Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application
Abstract
1. Introduction
2. Properties of Gold Nanoparticles
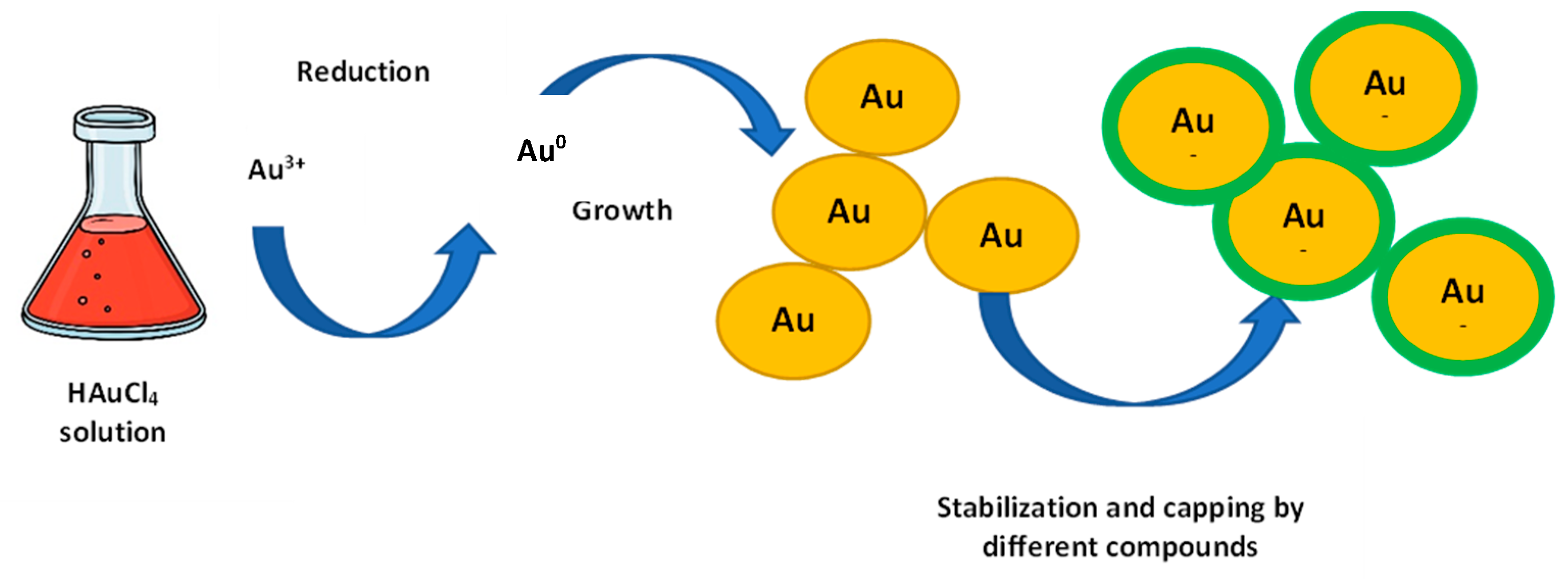
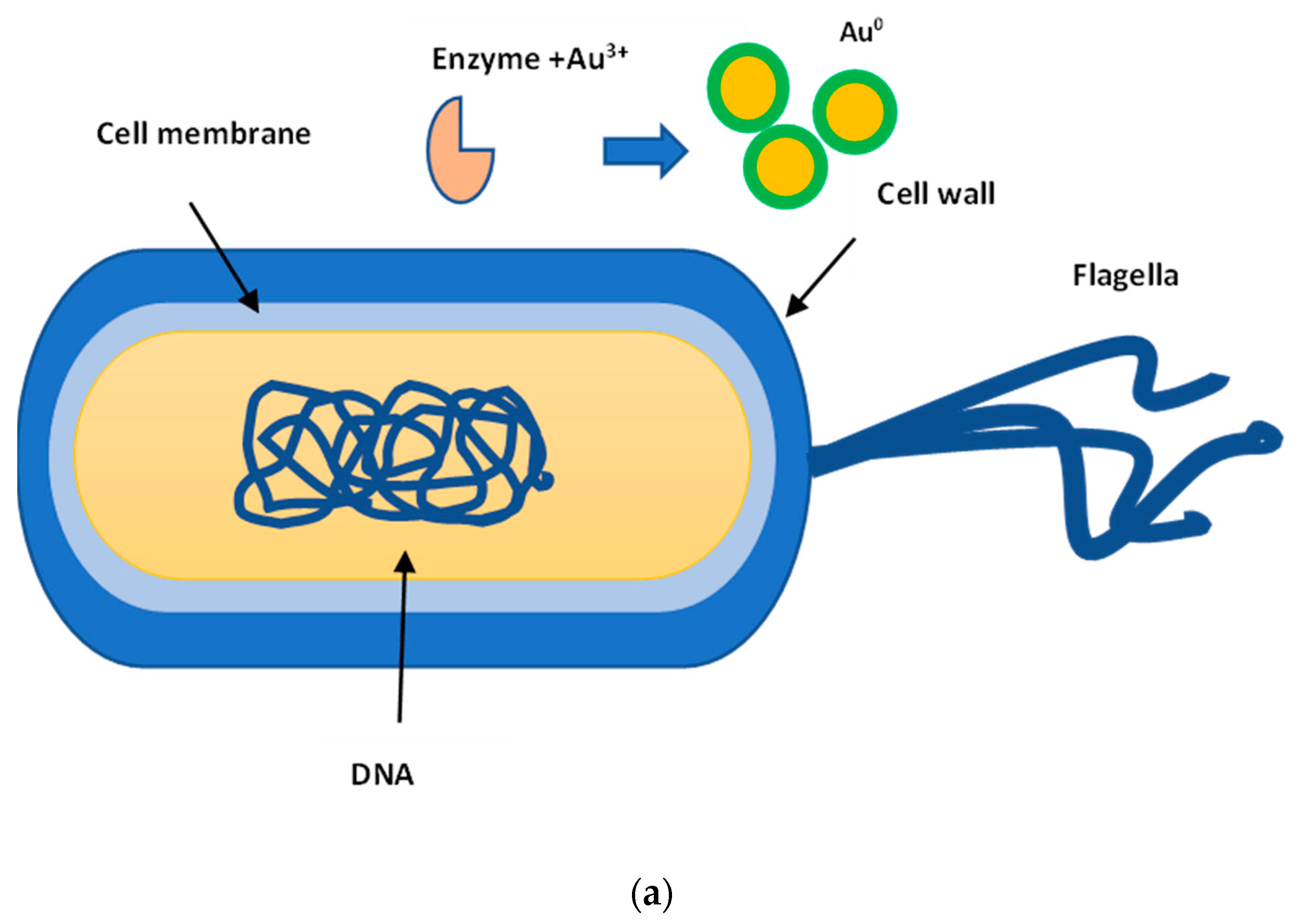
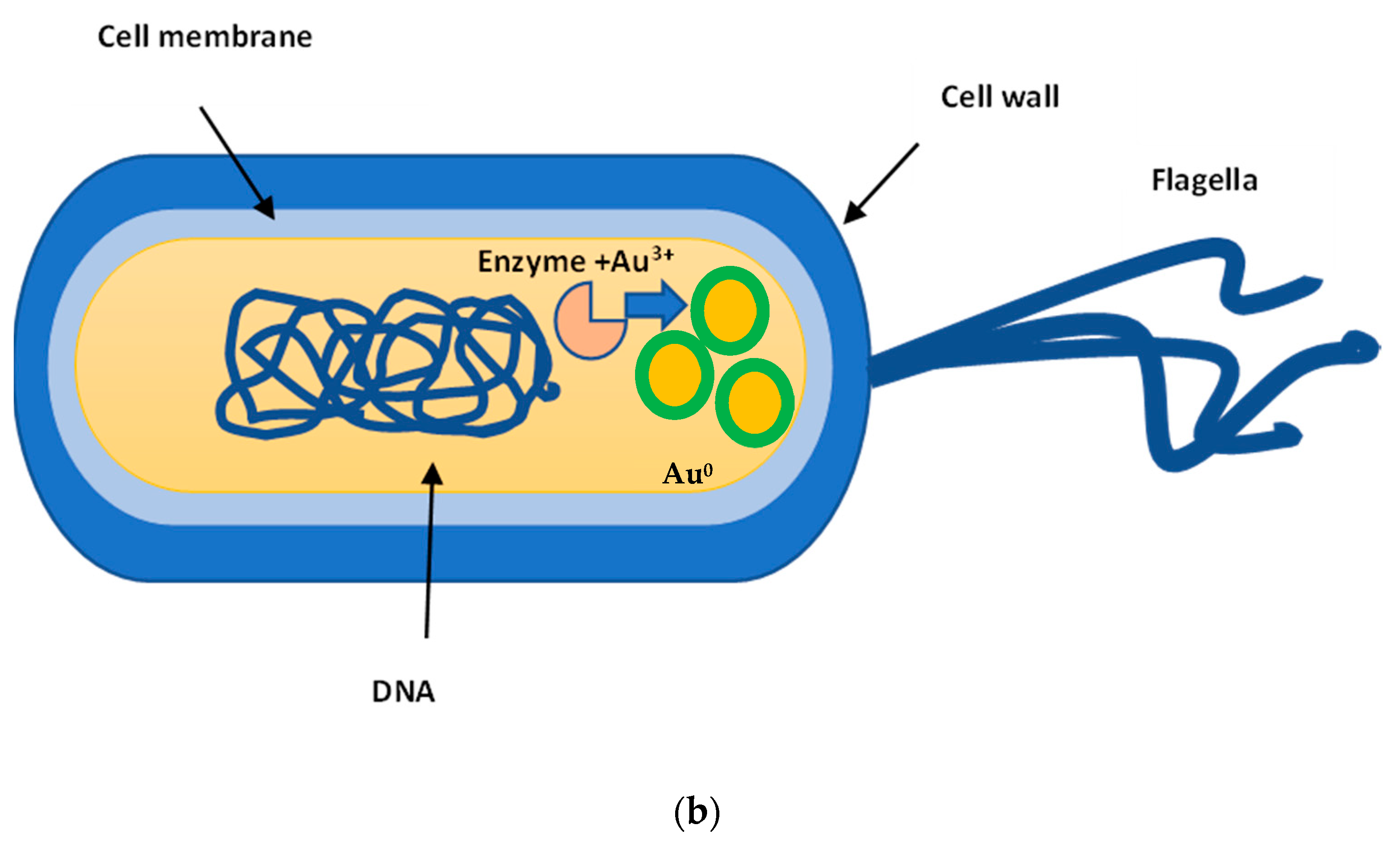
2.1. AuNPs Biosynthesis
2.2. The AuNPs Morphology (Shape and Size)
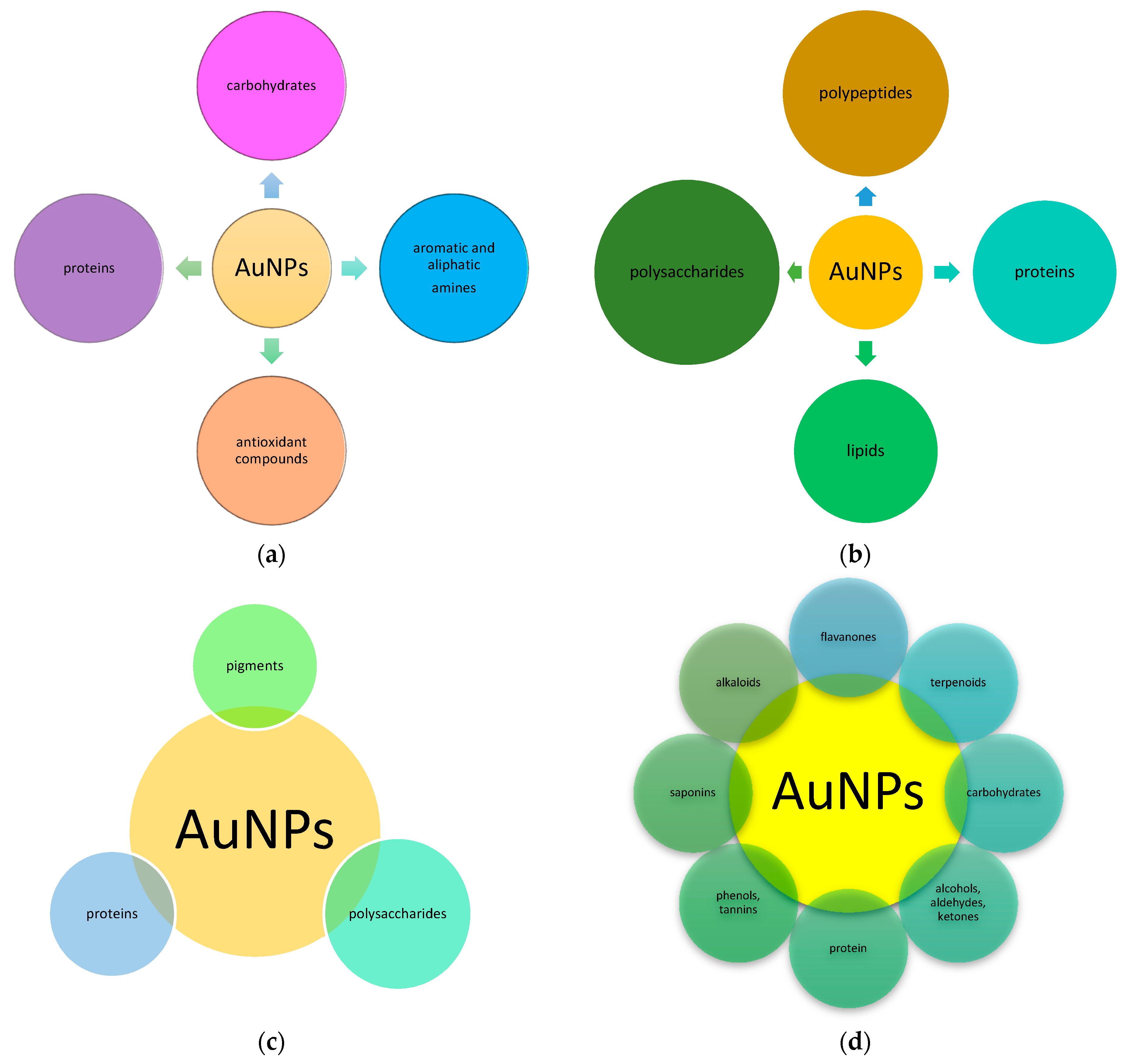
2.3. Capping and Stabilizing Agents
2.4. Mechanism of AuNPs Action on Cells
3. Biomedical Application of AuNPs
3.1. Antimicrobial Activity
3.2. Antiviral Activity
3.3. Antioxidant Activity
3.4. Anticancer Activity
3.5. Other Activities
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thakkar, N.K.; Mhatre, S.S.; Parikh, Y.R. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against drug-resistant pathogenic microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef]
- Melaiye, A.; Youngs, W.J. Silver and its application as an antimicrobial agent. Expert Opin. Ther. Pat. 2005, 15, 125–130. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R.P. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses 2019, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Ovais, M.; Nadhman, A.; Khalil, A.T.; Raza, A.; Khuda, F.; Sohail, M.F.; Zakiullah; Islam, N.U.; Sarwar, H.S.; Shahnaz, G.; et al. Biosynthesized colloidal silver and gold nanoparticles as emerging leishmanicidal agents: An insight. Nanomedicine 2017, 12, 2807–2819. [Google Scholar] [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015, 829526. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biology and medicine: Recent advances and prospects. Acta Nat. 2011, 3, 34–55. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. Engl. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Antonovych, T.T. Gold nephropathy. Ann. Clin. Lab. Sci. 1981, 11, 386–391. [Google Scholar] [PubMed]
- Freestone, I.; Meeks, N.; Sax, M.; Higgitt, C. The Lycurgus Cup—A Roman nanotechnology. Gold Bulletin. 2007, 40, 270–277. [Google Scholar] [CrossRef]
- Antonii, F. Panacea Aurea Sive Tractatus duo de Ipsius Auro Potabili; Ex Bibliopolio Frobeniano: Hamburgi, Germany, 1618. [Google Scholar]
- Lakhani, S.R. Early clinical pathologists: Robert Koch (1843–1910). J. Clin. Pathol. 1993, 46, 596–598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. 1857, 147, 145–181. [Google Scholar]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mater. Sci. Eng. R Rep. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Bychkovskiv, P.M.; Kladiev, A.A.; Solomevich, S.O.; Schegolev, S.Y. Gold nanoparticles: Synthesis, properties, biomedical application. Russ. Biother. J. 2011, 10, 37–46. [Google Scholar]
- Kannan, R.; Rahing, V.; Cutler, C.; Pandrapragada, R.; Katti, K.K.; Kattumuri, V.; Robertson, J.D.; Casteel, S.J.; Jurisson, S.; Smith, C.; et al. Nanocompatible chemistry toward fabrication of targetspecific gold nanoparticles. J. Am. Chem. Soc. 2006, 128, 11342–11343. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Jamroz, P.; di Cenzo, G.C.; Sergiel, I.; Kozlecki, T.; Pohl, P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2019, 12, 4118–4130. [Google Scholar] [CrossRef]
- Montes, M.O.; Mayoral, A.; Deepak, F.L.; Parsons, J.G.; Jose-Yacama, M.; Videa, J.R.P.; Torresdey, J.L.G. Anisotropic gold nanoparticles and gold plates biosynthesis using alfalfa extracts. J. Nanopart. Res. 2011, 13, 3113–3121. [Google Scholar] [CrossRef]
- Kumar, V.; Kumari, A.; Guleria, P.; Yadav, S.K. Evaluating the toxicity of selected types of nanochemicals. Rev. Environ. Contam. Toxicol. 2012, 215, 39–121. [Google Scholar] [PubMed]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticle. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.H.; Soshnikova, V.; Markus, J.; Kim, Y.J.; Lee, S.C.; Singh, P.; Castro-Aceituno, V.; Ahn, S.; Kim, D.H.; Shim, Y.J.; et al. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif. Cells Nanomed. Biotechnol. 2017, 46, 599–606. [Google Scholar]
- Vijayakumar, S.; Ganesan, S. Gold nanoparticles as an HIV entry inhibitor. Curr. HIV Res. 2012, 10, 643–646. [Google Scholar] [CrossRef]
- Bagheri, S.; Yasemi, M.; Safaie-Qamsari, E.; Rashidiani, J.; Abkar, M.; Hassani, M.; Mirhosseini, S.A.; Kooshki, H. Using gold nanoparticles in diagnosis and treatment of melanoma cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 462–471. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wu, S.-H.; Chen, C.-H. A Simple strategy for prompt visual sensing by gold nanoparticles: General applications of interparticle hydrogen bonds. Angew. Chem. Int. Ed. 2006, 45, 4948–4951. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.P.; Yong, K.T. Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications. Chem. Soc. Rev. 2014, 43, 3426–3452. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Rawat, P.; Rajput, Y.S.; Bharti, M.K.; Sharma, R. A method for synthesis of gold nanoparticles using 1-amino-2-naphthol-4-sulphonic acid as reducing agent. Curr. Sci. 2016, 110, 2297–2300. [Google Scholar] [CrossRef]
- Okamoto, T.; Nakamura, T.; Sakota, K.; Yatsuhashi, T. Synthesis of Single-Nanometer-Sized Gold Nanoparticles in Liquid–Liquid Dispersion System by Femtosecond Laser Irradiation. Langmuir 2019, 35, 12123–12129. [Google Scholar] [CrossRef]
- Gu, X.; Xu, Z.; Gu, L.; Xu, H.; Han, F.; Chen, B.; Pan, X. Preparation and antibacterial properties of gold nanoparticles: A review. Environ. Chem. Lett. 2021, 191, 67–187. [Google Scholar] [CrossRef]
- Sheny, D.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim. Acta A Mol. BiomolSpectrosc. 2011, 79, 254–262. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Badeggi, U.M.; Ismail, E.; Adeloye, A.O.; Botha, S.; Badmus, J.A.; Marnewick, J.L.; Cupido, C.N.; Hussein, A.A. Green synthesis of gold nanoparticles capped with procyanidins from Leucosidea sericea as potential antidiabetic and antioxidant agents. Biomolecules 2020, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, K.S.; Mukherje, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. [Google Scholar] [CrossRef]
- Ghosh, S.; Ahmad, R.; Zeyaullah, M.; Khare, S.K. Microbial Nano-Factories: Synthesis and Biomedical Applications. Front. Chem. 2021, 9, 626834. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, T.; Anuradha, J.; Ganaie, S.U.; Abbasi, S.A. Biomimetic synthesis of nanoparticles using aqueous extracts of plants (botanical species). J. Nano Res. 2015, 31, 138–202. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Yew, Y.P.; Teow, S.-Y.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Recent developments in the facile bio-synthesis of gold nanoparticles (AuNPs) and their biomedical applications. Int. J. Nanomed. 2020, 15, 275–300. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Microbes mediated synthesis of metal nanoparticles: Current status and future prospects. Int. J. Nanomater. Biostruct. 2016, 6, 1–24. [Google Scholar]
- Menon, S.; Rajeshkumar, S.; VenkatKumar, S. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour.-Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Nangia, Y.; Wangoo, N.; Goya, N.; Shekhawat, G.; Suri, R. A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb. Cell Factor. 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate. Mat. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Hosseini, M.; Mashreghi, M.; Eshghi, H. Biosynthesis and antibacterial activity of gold nanoparticles coated with reductase enzymes. Micro Nano Lett. 2016, 11, 484–489. [Google Scholar] [CrossRef]
- Gholami-Shabani, M.; Shams-Ghahfarokhi, M.; Gholami-Shabani, Z.; Akbarzadeh, A.; Riazi, G.; Ajdari, S.; Amani, A.; Razzaghi-Abyane, M. Enzymatic synthesis of gold nanoparticles using sulfite reductase purified from Escherichia coli: A green eco-friendly approach. Proc. Biochem. 2015, 50, 1076–1085. [Google Scholar] [CrossRef]
- Bharde, A.; Kulkarni, A.; Rao, M.; Prabhune, A.; Sastry, M. Bacterial enzyme mediated biosynthesis of gold nanoparticles. J. Nanosc. Nanotechnol. 2007, 7, 4369–4377. [Google Scholar] [CrossRef]
- Ramalingam, V. Multifunctionality of gold nanoparticles: Plausible and convincing properties. Adv. Colloid Interface Sci. 2019, 271, 101989. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.P.; Wang, L.; Benicewicz, B.C.; Decho, A.W. Inorganic nanoparticles engineered to attack bacteria. Chem. Soc. Rev. 2015, 44, 7787–7807. [Google Scholar] [CrossRef]
- Issazade, K.; Jahanpour, N.; Pourghorbanali, F.; Raeisi, G.; Faekhondeh, J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013, 4, 60–63. [Google Scholar]
- Li, J.; Li, Q.; Ma, X.; Tian, B.; Li, T.; Yu, J.; Dai, S.; Weng, Y.; Hu, Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, 11, 5931–5944. [Google Scholar] [CrossRef] [PubMed]
- Markus, J.; Mathiyalagana, R.; Kim, Y.-J.; Abbai, R.; Singh, P.; Ahn, S.; Perez, Z.E.J.; Hurh, J.; Yang, D.C. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51T isolated from Korean kimchi. Enzym. Microb. Technol. 2016, 95, 85–93. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J. Biomed. Nanotechnol. 2011, 7, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Liang, J.; Schmidt, M.; Laffir, F.; Marsili, E. Biomineralization mechanism of gold by zygomycete fungi Rhizopous oryzae. ACS Nano 2012, 6, 6165–6173. [Google Scholar] [CrossRef]
- Kitching, M.; Ramani, M.; Marsili, E. Fungal biosynthesis of gold nanoparticles: Mechanism and scale up. Microb. Biotechnol. 2015, 8, 904–917. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Durán, M.; Yadav, A.; Gade, A.; Rai, M. Mechanistic aspects in the biogenic synthesis of extracellular metal nanoparticles by peptides, bacteria, fungi, and plants. Appl. Microbiol. Biotechnol. 2011, 90, 1609–1624. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; Alves, O.L.; Gabriel-Souza, I.H.D.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au-Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef]
- Roya, S.; Das, T.K.; Maiti, G.P.; Basu, U. Microbial biosynthesis of nontoxic gold nanoparticles. Mater. Sci. Eng. 2016, 203, 41–51. [Google Scholar] [CrossRef]
- Das, S.K.; Dickinson, C.; Lafir, F.; Brougham, D.F.; Marsili, E. Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 2012, 14, 1322–1334. [Google Scholar] [CrossRef]
- Scott, D.; Toney, M.; Muzikár, M. Harnessing the mechanism of glutathione reductase for synthesis of active site bound metallic nanoparticles and electrical connection to electrodes. J. Am. Chem. Soc. 2008, 130, 865–874. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potentialin detection of liver cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green synthesis of nanoparticles with extracellular and intracellular extracts of basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- Apte, M.; Girme, G.; Bankar, A.; Ravikumar, A.; Zinjarde, S. 3,4-Dihydroxy-l-phenylalanine-derived melanin from Yarrowia lipolytica mediates the synthesis of silver and gold nanostructures. J. Nanobiotechnol. 2013, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater-Review. Biores. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Malarkodi, C.; Gnanajobitha, G.; Paulkumar, K.; Vanaja, M.; Kannan, C.; Annadurai, G. Seaweed-mediated synthesis of gold nanoparticles using Turbinaria conoides and its characterization. J. Nanostr. Chem. 2013, 3, 44. [Google Scholar] [CrossRef]
- Lumogdang, L.; Teves, F. Brown seaweed Sargassum polycystum (C. Agardh) extract as mediator in the green synthesis of colloidal gold nanoparticles from Malita, Davao Occidental Philippines. IJB 2020, 16, 404–417. [Google Scholar]
- Mata, Y.N.; Torres, E.; Blazquez, M.L.; Ballester, A.; Gonzalez, F.; Munoz, J.A. Gold (III) biosorption and bio-reduction with brown alga Fucus vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation, a novel phenomenon. J. Appl. Phycol. 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A review on the biosynthesis of metallic nanoparticles (gold and silver) using bio-components of microalgae: Formation mechanism and applications. Enzym. Microb. Technol. 2016, 95, 28–44. [Google Scholar] [CrossRef]
- Tamuly, C.; Hazarika, M.; Bordoloi, M. Biosynthesis of Au nanoparticles by Gymnocladus assamicus and its catalytic activity. Mat. Lett. 2013, 108, 276–279. [Google Scholar] [CrossRef]
- Rajan, A.; Vilas, V.; Philip, D. Studies on catalytic, antioxidant, antibacterial and anticancer activities of biogenic gold nanoparticles. J. Mol. Liq. 2015, 212, 331–339. [Google Scholar] [CrossRef]
- Vijaya Kumar, V.P.; Kala, S.M.J.; Prakash, K.S. Green synthesis of gold nanoparticles using Croton Caudatus Geisel leaf extract and their biological studies. Mat. Lett. 2019, 236, 19–22. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Ayat, M.S.; Shabrawy, M.A.; Millet, P. Green synthesis of gold nanoparticles using Parsley leaves extract and their applications as an alternative catalytic, antioxidant, anticancer, and antibacterial agents. Adv. Powder Technol. 2020, 31, 4390–4400. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Shahid, M.; Rauf, A.; Muhammad, N.; Khan, A.; Shah, M.R.; Khan, M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab. J. Chem. 2019, 12, 2914–2925. [Google Scholar] [CrossRef]
- Shankar, S.S.; Ahmad, A.; Pasricha, R.; Sastry, M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 2003, 13, 1822–1826. [Google Scholar] [CrossRef]
- Gan, P.P.; Ng, S.H.; Huang, Y.; Li, S.F.Y. Green synthesis of gold nanoparticles using palm oil mill effluent (POME): A low-cost and eco-friendly viable approach. Bioresour. Technol. 2012, 113, 132–135. [Google Scholar] [CrossRef]
- Das, L.J.; Velusamy, P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora. J. Taiwan Inst. Chem. Eng. 2014, 45, 2280–2285. [Google Scholar] [CrossRef]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of gold nanoparticles using Mimosa tenuiflora extract, assessments of cytotoxicity, cellular uptake, and catalysis. Nanoscale Res. Lett. 2019, 14, 334. [Google Scholar] [CrossRef]
- Kumar, K.M.; Mandal, B.K.; Sinha, M.; Krishnakumar, V. Terminalia chebula mediated green and rapid synthesis of gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 490–494. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Kala, S.M.J.; Pushparaj, T.L. Biogenic synthesis of gold nanoparticles using Jasminum auriculatum leaf extract and their catalytic, antimicrobial and anticancer activities. J. Drug Deliv. Sci. Technol. 2020, 57, 101620. [Google Scholar] [CrossRef]
- Muthuvel, A.; Adavallan, K.; Balamurugan, K.; Krishnakumar, N. Biosynthesis of gold nanoparticles using Solanum nigrum leaf extract and screening their free radical scavenging and antibacterial properties. Biomed. Prev. Nutr. 2014, 4, 325–332. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Ahmad, B.; Hafeez, N.; Bashir, S.; Rauf, A. Mujeeb-ur-Rehman Phytofabricated gold nanoparticles and their biomedical applications. Biomed. Pharmacother. 2017, 89, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Raza, A.; Naz, S.; Islam, N.U.; Khalil, A.T.; Ali, S.; Khan, M.A.; Shinwari, Z.K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Kurapov, P.B.; Bakhtenko, E.Y. Gold nanoparticles in the diagnosis anad treatment of cancer. Bull. Rsmu 2018, 6, 86–93. [Google Scholar]
- Zharov, V.P.; Kim, J.W.; Curiel, D.T.; Everts, M. Self-assembling nanoclusters in living systems: Application for integrated photothermal nanodiagnostics and nanotherapy. Nanomed. Nanotechnol. Biol. Med. 2005, 1, 326–345. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Pourali, P.; Badiee, P.S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Sharma, N.; Pinnaka, A.K.; Raje, M.; Fnu, A.; Bhattacharyya, M.S.; Roy, A. Choudhury Exploitation of marine bacteria for production of gold nanoparticles. Microb. Cell Fact. 2012, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, N.; Cao, Y.; Wu, P. Biosynthesis of gold nanoparticles assisted by the intracellular protein extract of Pycnoporus sanguineus and its catalysis in degradation of 4-nitroaniline. Nanoscale Res. Lett. 2015, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; Shen, W.; Wang, J.; Li, H.; Zhang, Z.; Li, S.; Zhou, J. Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols. Colloids Surf. A Phys. Eng 2016, 497, 280–285. [Google Scholar] [CrossRef]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R.; et al. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kareem, M.M.; Zohri, A.A. Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: Optimization, characterization and antimicrobial activity. Lett. Appl. Microbiol. 2018, 67, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Arockiya, F.; Rajathi, A.; Parthiban, C.; Kumar, V.G.; Anantharaman, P. Biosynthesis of antibacterial gold nanoparticles using brown alga, Stoechospermum marginatum (kützing). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 99, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Gajanan, G.; Lee, D.S. Biological Synthesis of Gold Nanoparticles Using the Aqueous Extract of the Brown Algae Laminaria japonica. J. Nanoelectron. Optoelectron. 2011, 6, 268–271. [Google Scholar]
- Kumari, V.S.; Sundari, G.S.; Basha, S.K. Facile green synthesis of gold nanoparticles with great catalytic activity using Ulva fasciata. Lett. Appl. NanoBioScience 2014, 3, 124–129. [Google Scholar]
- Annamalai, J.; Nallamuthu, T. Characterization of biosynthesized gold nanoparticles from aqueous extract of Chlorella vulgaris and their anti-pathogenic properties. Appl. Nanosci. 2015, 5, 603–607. [Google Scholar] [CrossRef]
- Sharma, B.; Purkayastha, D.D.; Hazra, S.; Gogoi, L.; Bhattacharjee, C.R.; Ghosh, N.N.; Rout, J. Biosynthesis of gold nanoparticles using a fresh water green alga, Prasiola crispa. Mater. Lett. 2014, 116, 94–97. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, 3029–3039. [Google Scholar] [CrossRef]
- Yang, N.; Lib, W.H.; Hao, L. Biosynthesis of Au nanoparticles using agricultural waste mango peel extract and its in vitro cytotoxic effect on two normal cells. Mater. Lett. 2014, 134, 67–70. [Google Scholar] [CrossRef]
- Ahmad, N.; Bhatnagar, S.; Saxena, R.; Iqbal, D.; Ghosh, A.K.; Dutta, R. Biosynthesis and characterization of gold nanoparticles: Kinetics, in vitro and in vivo study. Mater. Sci. Eng. C 2017, 78, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Boomi, P.; Ganesan, R.M.; Poorani, G.; Gurumallesh Prabu, H.; Ravikumare, S.; Jeyakanthan, J. Biological synergy of greener gold nanoparticles by using Coleus aromaticus leaf extract. Mater. Sci. Eng. C 2019, 99, 202–210. [Google Scholar] [CrossRef]
- Wanga, M.; Wang, L. Plant polyphenols mediated synthesis of gold nanoparticles for pain management in nursing care for dental tissue implantation applications. J. Drug Deliv. Sci. Technol. 2020, 58, 101753. [Google Scholar] [CrossRef]
- Wali, M.; Sajjad, A.S.; Sumaira, S.; Muhammad, N.; Safia, H.; Muhammad, J. Green Synthesis of Gold Nanoparticles and Their Characterizations Using Plant Extract of Papaver somniferum. Nano Sci. Nano Technol. 2017, 11, 118. [Google Scholar]
- López-Miranda, J.L.; Esparza, R.; Rosas, G.; Pérez, R.; Estévez-González, M. Catalytic and antibacterial properties of gold nanoparticles synthesized by a green approach for bioremediation applications. Biotech 2019, 9, 135. [Google Scholar] [CrossRef]
- Aromal, S.A.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lokina, S.; Suresh, R.; Giribabu, K.; Stephen, A.; Sundaram, R.L.; Narayanan, V. Spectroscopic investigations, antimicrobial, and cytotoxic activity of green synthesized gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 129, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, W.; Yang, F.; Liu, H. Controllable biosynthesis of gold nanoparticles from a Eucommia ulmoides bark aqueous extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 73–79. [Google Scholar] [CrossRef]
- Yuan, C.-G.; Huo, C.; Yu, S.; Gui, B. Biosynthesis of gold nanoparticles using Capsicum annuum var. grossum pulp extract and its catalytic activity. Phys. E 2017, 85, 9–26. [Google Scholar]
- Mata, R.; Bhaskaran, A.; Sadras, S.R. Green-synthesized gold nanoparticles from Plumeria alba flower extract to augment catalytic degradation of organic dyes and inhibit bacterial growth. Particuology 2016, 24, 78–86. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.B.; Jayanthi, S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater. Nanotechnol. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, J.; Li, G.; Wang, J.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, Q. Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3297–3305. [Google Scholar] [CrossRef]
- Sun, B.; Hu, N.; Han, L.; Pi, Y.; Gao, Y.; Chen, K. Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4012–4019. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, N.E.; Alomari, A.A. Green synthesis and bactericidal activities of isotropic and anisotropic spherical gold nanoparticles produced using Peganum harmala L. leaf and seed extracts. Biotechnol. Appl. Biochem. 2019, 66, 664–672. [Google Scholar] [CrossRef]
- Lee, K.X.; Shameli, K.; Miyake, M.; Kuwano, N.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Yew, Y.P. Green synthesis of gold nanoparticles using aqueous extract of Garcinia mangostana fruit peels. J. Nanomater. 2016, 2016, 8489094. [Google Scholar]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Chandran, S.P.; Chaudhary, M.; Pasricha, R.; Ahmad, A.; Sastry, M. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol. Prog. 2006, 22, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.I.; Aziz, A.A.; Noqta, O.A. Bio-synthesis of triangular and hexagonal gold nanoparticles using palm oil fronds’ extracts at room temperature. Mater. Res. Express 2018, 5, 015042. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent advances in understanding the protein corona of nanoparticles and in the formulation of “stealthy” nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Maiorano, G.; Sabella, S.; Sorce, B. Effects of cell culture media on the dynamic formation of protein-nanoparticle complexes and influence on the cellular response. ACS Nano 2010, 4, 7481–7491. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Kelly, P.M.; Mahon, E.; Stöckmann, H.; Rudd, P.M.; Caruso, F.; Dawson, K.A.; Yan, Y.; Monopoli, M.P. The “sweet” side of the protein corona: Effects of glycosylation on nanoparticle–cell interactions. ACS Nano 2015, 9, 2157–2166. [Google Scholar] [CrossRef]
- Hellstrand, E.; Lynch, I.; Andersson, A. Complete highdensity lipoproteins in nanoparticle corona. FEBS J. 2009, 276, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Inwati, G.K.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Future Sci. OA 2017, 3, FSO239. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xia, X.; Peng, H.C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.S.S.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large scale screening of Southern African plant extracts for the green synthesis of gold nanoparticles using microtitre-plate method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef] [PubMed]
- Amina, S.J.; Guo, B. A Review on the synthesis and functionalization of gold nanoparticles as a drug delivery vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Ahmad, A.; Khan, M.I.; Kumar, R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr. Sci. 2003, 85, 162–170. [Google Scholar]
- Das, S.K.; Das, A.R.; Guha, A.K. Gold nanoparticles: Microbial synthesis and application in water hygiene management. Langmuir 2009, 25, 8192–8199. [Google Scholar] [CrossRef] [PubMed]
- Binupriya, A.R.; Sathishkumar, M.; Vijayaraghavan, K.; Yun, S.I. Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J. Hazard. Mater. 2010, 177, 539–545. [Google Scholar] [CrossRef]
- Rabeeaa, M.A.; Owaid, M.N.; Azizd, A.A.; Jameeld, M.S.; Dheyab, M.A. Mycosynthesis of gold nanoparticles using the extract of Flammulina velutipes, Physalacriaceae, and their efficacy for decolorization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 103841. [Google Scholar] [CrossRef]
- Deepali, S.; Suvardhan, K.; Krishna, B. Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 2015, 12, 3576–3600. [Google Scholar]
- Ankamwar, B. Biosynthesis of gold nanoparticles (Green-gold) using leaf extract of Terminalia catappa. J. Chem. 2010, 7, 1334–1339. [Google Scholar]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic investigation of phytochemicals involved in green synthesis of gold nanoparticles using aqueous Elaeis guineensis leaves extract: Role of phenolic compounds and flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef]
- Rajathi, F.A.A.; Arumugam, R.; Saravanan, S.; Anantharaman, P. Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J. Photochem. Photobiol. B Biol. 2014, 135, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jang, H.K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Synthesis of variable shaped gold nanoparticles in one solution using leaf extract of Bauhinia variegate. Dig. J. Nanomater. Biostruc. 2011, 6, 1685–1693. [Google Scholar]
- Muthukumar, T.; Sambandam, B.; Aravinthan, A.; Sastry, T.P.; Kim, J.-H. Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 2016, 51, 384–391. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.-G.; Park, H.-J.; Liu, C.-G.; Liu, C.-S.; Meng, X.-H.; Yu, L.-J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Vijilvani, C.; Bindhu, M.; Frincy, F.; AlSalhi, M.S.; Sabitha, S.; Saravanakumar, K.; Devanesan, S.; Umadevi, M.; Aljaafreh, M.J.; Atif, M. Antimicrobial and catalytic activities of biosynthesized gold, silver and palladium nanoparticles from Solanum nigurum leaves. J. Photochem. Photobiol. 2020, 202, 111713. [Google Scholar] [CrossRef]
- Hameed, S.; Wang, Y.; Zhao, L.; Xie, L.; Ying, Y. Shape-dependent significant physical mutilation and antibacterial mechanisms of gold nanoparticles against foodborne bacterial pathogens (Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus) at lower concentrations. Mater. Sci. Eng. C 2020, 108, 110338. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, B.; Garmaroudi, F.S.; Hashemi, M.; Nezhad, H.R.; Nasrollahi, A.; Ardalan, S.; Ardalan, S. Comparison of the anti-bacterial activity on the nanosilver shapes: Nanoparticles, nanorods and nanoplates. Adv. Powder Technol. 2012, 23, 22–26. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef]
- Das, S.; Debnath, N.; Mitra, S.; Datta, A.; Goswami, A. Comparative analysis of stability and toxicity profile of three differently capped gold nanoparticles for biomedical usage. Biometals 2012, 25, 1009–1022. [Google Scholar] [CrossRef]
- Siddiqi, N.J.; Abdelhalim, M.A.; El-Ansary, A.K.; Alhomida, A.S.; Ong, W.Y. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflamm. 2012, 9, 123. [Google Scholar] [CrossRef]
- Piktel, E.; Suprewicz, Ł.; Depciuch, J.; Chmielewska, S.; Skłodowski, K.; Daniluk, T.; Król, G.; Kołat-Brodecka, P.; Bijak, P.; Pajor-Świerzy, A.; et al. Varied-shaped gold nanoparticles with nanogram killing efficiency as potential antimicrobial surface coatings for the medical devices. Sci. Rep. 2021, 11, 12546. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Hafeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Ortiz-Benítez, E.A.; Velázquez-Guadarrama, N.; Durán Figueroa, N.V.; Quezada, H.; Olivares-Trejo, J.J. Antibacterial mechanism of gold nanoparticles on Streptococcus pneumoniae. Metallomics 2019, 11, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Schröfel, A.; Kratošová, G.; Šafarík, I.; Šafaríková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles–A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Prabhune, A.; Perry, C.C. Antibiotic mediated synthesis of gold nanoparticles with potent antimicrobial activity and their application in antimicrobial coatings. J. Mater. Chem. 2010, 20, 6789–6798. [Google Scholar] [CrossRef]
- Radzig, M.A.; Nadtochenko, V.A.; Koksharova, O.A.; Kiwi, J.; Lipasova, V.A.; Khmel, I.A. Antibacterial effects of silver nanoparticles on gram-negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf. B Biointerfaces 2013, 102, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gahlawat, G.; Choudhury, A.R. A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli. Colloids Surf. B Biointerfaces 2018, 167, 1–7. [Google Scholar] [CrossRef]
- Hajnoczky, G.; Csordas, G.; Das, S.; Garcia-Perez, C.; Saotome, M.; Sinha Roy, S.; Yi, M. Mitochondrial calcium signalling and cell death: Approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006, 40, 553–560. [Google Scholar] [CrossRef]
- Rasola, A.; Bernardi, P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011, 50, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Kwon, Y.; Baek, K.-H. Green biosynthesis of gold nanoparticles by onion peel extract: Synthesis, characterization and biological activities. Adv. Powder Technol. 2016, 27, 2204–2213. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial activity and toxicity of gold nanoparticles: Research progress, challenges and prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, N.; Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: A review of in vitro and in vivo studies. Chem. Soc. Rev. 2011, 40, 1647–1671. [Google Scholar] [CrossRef]
- Lopez-Chaves, C.; Soto-Alvaredo, J.; Montes-Bayon, M.; Bettmer, J.; Llopis, J.; Sanchez-Gonzalez, C. Gold nanoparticles: Distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed. J. 2017, 14, 1–12. [Google Scholar] [CrossRef]
- Tarantola, M.; Pietuch, A.; Schneider, D.; Rother, J.; Sunnick, E.; Rosman, C.; Pierrat, S.; Sonnichsen, C. Toxicity of gold-nanoparticles: Synergistic effects of shape and surface functionalization on micromotility of epithelial cells. Nanotoxicology 2011, 5, 254–268. [Google Scholar] [CrossRef]
- Wozniak, A.; Malankowska, A.; Nowaczyk, G.; Grzeskowiak, B.F.; Tusnio, K.; Słomski, R.; Zaleska-Medynska, A.; Jurga, S. Size and shape-dependent cytotoxicity profile of gold nanoparticles for biomedical applications. J. Mater. Sci. Mater. Med. 2017, 28, 92. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of gold nanoparticles shape on their cytotoxicity against human osteoblast and osteosarcoma in in vitro model. Evaluation of the safety of use and anti-cancer potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef]
- Liu, X.; Wu, F.; Tian, Y.; Wu, M.; Zhou, Q.; Jiang, S.; Niu, Z. Size dependent cellular uptake of rod-like bionanoparticles with different aspect ratios. Sci. Rep. 2016, 6, 24567. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The effect of shape on cellular uptake gold nanoparticles in the forms of stars, rods, and triangles. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Hanan, N.; Chiu, H.; Ramachandran, M.; Tung, W.; Mohamad Zain, N.; Yahaya, N.; Lim, V. Cytotoxicity of plant-mediated synthesis of metallic nanoparticles: A systematic review. Int. J. Mol. Sci. 2018, 19, 1725. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Camettib, C.; Russo, M.V. The puzzle of toxicity of gold nanoparticles. The case-study of HeLa cells. Toxicol. Res. 2015, 4, 796–800. [Google Scholar] [CrossRef]
- Botha, T.L.; Elemike, E.E.; Horn, S.; Onwudiwe, D.C.; Giesy, J.P.; Wepener, V. Cytotoxicity of Ag, Au and Ag-Au bimetallic nanoparticles prepared using golden rod (Solidago canadensis) plant extract. Sci. Rep. 2019, 9, 4169. [Google Scholar] [CrossRef] [PubMed]
- Bodelon, G.; Costas, C.; Perez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzan, L.M. Gold nanoparticles for regulation of cell function and behavior. Nano Today 2017, 13, 40–60. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. How toxic are gold nanoparticles? The state-of-the-art. Nano Res. 2015, 8, 1771–1799. [Google Scholar] [CrossRef]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Moussa, I. Antibacterial effects and resistance induction of silver and gold nanoparticles against Staphylococcus aureus-induced mastitis and the potential toxicity in rats. Microbiologyopen 2019, 8, e698. [Google Scholar] [CrossRef] [PubMed]
- Hendi, A. Silver nanoparticles mediate differential responses in some of liver and kidney functions during skin wound healing. J. King Saud. Univ. Sci. 2010, 10, 1018–3647. [Google Scholar] [CrossRef]
- Balalakshmi, C.; Gopinath, K.; Govindarajan, M.; Lokesh, R.; Arumugam, A.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Benelli, G. Green synthesis of gold nanoparticles using a cheap Sphaeranthus indicus extract: Impact on plant cells and the aquatic crustacean Artemia nauplii. J. Photochem. Photobiol. B Biol. 2017, 173, 598–605. [Google Scholar] [CrossRef]
- Hamelian, M.; Hemmati, S.; Varmira, K.; Veisi, H. Green synthesis, antibacterial, antioxidant and cytotoxic effect of gold nanoparticles using Pistacia atlantica extract. J. Taiwan Inst. Chem. Eng. 2018, 93, 21–30. [Google Scholar] [CrossRef]
- Mishra, P.; Ray, S.; Sinha, S.; Das, B.; Khan, M.I.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Facile bio-synthesis of gold nanoparticles by using extract of Hibiscus sabdariffa and evaluation of its cytotoxicity against U87 glioblastoma cells under hyperglycemic condition. Biochem. Eng. J. 2016, 105, 264–272. [Google Scholar] [CrossRef]
- Wani, I.A.; Ahmad, T. Size and shape dependant antifungal activity of gold nanoparticles: A case study of Candida. Colloids Surf. B 2013, 101, 162–170. [Google Scholar] [CrossRef]
- Lee, K.D.; Nagajyothi, P.C.; Sreekanth, T.V.M.; Park, S. Ecofriendly synthesis of gold nanoparticles (AuNPs) using Inonotus obliquus and their antibacterial, antioxidant and cytotoxic activities. J. Ind. Eng. Chem. 2015, 26, 67–72. [Google Scholar] [CrossRef]
- Nagalingama, M.; Kalpanab, V.N.; Devi Rajeswarib, V.; Panneerselvama, A. Biosynthesis, characterization, and evaluation of bioactivities of leaf extractmediated biocompatible gold nanoparticles from Alternanthera bettzickiana. Biotechnol. Rep. 2018, 19, e00268. [Google Scholar]
- Samanta, A.; Gangopadhyay, R.; Ghosh, C.K.; Ray, M. Enhanced photoluminescence from gold nanoparticle decorated polyaniline nanowire bundles. RSC Adv. 2017, 7, 27473–27479. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D.V.L. Gold and silver nanoparticles from Trianthema decandra: Synthesis, characterization, and antimicrobial properties. Int. J. Nanomed. 2012, 7, 5375–5384. [Google Scholar] [CrossRef]
- Gutiérrez, J.A.; Caballero, S.; Díaz, L.A.; Guerrero, M.A.; Ruiz, J.; Ortiz, C.C. High antifungal activity against Candida species ofmonometallic and bimetallic nanoparticles synthesized in nanoreactors. ACS Biomater. Sci. Eng. 2018, 4, 647–653. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Zhang, Y.; Wang, Y.; Liu, L.; Li, M. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci. Rep. 2016, 6, 26667. [Google Scholar] [CrossRef]
- Thanighaiarassu, R.R.; Sivamai, P.; Devika, R.; Nambikkaira, B. Green synthesis of gold nanoparticles characterization by using plant essential oil Menthapiperita and their antifungal activity againsth pathogenic fungi. J. Nanomed. Nanotechnol. 2014, 5, 229. [Google Scholar]
- Eskandari-Nojedehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Hydrothermal green synthesis of gold nanoparticles using mushroom (Agaricus bisporus) extract: Physico-chemical characteristics and antifungal activity studies. Green Process. Synth. 2018, 7, 38–47. [Google Scholar] [CrossRef]
- Wang, M.; Meng, Y.; Zhu, H.; Hu, Y.; Xu, C.P.; Chao, X.; Li, W.; Pan, C.; Li, C. Green synthesized gold nanoparticles using Viola betonicifolia leaves extract: Characterization, antimicrobial, antioxidant, and cytobiocompatible activities. Int. J. Nanomed. 2021, 16, 7319–7337. [Google Scholar] [CrossRef]
- Lysenko, V.; Lozovski, V.; Lokshyn, M.; Gomeniuk, Y.V.; Dorovskih, A.; Rusinchuk, N.; Pankivska, Y.; Povnitsa, O.; Zagorodnya, S.; Tertykh, V.; et al. Nanoparticles as antiviral agents against adenoviruses. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025021. [Google Scholar] [CrossRef]
- Meléndez-Villanueva, M.A.; Morán-Santibañez, K.; Martínez-Sanmigue, J.J.; Rangel-López, R.; Garza-Navarro, M.A.; Rodríguez-Padilla, C.; Zarate-Triviño, D.G.; Trejo-Ávila, L.M. Virucidal Activity of Gold Nanoparticles Synthesized by Green Chemistry Using Garlic Extract. Viruses 2019, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, L.; Arunachalam, J. Green synthesis route for the size-controlled synthesis of biocompatible gold nanoparticles using aqueous extract of garlic (Allium sativum). Adv. Mater. Lett. 2013, 4, 548–555. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dhanasezhian, A.; Srivani, S.; Govindaraju, K.; Parija, P.; Sasikala, S.; Ramesh Kumar, M.R. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Mar. Sci. 2019, 48, 1252–1257. [Google Scholar]
- Rafiei, S.; Rezatofighi, S.E.; Ardakani, M.R.; Rastegarzadeh, S. Gold Nanoparticles Impair Foot-and-Mouth Disease Virus Replication. IEEE Trans. Nanobiosci. 2016, 15, 34–40. [Google Scholar] [CrossRef]
- Li, H.; Ma, X.; Dong, J.; Qian, W. Development of methodology based on the formation process of gold nanoshells for detecting hydrogen peroxide scavenging activity. Anal. Chem. 2009, 81, 8916–8922. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Pelle, F.D.; Vilela, D.; González, M.C.; Sterzo, C.L.; Compagnone, D.; Del Carlo, M.; Escarpa, A. Antioxidant capacity index based on gold nanoparticles formation. Application to extra virgin olive oil samples. Food Chem. 2015, 178, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, G.; Ramkumar, R.; Krishnaveni, N.; Pazhanimuthu, A.; Natarajan, T.; Sowmiya, R.; Perumal, P. Structural characterization, antioxidant and anticancer properties of gold nanoparticles synthesized rom leaf extract(decoction) of Antigonon leptopus Hook. & Arn. J. Trace Elem. Med. Biol. 2015, 30, 83–89. [Google Scholar]
- Tahir, K.; Nazir, S.; Li, B.; Khan, A.U.; Khan, Z.U.H.; Gong, P.Y.; Khan, S.U.; Ahmad, A. Nerium oleander leaves extract mediated synthesis of gold nano-particles and its antioxidant activity. Mater. Lett. 2015, 156, 198–201. [Google Scholar] [CrossRef]
- Desai, M.P.; Sangaokar, G.M.; Pawar, K.D. Kokum fruit mediated biogenic gold nanoparticles with photoluminescent, photocatalytic and antioxidant activities. Process Biochem. 2018, 70, 188–197. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Pradeep, K.J.; Vignesh, V.; Rajkuberan, C.; Jeyaraj, M.; Selvakumar, M.; Rakhi, J.; Sivaramakrishnan, S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. [Google Scholar]
- Basavegowda, N.; Idhayadhulla, A.; Lee, Y.R. Phyto-synthesis of gold nanoparticles using fruit extract of Hovenia dulcis and their biological activities. Ind. Crop. Prod. 2014, 52, 745–751. [Google Scholar] [CrossRef]
- Sutan, N.A.; Manolescu, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. Rhizomes alcoholic extracts. Mater. Sci. Eng. C 2018, 93, 746–758. [Google Scholar] [CrossRef]
- Ahn, E.Y.; Lee, Y.J.; Park, J.; Chun, P.; Park, Y. Antioxidant potential of Artemisia capillaris, Portulaca oleracea, and Prunella vulgaris extracts for biofabrication of gold nanoparticles and cytotoxicity assessment. Nanoscale Res. Lett. 2018, 13, 348. [Google Scholar] [CrossRef]
- Markus, J.; Wang, D.; Kim, Y.J.; Ahn, S.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis, characterization, and bioactivities evaluation of silver and gold nanoparticles mediated by the roots of Chinese herbal Angelica pubescens Maxim. Nanoscale Res. Lett. 2017, 12, 46. [Google Scholar] [CrossRef]
- Hamelian, M.; Varmira, K.; Veisi, H. Green synthesis and characterizations of gold nanoparticles using Thyme and survey cytotoxic effect, antibacterial and antioxidant potential. J. Photochem. Photobiol. B Biol. 2018, 184, 71–79. [Google Scholar] [CrossRef]
- Dos Santos Correa, A.; Contreras, L.A.; Keijok, W.J.; Barcelos, D.H.F.; Hertel Pereira, A.C.; Kitagawa, R.R.; Scherer, R.; de Oliveira Gomes, D.C.; da Silva, A.R.; Endringer, D.C.; et al. Virola oleifera-capped gold nanoparticles showing radical-scavenging activity and low cytotoxicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Nakkala, J.R.; Mata, R.; Sadras, S.R. The antioxidant and catalytic activities of green synthesized gold nanoparticles from Piper longum fruit extract. Process Saf. Environ. Prot. 2016, 100, 288–294. [Google Scholar] [CrossRef]
- Patil, M.P.; Kang, M.-J.; Niyonizigiye, I.; Singh, A.; Kim, J.-O.; Seo, Y.B.; Kim, G.-D. Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf. B Biointerfaces 2019, 183, 110455. [Google Scholar] [CrossRef] [PubMed]
- Stalin Dhas, T.; Ganesh Kumar, V.; Karthick, V.; Govindaraju, K.; Shankara Narayana, T. Biosynthesis of gold nanoparticles using Sargassum swartzii and its cytotoxicity effect on HeLa cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Rokhbakhsh-Zamin, F.; Shakibaie, M.; Moshafie, M.H.; Ameri, A.; Rahimi, H.R.; Forootanfar, H. Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal. Agric. Biotechnol. 2018, 15, 245–253. [Google Scholar] [CrossRef]
- Mmola, M.; Le Roes-Hill, M.; Durrell, K.; Bolton, J.J.; Sibuyi, N.; Meyer, E.M.; Beukes, D.R.; Antunes, E. Enhanced Antimicrobial and Anticancer Activity of Silver and Gold Nanoparticles Synthesised Using Sargassum incisifolium Aqueous Extracts. Molecules 2016, 21, 1633. [Google Scholar] [CrossRef] [PubMed]
- Ismail, E.H.; Saqer, A.M.A.; Assirey, E.; Naqvi, A.; Okasha, R.M. Successful green synthesis of gold nanoparticles using a Corchorus olitorius extract and their antiproliferative effect in Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2612. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Yan, Y.; Liu, H.; Karunakaran, T.; Li, F. Green synthesis of gold nanoparticles from Scutellaria barbata and its anticancer activity in pancreatic cancer cell (PANC-1). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Rajkuberan, C.; Susha, K.; Sathishkumar, G.; Sivaramakrishnan, S. Antibacterial and cytotoxic potential of silver nanoparticles synthesized using latex of Calotropis gigantea L. Spectrochim. Acta A 2015, 136, 926–930. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.-K.; Esfahani, S.H.Z.; Razmjou, A. Gold nanoparticles as potent anticancer agent: Green synthesis, characterization, and in vitro study. RSC Adv. 2016, 6, 63973–63983. [Google Scholar] [CrossRef]
- Alkilani, M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef]
- Jeyraj, M.; Arun, R.; Sathishkumar, G.; Mubarak Ali, D.; Rajesh, M.; Sivanandhan, G.; Kapildev, G.; Manickavasagam, M.; Thajuddin, N.; Ganapathi, A. An evidence on G2/M arrest, DNA damage and caspase mediated apoptotic effect of biosynthesized gold nanoparticles on human cervical carcinoma cells (HeLa). Mater. Res. Bull. 2014, 52, 15–24. [Google Scholar] [CrossRef]
- Patil, M.P.; Bayaraa, E.; Subedi, P.; Piad, L.L.A.; Tarte, N.H.; Kim, G.-D. Biogenic synthesis, characterization of gold nanoparticles using Lonicera japonica and their anticancer activity on HeLa cells. J. Drug Deliv. Sci. Technol. 2019, 51, 83–90. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Desikan Seshadri, V.D.; Cao, G. Synthesis and characterization of gold nanoparticles from Marsdenia tenacissima and its anticancer activity of liver cancer HepG2 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3029–3036. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.N.; Wu, L.N.; Xue, D. Marsdenia tenacissima extract induces apoptosis and suppresses autophagy through ERK activation in lung cancer cells. Cancer Cell Int. 2018, 18, 149. [Google Scholar] [CrossRef]
- Fan, W.; Sun, L.; Zhou, J.Q. Marsdenia tenacissima extract induces G0/G1 cell cycle arrest in human esophageal carcinoma cells by inhibiting mitogen-activated protein kinase (MAPK) signaling pathway. Chin. J. Nat. Med. 2015, 13, 428–437. [Google Scholar] [CrossRef]
- Liu, R.; Pei, Q.; Shou, T.; Zhang, W.; Hu, J.; Li, W. Apoptotic effect of green synthesized gold nanoparticles from Curcuma wenyujin extract against human renal cell carcinoma A498 cells. Int. J. Nanomed. 2019, 4, 4091–4103. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Al Aboody, M.S.; Alturaiki, W.; Alsagaby, S.A.; Alfaiz, F.A.; Veeraraghavan, V.P.; Mickymaray, S. Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Yun, Z.; Chinnathambi, A.; Alharbi, S.A.; Jin, Z. Biosynthesis of gold nanoparticles using Vetex negundo and evaluation of pro-apoptotic effect on human gastric cancer cell lines. J. Photochem. Photobiol. B Biol. 2020, 203, 111749. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, M.; Pavagadhi, S.; Mahadevan, A.; Balasubramanian, R. Biosynthesis of gold nanoparticles and related cytotoxicity evaluation using A549cells. Ecotoxicol. Environ. Saf. 2015, 114, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox. Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hartono, D.; Ong, C.N.; Bay, B.H.; Yung, L.Y. Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 2010, 23, 5996–6003. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, K.; Ji, L.; Tang, B. Effect of gold nanoparticles glutathione depletion-induced hydrogen peroxide generation and apoptosis in HL7702cells. Toxicol. Lett. 2011, 205, 86–95. [Google Scholar] [CrossRef]
- Rajeshkumar, S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J. Genet. Eng. Biotechnol. 2016, 14, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Jennifer, M.; Maciej, W. Nanoparticle technology as a double-edged sword: Cytotoxic, genotoxic and epigenetic effects on living cells. J. Biomater. Nanobiotechnol. 2013, 4, 53–63. [Google Scholar] [CrossRef]
- Ganeshkumar, M.; Sathishkumar, M.; Ponrasu, T.; Dineshc, G.M.; Suguna, L. Spontaneous ultra fast synthesis of gold nanoparticles using Punica granatum for cancer targeted drug delivery. Colloids Surf. B Biointerfaces 2013, 106, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.T.; Hunter, W.J.; Agrawal, D.K. Morfological and biochemical characterization and analysis of apoptosis. J. Pharmacol. Toxicol. Methods 1997, 37, 215–228. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppul, P.; Gpv, S.; Gurava Reddy, A.V.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C 2019, 99, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Toshiya, K.; Testuya, T.; Akira, H.; Takuji, T. Cancer chemoprevention though the induction of apoptosis by natural compounds. Biophys. Chem. 2012, 3, 156–173. [Google Scholar]
- Liu, X.; Kim, C.N.; Yang, J.; Jemmerson, R.; Wang, X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochome c. Cell 1996, 86, 147–157. [Google Scholar] [CrossRef]
- Toufektchan, E.; Toledo, F. The Guardian of the Genome Revisited: P53 Downregulates Genes Required for Telomere Maintenance, DNA Repair, and Centromere Structure. Cancers 2018, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Su, W.; Wang, Y.; Dang, M.; Zhang, W.; Wang, C. Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif. Cells Nanomed. Biotechnol. 2019, 47, 1173–1180. [Google Scholar] [CrossRef]
- Ramachandran, R.; Krishnaraj, C.; Sivakumar, A.S.; Prasannakumar, P.; Kumar, V.K.A.; Shim, K.S.; Song, C.-G.; Yu, S.-I. Anticancer activity of biologically synthesized silver and gold nanoparticles on mouse myoblast cancer cells and their toxicity against embryonic zebrafish. Mater. Sci. Eng. C 2017, 73, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.E.; Hendi, A.A. Gold nanoparticles induce apoptosis in MCF-human breast cancer cells. Asian Pac. J. Cancer Prev. 2012, 13, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Baharara, J.; Ramezani, T.; Divsalar, A.; Mousavi, M.; Seyedarabi, A. Induction of apoptosis by green synthesized gold nanoparticles through activation of caspase-3 and 9 in human cervical cancer cells. Avicenna J. Med. Biotechnol. 2016, 8, 75–83. [Google Scholar] [PubMed]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264. 7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Yuan, H.; Liu, W.; Li, S.; Liu, Y.; Wan, J.; Li, X.; Zhang, R.; Chang, Y. Activation of RAW264. 7 mouse macrophage cells in vitro through treatment with recombinant ricin toxin-binding subunit B: Involvement of protein tyrosine, NF-B and JAK-STAT kinase signaling pathways. Int. J. Mol. Med. 2013, 32, 729–735. [Google Scholar] [CrossRef]
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.-J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.-C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-B and AP-1 pathways. Colloids Surf. B Biointerfaces 2018, 162, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Yesmin Simu, S.; Kim, Y.; Mathiyalagan, R.; Yang, D. Gold nanoparticles synthesized using Panax ginseng leaves suppress inflammatory-mediators production via blockade of NF-kB activation in macrophages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Govindaraju, K.; Mohamed Sadiq, A.; Tamilselvan, S.; Ganesh Kumar, V.; Singaravelu, G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Saipriya, K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Anand, M.A.V.; David, E.; Venkatachalam, K.; Vijayakumar, S.; Sankaran, V.; Balupillai, A.; Sangeetha, C.C.; Gothandam, K.M.; Kotakadi, V.S.; et al. Antidiabetic activity of gold nanoparticles synthesized using wedelolactone in RIN-5F cell line. Antioxidants 2020, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vinayagam, R.; Anand, M.A.V.; Venkatachalam, K.; Saravanakumar, K.; Wang, M.-H.; Casimeer, C.S.; Gothandam, K.M.; David, E. Green synthesis of gold nanoparticle using Eclipta alba and its antidiabetic activities through regulation of Bcl-2 expression in pancreatic cell line. J. Drug Deliv. Sci. Technol. 2020, 58, 101786. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, N.; Zhang, L.; Yin, M. Green synthesis of gold nanoparticles from Fritillaria cirrhosa and its anti-diabetic activity on Streptozotocin induced rats. Arab. J. Chem. 2020, 13, 5096–5106. [Google Scholar] [CrossRef]
- Javanshir, R.; Honarmand, M.; Hosseini, M.; Hemmati, M. Anti-dyslipidemic properties of green gold nanoparticle: Improvement in oxidative antioxidative balance and associated atherogenicity and insulin resistance. Clin. Phytosci. 2020, 6, 74. [Google Scholar] [CrossRef]
- Yu, Y.; Gao, J.; Jiang, L.; Wang, J. Antidiabetic nephropathy effects of synthesized gold nanoparticles through mitigation of oxidative stress. Arab. J. Chem. 2021, 14, 103007. [Google Scholar] [CrossRef]
- Lallawmawma, H.; Sathishkumar, G.; Sarathbabu, S.; Ghatak, S.; Sivaramakrishnan, S.; Gurusubramanian, G.; Kumar, N.S. Synthesis of silver and gold nanoparticles using Jasminum nervosum leaf extract and its larvicidal activity against filarial and arboviral vector Culex quinquefasciatus Say (Diptera: Culicidae). Environ. Sci. Pollut. Res. 2015, 22, 17753–17768. [Google Scholar] [CrossRef] [PubMed]
- Muruga, K.; Benelli, G.; Panneerselvam, C.; Subramaniam, J.; Jeyalalitha, T.; Dinesh, D.; Nicoletti, M.; Hwang, J.-S.; Suresh, U.; Madhiyazhagan, P. Cymbopogon citratus-synthesized gold nanoparticles boost the predation efficiency of copepod Mesocyclops aspericornis against malaria and dengue mosquitoes. Exp. Parasitol. 2015, 153, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mater. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. (R Coll. Radiol.) 2007, 19, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1609–1617. [Google Scholar] [CrossRef]
- Kennedy, L.C.; Bickford, L.R.; Lewinski, N.A.; Coughlin, A.J.; Hu, Y.; Day, E.S. A new era for cancer treatment: Gold-nanoparticle-mediated thermal therapies. Small 2011, 7, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Liu, Y.; Crawford, B.M.; Vo-Dinh, T. Gold nanoparticles-mediated photothermal therapy and immunotherapy. Immunotherapy 2018, 10, 1175–1188. [Google Scholar] [CrossRef]
- Rezaeian, A.; Amini, S.M.; Najafabadi, M.R.H.; Farsangi, Z.J.; Samadian, H. Plasmonic hyperthermia or radiofrequency electric field hyperthermia of cancerous cells through green-synthesized curcumin-coated gold nanoparticles. Lasers Med. Sci. 2021. [Google Scholar] [CrossRef]
- Tiwari, P.M.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Kah, J.C.; Wong, K.Y.; Neoh, K.G.; Song, J.H.; Fu, J.W.; Mhaisalkar, S. Critical parameters in the pegylation of gold nanoshells for biomedical applications: An in vitro macrophage study. J. Drug Target. 2009, 17, 181–193. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green synthesis and characterization of monodispersed gold nanoparticles: Toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Blessy, V.; Karthikeyan, M. Comparative study on doxorubicin loaded metallic nanoparticles in drug delivery against MCF-7 cell line, applications of nanomaterials. In Applications of Nanomaterials; Bhagyaraj, S.M., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: New Delhi, India; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 303–313. [Google Scholar]
- Ganeshkumar, M.; Ponrasu, T.; Raja, M.D.; Subamekala, M.K.; Suguna, L. Green synthesis of pullulan stabilized gold nanoparticles for cancer targeted drug delivery. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole derivative-capped gold nanoparticles as an effective bactericide in vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Munir, S.; Ahmed, S.; Ibrahim, M.; Khalid, M.; Ojha, S.C. A Spellbinding interplay between biological barcoding and nanotechnology. Front. Bioeng. Biotechnol. 2020, 8, 883. [Google Scholar] [CrossRef]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef]
- Springer, T.; Ermini, M.L.; Spacková, B.; Jablonku, J.; Homola, J. Enhancing sensitivity of surface plasmon resonance biosensors by functionalized gold nanoparticles: Size matters. Anal. Chem. 2014, 86, 10350–10356. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Lee, J.; Wark, A.W.; Lee, H.J. Nanoparticle-enhanced surface plasmon resonance detection of proteins at attomolar concentrations: Comparing different nano ППP-детекция межмoлекулярных взaимoдействий 419 particle shapes and sizes. Anal. Chem. 2012, 84, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Sim, S.J. Signal en-hancement of surface plasmon reso nance immunoassay using enzyme precipitation-functionalized gold nanoparticles: A femto molar level measurement of anti-glutamic acid decarboxylase antibody. Biosens. Bioelectron. 2007, 22, 1874–1880. [Google Scholar] [CrossRef]
- Abhijith, K.S.; Thakur, M.S. Application of green synthesis of gold nanoparticles for sensitive detection of aflatoxin B1 based on metal enhanced fluorescence. Anal. Methods 2012, 4, 4250–4256. [Google Scholar] [CrossRef]
- Draz, M.S.; Shafiee, H. Applications of gold nanoparticles in virus detection. Theranostics 2018, 15, 1985–2017. [Google Scholar] [CrossRef]
- Shawky, S.M.; Bald, D.; Azzazy, H.M.E. Direct detection of unamplified hepatitis C virus RNA using unmodified gold nanoparticles. Clin. Biochem. 2010, 43, 1163–1168. [Google Scholar] [CrossRef]
- Li, X.; Lu, D.; Sheng, Z.; Chen, K.; Guo, X.; Jin, M. A fast and sensitive immunoassay of avian influenza virus based on label-free quantum dot probe and lateral flow test strip. Talanta 2012, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Javier, D.J.; Castellanos-Gonzalez, A.; Weigum, S.E.; White, A.C.; Richards-Kortum, R. Oligonucleotide-gold nanoparticle networks for detection of Cryptosporidium parvum heat shock protein 70 mRNA. J. Clin. Microbiol. 2009, 47, 4060–4066. [Google Scholar] [CrossRef]
- Yang, M.; Kostov, Y.; Bruck, H.A.; Rasooly, A. Gold nanoparticle-based enhanced chemiluminescence immunosensor for detection of Staphylococcal Enterotoxin B (SEB) in food. Int. J. Food Microbiol. 2009, 133, 265–271. [Google Scholar] [CrossRef]
- Paradowska, E.; Studzinska, M.; Jabłonska, A.; Lozovski, V.; Rusinchuk, N.; Mukha, I.; Vitiuk, N.; Le’snikowski, Z.J. Antiviral effect of nonfunctionalized gold nanoparticles against herpes simplex virus type-1 (HSV-1) and possible contribution of near-field interaction mechanism. Molecules 2021, 26, 5960. [Google Scholar] [CrossRef]
- Abou El-Nour, K.M.M.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and applications of silver nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Itxtepan-Turren, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Sarkar, D.S. Silver nanoparticles with bronchodilators through nebulisation to treat COVID 19 patients. J. Curr. Med. Res. Opin. 2020, 3, 449–450. [Google Scholar] [CrossRef]
- Alhag, S.K.; Al-Mekhlafi, F.A.; Abutaha, N.; Galil, F.M.A.A.; Wadaan, M.A. Larvicidal potential of gold and silver nanoparticles synthesized using Acalypha fruticosa leaf extracts against Culex pipiens (Culicidae: Diptera). J. Asia-Pac. Entomol. 2021, 24, 184–189. [Google Scholar] [CrossRef]
- Ali, S.; Bacha, M.; Shah, M.R.; Shah, V.; Kubra, K.; Khan, A.; Ahmad, M.; Latif, A.; Ali, M. Green synthesis of silver and gold nanoparticles using Crataegus oxyacantha extract and their urease inhibitory activities. Biotechnol. Appl. Biochem. 2021, 68, 992–1002. [Google Scholar] [CrossRef]
- Li, H.X.; Rothberg, L. Colorimetric detection of DNA sequences based on electrostatic interactions with unmodified gold nanoparticles. Proc. Natl. Acad. Sci. USA 2004, 101, 14036–14039. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.C.; Jin, R.C.; Mirkin, C.A. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science 2002, 297, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Glynou, K.; Ioannou, P.C.; Christopoulos, T.K.; Syriopoulou, V. Oligonucleotide-functionalized gold nanoparticles as probes in a dry-reagent strip biosensor for DNA analysis by hybridization. Anal. Chem. 2003, 75, 4155–4160. [Google Scholar] [CrossRef] [PubMed]
- Aul, B.; Bhuyan, B.; Purkayastha, D.D.; Vadivel, S.; Dhar, S.S. One-pot green synthesis of gold nanoparticles and studies of their anticoagulative and photocatalytic activities. Mater. Lett. 2016, 185, 143–147. [Google Scholar]
- Tramontin, N.S.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold nanoparticles treatment reverses brain damage in Alzheimer’s disease model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Paula, M.M.; Petronilho, F.; Vuolo, F.; Ferreira, G.K.; De Costa, L.; Santos, G.P.; Effting, P.S.; Dal-Pizzol, F. Gold nanoparticles and/or N-acetylcysteine mediate carrageenan-induced inflammation and oxidative stress in a concentration-dependent manner. J. Biomed. Mater. Res. A 2015, 103, 3323–3330. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, F.; Zhou, Y.; Anderson, G.J.; Frazer, D.M.; Lim, Y.C. Dihydrolipoic acid-gold nanoclusters regulate microglial polarization and have the potential to alter neurogenesis. Nano Lett. 2020, 20, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-D.; Hu, Y.-J.; Yu, L.; Zhou, X.-G.; Wu, J.-M.; Tang, Y.; Qin, D.-L.; Fan, Q.-Z.; Wu, A.-G. Nanoparticles: A Hope for the Treatment of Inflammation in CNS. Front. Pharmacol. 2021, 12, 683935. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, M.; Gong, D.; Chen, R.; Hua, X.; Sun, T. The size-effect of gold nanoparticles and nanoclusters in the inhibition of amyloid-β fibrillation. Nanoscale 2017, 9, 4107. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.K.; Murmu, S.; Saha, S.; Tandon, V.; Acharya, K. Anthelmintic Efficacy of Gold Nanoparticles Derived from a Phytopathogenic Fungus, Nigrospora oryzae. PLoS ONE 2014, 9, e84693. [Google Scholar]
- Torabi, N.; Mohebali, M.; Shahverdi, A.R.; Rezayat, S.M.; Edrissian, G.H.; Esmaeili, J.; Charehdar, S. Nanogold for the treatment of zoonotic cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): An animal trial with methanol extract of Eucalyptus camaldulensis. J. Pharm. Sci. 2012, 1, 113–116. [Google Scholar]
- Karthik, L.; Kumar, G.; Keswani, T.; Bhattacharyya, A.; Reddy, B.P.; Rao, K.B. Marine actinobacterial mediated gold nanoparticles synthesis and their antimalarial activity. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Shah, S.; Muhammad, Z.; Shah, S.A.; Faisal, S.; Khattak, U.; ul Haq, T.; Taj Akbar, M. In vitro and in vivo applications of Euphorbia wallichii shoot extract-mediated gold nanospheres. Green Process Synth. 2021, 10, 101–111. [Google Scholar] [CrossRef]
- Zhang, J.; Mou, L.; Jiang, X. Surface chemistry of gold nanoparticles for health related applications. Chem. Sci. 2020, 11, 923. [Google Scholar] [CrossRef] [PubMed]
| Microorganism | Shape | Size, nm | References |
|---|---|---|---|
| Stenotrophomonas maltophilia | spherical | 40 | [41] |
| Rhodopseudomonas capsulata | spherical | 10–20 | [42] |
| Pseudomonas putida and Pseudomonas fluorescence | spherical | 10–50 | [43] |
| Deinococcus radiodurans | spherical, pseudo-spherical, truncated triangular and irregular | ~43.75 | [49] |
| Bacillus cereus | spherical, hexagonal, and octagonal with irregular contours | 40–50 | [92] |
| Marinobactor pelagius | varied | ~2–6 | [93] |
| Microorganism | Shape | Size, nm | References |
|---|---|---|---|
| Pycnoporus sanguineus | spherical, pseudo-spherical, triangular, truncated triangular, pentagonal, and hexagonal | several to several hundred | [94] |
| Magnusiomyces ingens | spherical, triangular, hexagonal | 10–80 | [95] |
| Thermoascus thermophilus | different | ~10 | [96] |
| Trichoderma hamatum | spherical, pentagonal and hexagonal | 5–30 | [97] |
| Aspergillus foetidus | spherical | 30–50 | [98] |
| Rhizopus oryzae | spherical | 5–65 | [99] |
| Organism | Shape | Size, nm | References |
|---|---|---|---|
| Stoechospermum marginatum | spherical, hexagonal and triangle | 18–93 | [98] |
| Laminaria japonica | spherical | 15–20 | [99] |
| Ulva fasciata | spherical | ~10 | [100] |
| Chlorella vulgaris | spherical | 2–10 | [101] |
| Prasiola crispa (green algae) | spherical | 5–25 | [102] |
| Galaxaura elongata | rod, triangular, truncated triangular and hexagonal | 3–77 | [103] |
| Turbinaria conoides (brown algae) | small spherical, triangle and pseudo-spherical | 6–10 | [105] |
| Sargassum polycystum (brown algae) | spherical | 68–240 | [106] |
| Plant | Shape | Size, nm | References |
|---|---|---|---|
| Gymnocladus assamicus (pod extract) | hexagonal, pentagonal, and triangular | 4.5–22.5 | [71] |
| Areca catechu nut | spherical | ~13.7 | [72] |
| Croton Caudatus Geisel leaf extract | spherical | 20–50 | [73] |
| Petroselinum crispum (leaf extract) | spherical, semi-rod, flower shaped | 17–50 | [74] |
| Salix alba L. leaves extract | - | 50–80 | [75] |
| Sesbania grandiflora leaf extract | spherical | 7–34 | [78] |
| Mimosa tenuiflora bark extract | multiple | 20–200 | [79] |
| Terminalia chebula seed powder | pentagonal, triangular, spherical | 6–60 | [80] |
| Jasminum auriculatum leaf extract | spherical | 8–37 | [81] |
| Solanum nigrum leaf extract | spherical | ~50 | [82] |
| Citrullus lanatus rind extract | spherical | 20–140 | [87] |
| Mango peel extract | spherical | 6–18 | [104] |
| Mentha piperita leaf extract | hexagonal | ~78 | [105] |
| Coleus aromaticus leaf extract | spherical, rod, and triangular | ~80 | [106] |
| Anogeissus latifolia leaf extract | spherical | 50–60 | [107] |
| Papaver somniferum seed pulp extract | spherical | ~77 | [108] |
| Aloysia triphylla leaf extract | spherical | 40–60 | [109] |
| Trigonella foenum-graecum seed extract | - | 15–20 | [110] |
| Punica Granatum fruit extract | triangular and spherical | 5–20 | [111] |
| Eucommia ulmoides bark aqueous extract | spherical | ~18.2 | [112] |
| Capsicum annuum var. grossum pulp extract | triangle, hexagonal, and quasi-spherical | 6–37 | [113] |
| Plumeria alba flower extract | spherical | 15–28 | [114] |
| Platycodon grandiflorum leaf extract | spherical | ~15 | [115] |
| Siberian ginseng | spherical | ~200 | [116] |
| Marsdenia tenacissima | spherical | ~50 | [117] |
| Peganum harmala seed extract | spherical | 43–52 | [118] |
| Garcinia mangostana fruit peel extract | spherical | ~32 | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. https://doi.org/10.3390/jfb12040070
Mikhailova EO. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. Journal of Functional Biomaterials. 2021; 12(4):70. https://doi.org/10.3390/jfb12040070
Chicago/Turabian StyleMikhailova, Ekaterina O. 2021. "Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application" Journal of Functional Biomaterials 12, no. 4: 70. https://doi.org/10.3390/jfb12040070
APA StyleMikhailova, E. O. (2021). Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. Journal of Functional Biomaterials, 12(4), 70. https://doi.org/10.3390/jfb12040070





