treatment with electrical stimulation and insulin-like growth factor-1 promotes bone regeneration in vitro. PLoS ONE 2018, 13, e0197006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srirussamee, K.; Mobini, S.; Cassidy, N.J.; Cartmell, S.H. Direct electrical stimulation enhances osteogenesis by inducing Bmp2 and Spp1 expressions from macrophages and preosteoblasts. Biotechnol. Bioeng. 2019, 116, 3421–3432. [Google Scholar] [CrossRef]
- Sahm, F.; Ziebart, J.; Jonitz-Heincke, A.; Hansmann, D.; Dauben, T.; Bader, R. Alternating Electric Fields Modify the Function of Human Osteoblasts Growing on and in the Surroundings of Titanium Electrodes. Int. J. Mol. Sci. 2020, 21, 6944. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, J.M.; Zohny, Z.; MacEwan, M.; Stephen, M.; Johnston, W.; Gamble, P.; Zeng, Y.; Yan, Y.; Ray, W.Z. Electrical Stimulation and Bone Healing: A Review of Current Technology and Clinical Applications. IEEE Rev. Biomed. Eng. 2018, 11, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, M.; Kang, E.T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef]
- Robling, A.G.; Turner, C.H. Mechanical signaling for bone modeling and remodeling. Crit Rev. Eukaryot Gene Expr. 2009, 19, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liedert, A.; Kaspar, D.; Augat, P.; Ignatius, A.; Claes, L. Mechanobiology of Bone Tissue and Bone Cells. In Mechanosensitivity in Cells and Tissues; Kamkin, A., Kiseleva, I., Eds.; Academia: Moscow, Russia, 2005. [Google Scholar]
- Ozcivici, E.; Luu, Y.K.; Adler, B.; Qin, Y.X.; Rubin, J.; Judex, S.; Rubin, C.T. Mechanical signals as anabolic agents in bone. Nat. Rev. Rheumatol. 2010, 6, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Palomares, K.T.; Gleason, R.E.; Mason, Z.D.; Cullinane, D.M.; Einhorn, T.A.; Gerstenfeld, L.C.; Morgan, E.F. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J. Orthop. Res. 2009, 27, 1123–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, C.D.; Zhang, N.; Tong, W.X.; Zhang, Y.F.; Shan, S.Z.; Zhang, X.L.; Li, Q.F. Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis. 2016, 7, e2221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, I.; Cerqueira, G.; Varella Penteado, F.; Córdoba de Torresi, S.I. Electrical Stimulation and Conductive Polymers as a Powerful Toolbox for Tailoring Cell Behaviour in vitro. Front. Med Technol. 2021, 3, 670274. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhu, K.; Li, Y.; Zhu, Z.; Pan, L.; Pan, T.; Borgens, R.B.; Zhao, M. Optimization of Electrical Stimulation for Safe and Effective Guidance of Human Cells. Bioelectricity 2020, 2, 372–381. [Google Scholar] [CrossRef]
- Grant, P.F.; Lowery, M.M. Electric field distribution in a finite-volume head model of deep brain stimulation. Med. Eng. Phys. 2009, 31, 1095–1103. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Type | Polymer | Advantages | Reference |
|---|---|---|---|
| Synthetic | PLA | Biodegradable; controllable geometry | [59,62] |
| PCL | Biocompatibility; ease of manipulation | [67] | |
| PLGA | Controllable degradation | [59,68] | |
| PGA | Nontoxic in degradation | [62] | |
| PVA | Low protein absorption; high water solubility | [69] | |
| Natural | Collagen | Naturally found in ECM; improves biocompatibility; biodegradable | [70,71] |
| Fibrin | Growth factors; co-enzymes | [72] | |
| Gelatin | Improved osteoinduction | [73] | |
| Chitosan | Osteoconductivity; interaction with charged molecules; resistance to bacteria | [74,75] | |
| Silk | Strong natural fiber; ease of processing; controllable degradation | [76] |
| Ceramic | Strengths | Weaknesses | Reference |
|---|---|---|---|
| HA | Found in natural bone tissue; biocompatible; stimulates osteoconduction | Not suitable as stand-alone supportive scaffold (often used to tune degradation) | [85,86] |
| TCP | High solubility; biodegradable | Low mechanical resistance; α-TCP rapid degradation | [87,88] |
| CaCO3 | Flexibility in preparation; biodegradable | Reduction of compressive strength when used as additive to scaffold | [89] |
| BAGs | Antibacterial properties | Low fracture toughness limits implantation into load bearing bone alone | [90,91] |
| Name and Abbreviation | |
|---|---|
| Polypyrrole (PPy) | Poly(p-phenylene terephthalamide) (PPTA) |
| Polyaniline (PANI) | Polyacetylene (PAc) |
| Poly(3,4-ethylenedioxythiophene) (PEDOT) | Poly(isothianaphthene) (PITN) |
| Polythiophene (PTh) | Poly(a-naphthylamine) (PNA) |
| Polythiophene-vinylene (PTh-V) | Polyazulene (PAZ) |
| Poly(2,5-thienylenevinylene) (PTV) | Polyfuran (PFu) |
| Poly(3-alkylthiophene) (PAT) | Polyisoprene (PIP) |
| Poly(p-phenylene) (PPP) | Polybutadiene (PDB) |
| Poly(p-phenylene sulphide) (PPS) | Poly(3-octylthiophnene-3-methylthiophene) (POTMT) |
| Poly(p-phenylene vinylene) (PPV) | Poly(p-phenylene terephthalamide) (PPTA) |
| Tissue/Conductive Polymer | Conductivity (S cm−1) |
|---|---|
| * Cancellous Bone | 1.6 × 10−3–2.0 × 10−3 |
| * Cortical Bone | 5.8 × 10−4–6.3 × 10−4 |
| Polypyrrole (PPy) | 1 × 102–7.5 × 103 |
| Polyaniline (PANI) | 30–200 |
| † Poly(3,4-ethylenedioxythiophene) (PEDOT) | 10–1 × 103 |
| Polythiophene (PTh) | 10–1 × 103 |
| Poly(p-phenylene) (PPP) | 1 × 102–1 × 103 |
| Poly(p-phenylenevinylene) (PPV) | 3–5 × 103 |
| Polyacetylene (PAc) | 1 × 103–1.7 × 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2022, 13, 1. https://doi.org/10.3390/jfb13010001
Dixon DT, Gomillion CT. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. Journal of Functional Biomaterials. 2022; 13(1):1. https://doi.org/10.3390/jfb13010001
Chicago/Turabian StyleDixon, Damion T., and Cheryl T. Gomillion. 2022. "Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook" Journal of Functional Biomaterials 13, no. 1: 1. https://doi.org/10.3390/jfb13010001
APA StyleDixon, D. T., & Gomillion, C. T. (2022). Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. Journal of Functional Biomaterials, 13(1), 1. https://doi.org/10.3390/jfb13010001






