Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies
Abstract
1. Introduction
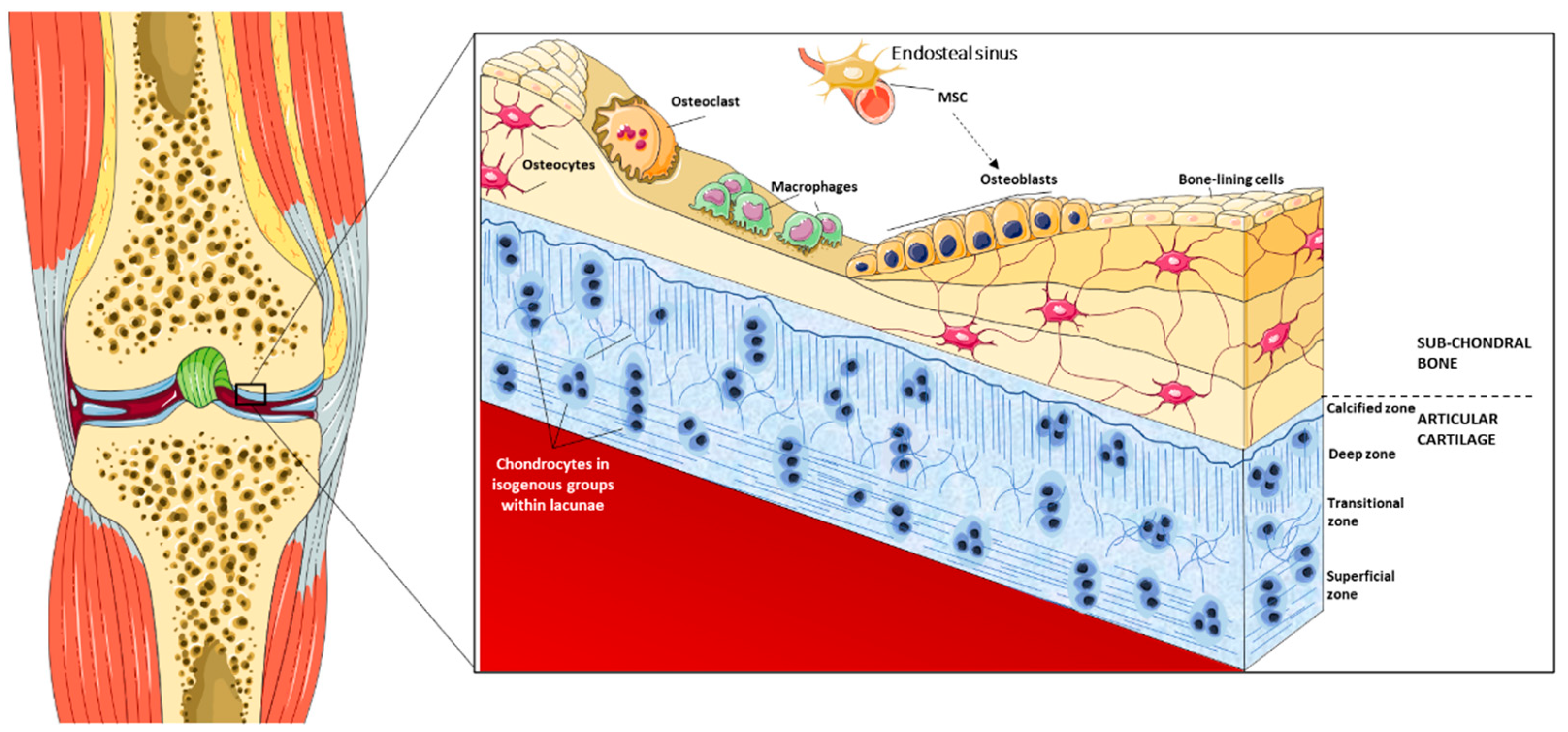
2. Overview of the Joint Surface Structure
2.1. Cartilage Structure and Functional Properties
2.2. Bone Structure and Functional Properties
2.3. Osteochondral Developmental Insights
2.4. Key Molecular Signaling in Osteochondral Development and Growth
3. Tissue Engineering for Osteochondral Regeneration
3.1. Biomaterials
3.2. Cell Types for Osteochondral Regeneration
3.3. Additive Manufacturing
4. Strategies for Osteochondral Regeneration
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brody, L.T. Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Phys. Ther. Sport 2015, 16, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- O’Neill, T.W.; McCabe, P.S.; McBeth, J. Update on the epidemiology, risk factors and disease outcomes of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 312–326. [Google Scholar] [CrossRef]
- Toh, W.S.; Lee, E.H.; Cao, T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. Rep. 2011, 7, 544–559. [Google Scholar] [CrossRef]
- Bryant, S.J.; Davis-Arehart, K.A.; Luo, N.; Shoemaker, R.K.; Arthur, J.A.; Anseth, K.S. Synthesis and characterization of photopolymerized multifunctional hydrogels: Water-soluble poly (vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules 2004, 37, 6726–6733. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Thomas, B.H.; Fryman, J.C.; Liu, K.; Mason, J. Hydrophilic–hydrophobic hydrogels for cartilage replacement. J. Mech. Behav. Biomed. Mater. 2009, 2, 588–595. [Google Scholar] [CrossRef]
- Janjanin, S.; Li, W.-J.; Morgan, M.T.; Shanti, R.M.; Tuan, R.S. Mold-shaped, nanofiber scaffold-based cartilage engineering using human mesenchymal stem cells and bioreactor. J. Surg. Res. 2008, 149, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Brophy, R.H. Decision making in cartilage repair procedures. In Cartilage Repair Strategies; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 37–53. [Google Scholar]
- Castro, N.J.; Hacking, S.A.; Zhang, L.G. Recent progress in interfacial tissue engineering approaches for osteochondral defects. Ann. Biomed. Eng. 2012, 40, 1628–1640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Schaefer, D.; Martin, I.; Shastri, P.; Padera, R.F.; Langer, R.; Freed, L.E.; Vunjak-Novakovic, G. In vitro generation of osteochondral composites. Biomaterials 2000, 21, 2599–2606. [Google Scholar] [CrossRef]
- Sargeant, T.D.; Desai, A.P.; Banerjee, S.; Agawu, A.; Stopek, J.B. An in situ forming collagen–PEG hydrogel for tissue regeneration. Acta Biomater. 2012, 8, 124–132. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Olsen, B.D.; Khademhosseini, A. The mechanical properties and cytotoxicity of cell-laden double-network hydrogels based on photocrosslinkable gelatin and gellan gum biomacromolecules. Biomaterials 2012, 33, 3143–3152. [Google Scholar] [CrossRef]
- Jeffrey, D.R.; Watt, I. Imaging hyaline cartilage. Br. J. Radiol. 2003, 76, 777–787. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Mizuno, M.; Kim, G.; Takagi, S.; Okumura, M.; Fujinaga, T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol. Bioeng. 2006, 93, 1152–1163. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Santos, T.C.; Martins, L.; Picciochi, R.; Marques, A.P.; Castro, A.G.; Neves, N.M.; Mano, J.F.; Reis, R.L. Gellan gum injectable hydrogels for cartilage tissue engineering applications: In vitro studies and preliminary in vivo evaluation. Tissue Eng. Part A 2009, 16, 343–353. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.G.; Peterson, L. Autologous chondrocyte implantation. In Cartilage Repair Strategies; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 137–165. [Google Scholar]
- Schrobback, K.; Klein, T.J.; Crawford, R.; Upton, Z.; Malda, J.; Leavesley, D.I. Effects of oxygen and culture system on in vitro propagation and redifferentiation of osteoarthritic human articular chondrocytes. Cell Tissue Res. 2012, 347, 649–663. [Google Scholar] [CrossRef]
- Baker, M.I.; Walsh, S.P.; Schwartz, Z.; Boyan, B.D. A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1451–1457. [Google Scholar] [CrossRef]
- Chang, C.-H.; Liu, H.-C.; Lin, C.-C.; Chou, C.-H.; Lin, F.-H. Gelatin–chondroitin–hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Biomaterials 2003, 24, 4853–4858. [Google Scholar] [CrossRef]
- Natoli, R.M.; Revell, C.M.; Athanasiou, K.A. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng. Part A 2009, 15, 3119–3128. [Google Scholar] [CrossRef]
- Vunjak-Novakovic, G.; Radisic, M. Cell seeding of polymer scaffolds. In Biopolymer Methods in Tissue Engineering; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 131–145. [Google Scholar]
- Lam, J.; Lee, E.J.; Clark, E.C.; Mikos, A.G. Honing Cell and Tissue Culture Conditions for Bone and Cartilage Tissue Engineering. Cold Spring Harb. Perspect. Med. 2017, 7, a025734. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ozbolat, I.T. Bioprinting of osteochondral tissues: A perspective on current gaps and future trends. Int. J. Bioprinting 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Lattanzi, W.; Parolisi, R.; Barba, M.; Bonfanti, L. Osteogenic and Neurogenic Stem Cells in Their Own Place: Unraveling Differences and Similarities between Niches. Front. Cell. Neurosci. 2015, 9, 455. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef]
- Tzaphlidou, M. Bone architecture: Collagen structure and calcium/phosphorus maps. J. Biol. Phys. 2008, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H. Osteoblasts, osteoclasts, and osteocytes: Unveiling their intimate-associated responses to applied orthodontic force. Semin. Orthod. 2012, 18, 237–248. [Google Scholar] [CrossRef]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast differentiation and bone matrix formation in vivo and in vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Osteoclasts: What do they do and how do they do it? Am. J. Pathol. 2007, 170, 427–435. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef] [PubMed]
- Alford, A.I.; Kozloff, K.M.; Hankenson, K.D. Extracellular matrix networks in bone remodeling. Int. J. Biochem. Cell Biol. 2015, 65, 20–31. [Google Scholar] [CrossRef]
- Hollander, A.P.; Dickinson, S.C.; Kafienah, W. Stem cells and cartilage development: Complexities of a simple tissue. Stem Cells 2010, 28, 1992–1996. [Google Scholar] [CrossRef]
- Akiyama, H.; Chaboissier, M.-C.; Martin, J.F.; Schedl, A.; de Crombrugghe, B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002, 16, 2813–2828. [Google Scholar] [CrossRef]
- Nishimura, R.; Hata, K.; Takahata, Y.; Murakami, T.; Nakamura, E.; Yagi, H. Regulation of cartilage development and diseases by transcription factors. J. Bone Metab. 2017, 24, 147–153. [Google Scholar] [CrossRef]
- Komori, T. Regulation of osteoblast differentiation by Runx2. In Osteoimmunology; Springer: Boston, MA, USA, 2009; pp. 43–49. [Google Scholar]
- Lattanzi, W.; Bernardini, C. Genes and molecular pathways of the osteogenic process. In Osteogenesis; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2012, 19, 254–263. [Google Scholar] [CrossRef]
- Omatsu, Y.; Nagasawa, T. The critical and specific transcriptional regulator of the microenvironmental niche for hematopoietic stem and progenitor cells. Curr. Opin. Hematol. 2015, 22, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Q.; Luo, S.; Liu, Z.; Luo, D.; Zhang, B.; Zhang, D.; Rao, P.; Xiao, J. PPARγ and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2016, 11, 216–225. [Google Scholar] [CrossRef]
- Levato, R.; Webb, W.R.; Otto, I.A.; Mensinga, A.; Zhang, Y.; van Rijen, M.; van Weeren, R.; Khan, I.M.; Malda, J. The bio in the ink: Cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater. 2017, 61, 41–53. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2017. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef] [PubMed]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, D.; Wang, K.; Niu, Y. Semi-degradable porous poly (vinyl alcohol) hydrogel scaffold for cartilage repair: Evaluation of the initial and cell-cultured tribological properties. J. Mech. Behav. Biomed. Mater. 2017, 68, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J. Orthop. Transl. 2019, 17, 15–25. [Google Scholar] [CrossRef]
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 1999, 354, S32–S34. [Google Scholar] [CrossRef]
- Deng, C.; Chang, J.; Wu, C.T. Trace element-based biomaterials for osteochondral regeneration. In Bioactive Materials for Bone Regeneration; Chang, J., Zhang, X., Dai, K., Eds.; Academic Press: London, UK, 2020. [Google Scholar]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Jung, Y. Bi-layered PLCL/(PLGA/β-TCP) composite scaffold for osteochondral tissue engineering. J. Bioact. Compat. Polym. 2015, 30, 178–187. [Google Scholar] [CrossRef]
- Abdulghani, S.; Morouço, P.G. Biofabrication for osteochondral tissue regeneration: Bioink printability requirements. J. Mater. Sci. Mater. Med. 2019, 30, 20. [Google Scholar] [CrossRef]
- Gomoll, A.H.; Madry, H.; Knutsen, G.; van Dijk, N.; Seil, R.; Brittberg, M.; Kon, E. The subchondral bone in articular cartilage repair: Current problems in the surgical management. Knee Surgery Sport. Traumatol. Arthrosc. 2010, 18, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Orth, P.; Cucchiarini, M. Role of the Subchondral Bone in Articular Cartilage Degeneration and Repair. J. Am. Acad. Orthop. Surg. 2016, 24, e45–e46. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Feng, K.; Liu, X.; Ma, P.X. Chondrogenic and osteogenic differentiations of human bone marrow-derived mesenchymal stem cells on a nanofibrous scaffold with designed pore network. Biomaterials 2009, 30, 5061–5067. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of Pore Size Effect on Chondrogenic Differentiation of Adipose Stem Cells Using a Pore Size Gradient Scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Discher, D.E. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Huebsch, N.; Arany, P.R.; Mao, A.S.; Shvartsman, D.; Ali, O.A.; Bencherif, S.A.; Rivera-Feliciano, J.; Mooney, D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010, 9, 518–526. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Stiffness Gradients Mimicking In Vivo Tissue Variation Regulate Mesenchymal Stem Cell Fate. PLoS ONE 2011, 6, e15978. [Google Scholar] [CrossRef]
- Longley, R.; Ferreira, A.M.; Gentile, P. Recent approaches to the manufacturing of biomimetic multi-phasic scaffolds for osteochondral regeneration. Int. J. Mol. Sci. 2018, 19, 1755. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, A.; Van Blitterswijk, C.; Moroni, L. The osteochondral interface as a gradient tissue: From development to the fabrication of gradient scaffolds for regenerative medicine. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 34–52. [Google Scholar] [CrossRef]
- Frassica, M.T.; Grunlan, M.A. Perspectives on Synthetic Materials to Guide Tissue Regeneration for Osteochondral Defect Repair. ACS Biomater. Sci. Eng. 2020, 6, 4324–4336. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- McGlohorn, J.B.; Holder, W.D.; Grimes, L.W.; Thomas, C.B.; Burg, K.J.L. Evaluation of Smooth Muscle Cell Response Using Two Types of Porous Polylactide Scaffolds with Differing Pore Topography. Tissue Eng. 2004, 10, 505–514. [Google Scholar] [CrossRef]
- Woodfield, T.; Moroni, L.; Malda, J. Combinatorial Approaches to Controlling Cell Behaviour and Tissue Formation in 3D via Rapid-Prototyping and Smart Scaffold Design. Comb. Chem. High Throughput Screen 2009, 12, 562–579. [Google Scholar] [CrossRef]
- Khetan, S.; Burdick, J.A. Patterning hydrogels in three dimensions towards controlling cellular interactions. Soft Matter. 2011, 7, 830–838. [Google Scholar] [CrossRef]
- Nichol, J.W.; Khademhosseini, A. Modular tissue engineering: Engineering biological tissues from the bottom up. Soft Matter. 2009, 5, 1312. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Narayan, R.J. Gradient scaffolds for osteochondral tissue engineering and regeneration. J. Mater. Chem. B 2020, 8, 8149–8170. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Abarrategi, A.; Lópiz-Morales, Y.; Ramos, V.; Civantos, A.; López-Durán, L.; Marco, F.; López-Lacomba, J.L. Chitosan scaffolds for osteochondral tissue regeneration. J. Biomed. Mater. Res. Part A 2010, 95, 1132–1141. [Google Scholar] [CrossRef]
- Liao, C.-T.; Ho, M.-H. The Fabrication of Biomimetic Chitosan Scaffolds by Using SBF Treatment with Different Crosslinking Agents. Membranes 2010, 1, 3–12. [Google Scholar] [CrossRef]
- Maia, F.R.; Carvalho, M.R.; Oliveira, J.M.; Reis, R.L. Tissue Engineering Strategies for Osteochondral Repair. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; pp. 353–371. [Google Scholar]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, J.; Jadhav, S.; Bodas, D.; Barhanpurkar-Naik, A.; Wani, M.; Paknikar, K.; Rajwade, J. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomedicine 2017, 12, 6437–6459. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Salvatore, L.; Veschini, L.; Serra, M.P.; Hobbs, C.; Madaghiele, M.; Sannino, A.; Di Silvio, L. Biomimetic gradient scaffold of collagen–hydroxyapatite for osteochondral regeneration. J. Tissue Eng. 2020, 11, 204173141989606. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [PubMed]
- Erickson, A.E.; Sun, J.; Lan Levengood, S.K.; Swanson, S.; Chang, F.-C.; Tsao, C.T.; Zhang, M. Chitosan-based composite bilayer scaffold as an in vitro osteochondral defect regeneration model. Biomed. Microdevices 2019, 21, 34. [Google Scholar] [CrossRef]
- Filardo, G.; Perdisa, F.; Gelinsky, M.; Despang, F.; Fini, M.; Marcacci, M.; Parrilli, A.P.; Roffi, A.; Salamanna, F.; Sartori, M.; et al. Novel alginate biphasic scaffold for osteochondral regeneration: An in vivo evaluation in rabbit and sheep models. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Pina, S.; Costa, J.B.; Cengiz, I.F.; García-Fernández, L.; Fernández-Gutiérrez, M.D.M.; Paiva, O.C.; Oliveira, A.L.; San-Román, J.; Oliveira, J.M.; et al. Enzymatically Cross-Linked Silk Fibroin-Based Hierarchical Scaffolds for Osteochondral Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 3781–3799. [Google Scholar] [CrossRef]
- Larson, B.L.; Yu, S.N.; Park, H.; Estes, B.T.; Moutos, F.T.; Bloomquist, C.J.; Wu, P.B.; Welter, J.F.; Langer, R.; Guilak, F.; et al. Chondrogenic, hypertrophic, and osteochondral differentiation of human mesenchymal stem cells on three-dimensionally woven scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, P.; Gao, J.; Guo, R.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp Composite Scaffold for Osteochondral Tissue Engineering and Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Cai, B.; Wang, L.; Cai, L.; Wang, Z.; Xie, J.; Lv, Q.; Yuan, Y.; Liu, C.; Shen, S.G. A viscoelastic PEGylated poly(glycerol sebacate)-based bilayer scaffold for cartilage regeneration in full-thickness osteochondral defect. Biomaterials 2020, 253, 120095. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, R.; Pruncu, C.I.; Yadav, A. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 2020, 195, 109026. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Fernandes, C.; Moura, C.; Ascenso, R.M.T.; Amado, S.; Alves, N.; Pascoal-Faria, P. Comprehensive Review on Full Bone Regeneration through 3D Printing Approaches. In Design and Mnufacturing; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Baker, B.M.; Shah, R.P.; Silverstein, A.M.; Esterhai, J.L.; Burdick, J.A.; Mauck, R.L. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14176–14181. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Lee, S.J.; Seok, J.M.; Lee, J.H.; Kim, W.D.; Kwon, I.K. Fabrication of 3D Printed PCL/PEG Polyblend Scaffold Using Rapid Prototyping System for Bone Tissue Engineering Application. J. Bionic Eng. 2018, 15, 435–442. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, J.; Narayan, R. Nanostructured biomaterials for regenerative medicine: Clinical perspectives. In Nanostructured Biomaterials for Regenerative Medicine; Elsevier: London, UK, 2020; pp. 47–80. [Google Scholar]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Gold and nano-gold in medicine: Overview, toxicology and perspectives. J. Appl. Biomed. 2009, 7, 75–91. [Google Scholar] [CrossRef]
- El-Sayed, M.A. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Lee, S.B.; Cho, S.G. The impact of metallic nanoparticles on stem cell proliferation and differentiation. Nanomaterials 2018, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Armentano, I.; Dottori, M.; Fortunati, E.; Mattioli, S.; Kenny, J.M. Biodegradable polymer matrix nanocomposites for tissue engineering: A review. Polym. Degrad. Stab. 2010, 95, 2126–2146. [Google Scholar] [CrossRef]
- Augustine, R.; Dominic, E.A.; Reju, I.; Kaimal, B.; Kalarikkal, N.; Thomas, S. Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Adv. 2014, 4, 51528–51536. [Google Scholar] [CrossRef]
- Buzarovska, A.; Gualandi, C.; Parrilli, A.; Scandola, M. Effect of TiO2 nanoparticle loading on Poly(l-lactic acid) porous scaffolds fabricated by TIPS. Compos. Part B Eng. 2015, 81, 189–195. [Google Scholar] [CrossRef]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Future Med. 2007, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.K.; Heo, D.N.; Moon, H.J.; Lee, S.J.; Bae, M.S.; Lee, J.B.; Sun, I.C.; Jeon, H.B.; Park, H.K.; Kwon, I.K. The effect of gold nanoparticle size on osteogenic differentiation of adipose-derived stem cells. J. Colloid Interface Sci. 2015, 438, 68–76. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, P.; Lui, V.C.H.; Chen, Y.; Liu, X.; Lok, C.N.; To, M.; Yeung, K.W.K.; Wong, K.K.Y. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1949–1959. [Google Scholar] [CrossRef]
- Bianco, P.; Cao, X.; Frenette, P.S.; Mao, J.J.; Robey, P.G.; Simmons, P.J.; Wang, C.-Y. The meaning, the sense and the significance: Translating the science of mesenchymal stem cells into medicine. Nat. Med. 2013, 19, 35. [Google Scholar] [CrossRef]
- Dupont, K.M.; Sharma, K.; Stevens, H.Y.; Boerckel, J.D.; García, A.J.; Guldberg, R.E. Human stem cell delivery for treatment of large segmental bone defects. Proc. Natl. Acad. Sci. USA 2010, 107, 3305–3310. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Eyre, D. Articular cartilage and changes in arthritis: Collagen of articular cartilage. Arthritis Res. Ther. 2001, 4, 30. [Google Scholar] [CrossRef][Green Version]
- Kalamegam, G.; Memic, A.; Budd, E.; Abbas, M.; Mobasheri, A. A comprehensive review of stem cells for cartilage regeneration in osteoarthritis. Cell Biol. Transl. Med. 2018, 2, 23–36. [Google Scholar]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Kristjánsson, B.; Honsawek, S. Mesenchymal stem cells for cartilage regeneration in osteoarthritis. World J. Orthop. 2017, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Z.; Xie, H.-Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.-C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: Current status and future perspectives. Stem Cell Rev. Rep. 2017, 13, 575–586. [Google Scholar] [CrossRef]
- Mizuno, M.; Katano, H.; Mabuchi, Y.; Ogata, Y.; Ichinose, S.; Fujii, S.; Otabe, K.; Komori, K.; Ozeki, N.; Koga, H. Specific markers and properties of synovial mesenchymal stem cells in the surface, stromal, and perivascular regions. Stem Cell Res. Ther. 2018, 9, 123. [Google Scholar] [CrossRef]
- Correa, D.; Lietman, S.A. Articular cartilage repair: Current needs, methods and research directions. In Seminars in Cell & Developmental Biology; Elsevier: London, UK, 2017; pp. 67–77. [Google Scholar]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Van Buul, G.M.; Villafuertes, E.; Bos, P.K.; Waarsing, J.H.; Kops, N.; Narcisi, R.; Weinans, H.; Verhaar, J.A.N.; Bernsen, M.R.; Van Osch, G. Mesenchymal stem cells secrete factors that inhibit inflammatory processes in short-term osteoarthritic synovium and cartilage explant culture. Osteoarthr. Cartil. 2012, 20, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Magatti, M.; Vertua, E.; Cargnoni, A.; Silini, A.; Parolini, O. The immunomodulatory properties of amniotic cells: The two sides of the coin. Cell Transplant 2018, 27, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Silini, A.R.; Magatti, M.; Cargnoni, A.; Parolini, O. Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transpl. 2017, 26, 531–539. [Google Scholar] [CrossRef]
- Tschon, M.; Brogini, S.; Parrilli, A.; Bertoldi, S.; Silini, A.; Parolini, O.; Faré, S.; Martini, L.; Veronesi, F.; Fini, M. Assessment of the in vivo biofunctionality of a biomimetic hybrid scaffold for osteochondral tissue regeneration. Biotechnol. Bioeng. 2021, 118, 465–480. [Google Scholar] [CrossRef]
- Yuan, L.; Ding, S.; Wen, C. Additive manufacturing technology for porous metal implant applications and triple minimal surface structures: A review. Bioact. Mater. 2019, 4, 56–70. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D Printing of Scaffolds and Tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Zhou, X.; Nowicki, M.; Cui, H.; Zhu, W.; Fang, X.; Miao, S.; Lee, S.; Keidar, M.; Grace, L. 3D bioprinted graphene oxide-incorporated matrix for promoting chondrogenic differentiation of human bone marrow mesenchymal stem cells. Carbon N. Y. 2017, 116, 615–624. [Google Scholar] [CrossRef]
- Graham, A.D.; Olof, S.N.; Burke, M.J.; Armstrong, J.P.K.; Mikhailova, E.A.; Nicholson, J.G.; Box, S.J.; Szele, F.G.; Perriman, A.W.; Bayley, H. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 2017, 7, 1–11. [Google Scholar]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; Van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J.A. Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Eng. Part C Methods 2012, 18, 33–44. [Google Scholar] [CrossRef]
- Kumar, S.; Choudhary, A.K.S.; Singh, A.K.; Gupta, A.K. A Comparison of Additive Manufacturing Technologies. Int. J. Innov. Res. Sci. Technol. 2016, 3, 147–152. [Google Scholar]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Daly, A.C.; Freeman, F.E.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, O.; Darwish, S.M. Fabrication of Tissue Engineering Scaffolds Using Rapid Prototyping Techniques. Int. J. Mech. Aerospace Ind. Mechatron. Manuf. Eng. 2011, 5, 2317–2325. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Cai, B.; Wang, G.; Fan, H.; Zhang, X. Preparation of porous PLGA/Ti biphasic scaffold and osteochondral defect repair. Biomater. Sci. 2013, 1, 703. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic Bone Scaffold Design Parameters: Osteogenic Differentiation and Signal Expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Marrella, A.; Lee, T.Y.; Lee, D.H.; Karuthedom, S.; Syla, D.; Chawla, A.; Khademhosseini, A.; Jang, H.L. Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration. Mater. Today 2018, 21, 362–376. [Google Scholar] [CrossRef]
- O’Reilly, A.; Kelly, D.J. A Computational Model of Osteochondral Defect Repair Following Implantation of Stem Cell-Laden Multiphase Scaffolds. Tissue Eng. Part A 2017, 23, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Zhu, W.; Sarkar, K.; Rao, R.; Zhang, L.G. 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting 2020, 17, e00066. [Google Scholar] [CrossRef]
| Biomaterial | In Vitro Studies | In Vivo Studies | Main Results | Ref. |
|---|---|---|---|---|
| Bacterial cellulose (BC-HA and BC-GAG) | Support attachment and in vitro proliferation of osteoblasts and hACs | Tissue ingrowth and induced no inflammation or immunological reactions after subcutaneous implantation in rats | BC could be modified to mimic two structurally different tissues, and BC exhibited highly desirable biodegradative resorption capability; data presented warrant further extensive and long-term evaluation of BC nanocomposite scaffolds in other animals as well as humans for the eventual translation into clinical practice. | [81] |
| Collagen matrix with a gradient distribution of low-crystalline HA particles | Ability of cells to be directed by the chondrogenic or osteogenic environment created by the gradient material composition and stiffness in the different regions of the gradient scaffold | Biocompatibility of the gradient scaffold was confirmed by its subcutaneous implantation in rats minimal inflammatory response observed and evidence of cellular differentiation; Scaffold has the potential to selectively differentiate recruited cells | Successful fabrication of a novel composite gradient scaffold with appropriate biomimetic physicochemical and biological properties, for potential application in osteochondral regeneration; Good biocompatibility; | [82] |
| Poli(N-acriloil 2-glycine) (PACG) and methacrylate gelatin (GelMA) (PACG-GelMA) | High compressive strength (up to 12.4 MPa), and compressive modulus (up to 837 kPa); Incorporating BG could improve the proliferation, ALP activities, and differentiation of hBMSCs, and loading Mn2+ facilitated chondrogenic differentiation of the hBMSCs. Supports cell attachment and spreading, enhances gene expression of chondrogenic-related and osteogenic-related differentiation of human bone marrow stem cells. | Facilitates concurrent regeneration of cartilage and subchondral bone in a rat model. | Superior performance for accelerating cartilage and subchondral bone repair simultaneously in rat knee osteochondral defect | [83] |
| Two regions: chitosan-hyaluronic acid (cartilage) and chitosan-alginate (bone) | Co-culture with chondrocyte-like (SW-1353 or mesenchymal stem cells) and osteoblast-like cells (MG63), cell proliferation and migration to the interface along with increased gene expression associated with relevant markers of osteogenesis and chondrogenesis | Bilayer scaffold for osteochondral tissue regeneration. | [84] | |
| Alginate-based biphasic scaffold | Good biocompatibility profile | Partial osteochondral regeneration in the rabbit; No evidence of adverse or inflammatory reactions | Limited subchondral bone formation was present, together with a slow scaffold resorption time; Further studies are necessary. | [85] |
| Horseradish peroxidase (HRP)-cross-linked silk fibroin (SF) cartilage-like layer (HRP-SF layer) + HRP-SF/ZnSr-doped β-tricalcium phosphate (β-TCP) subchondral bone-like layer (HRP-SF/dTCP layer) | Adequate structure, as well as controllable porosity and TCP distribution; Co-culturing of human osteoblasts and human articular chondrocytes showed cell adhesion, proliferation, and ECM production | Complementary in vivo evaluation is necessary to fully validate these structures and confirm the welfare of the ion presence; These hierarchical scaffolds make these constructs encouraging candidates for OC defect regeneration. | [86] | |
| Poly(ε-caprolactone; PCL) | Transwell in vitro culture system of MSC-based constructs enabled the study of soluble biological cues without the influences of mechanical forces, host systemic responses, or animal-to-animal variability that can result in difficulties in interpreting in vivo studies. | Implantation in rat showed that it could significantly shift the phenotype of MSCs from a chondrogenic phenotype to a hypertrophic, osteogenic one. | PCL scaffolds support cellularization as well as extracellular matrix synthesis, accumulation, and remodeling in vivo, regardless of whether or not MSCs were preseeded or precultured; | [87] |
| Bilayered PLGA/PLGA-HAp | Introduction of the extra cells led to better results | Exhibited satisfactory in vivo efficacy after implanted into the rabbit knee joints for 16 weeks | Potentiality for osteochondral TE, or in situ tissue induction, probably by recruiting the local cells toward chondrogenic and osteogenic differentiation in the porous biomaterials. | [88] |
| PEGylated poly(glycerol sebacate) (PEGS) | PEGS-12h with low crosslinking degree, hierarchical macro-/micro-porosities, and viscoelasticity significantly enhanced chondrogenic differentiation and cartilage matrix secretion compared to that with high crosslinking degree. | Exhibited extraordinary regenerative efficiency in an articular osteochondral defect model in vivo; Bilayer scaffold reconstructed successfully integrated articular hyaline cartilage and its subchondral bone in 12 weeks | [89] | |
| Silver nanorods (Ag-nr) incorporated wollastonite (CaSiO3) | Cytocompatible, interconnected porous structure and bioactivity of the scaffold. | Healthier substitute for bone tissue engineering compared to other similar materials | [90] |
| Technique | Materials | Advantages | Disadvantages |
|---|---|---|---|
| FFF | Ceramics; Polymers; Thermoplastic. | Speed; Low Cost; Simplicity; Flexibility. | Poor surface quality; Need for heating in the molding process. |
| SLA | Resins photocurable. | Fast processing times; Good surface finish; Geometrical accuracy; Higher resolution. | Very few materials; Requires support structures; Unable to process functional materials (like metals). |
| SLS | Polymers; Metals; Alloys; Particles in powder; Ceramics; Stainless steel. | No support structures are required during processing. | Post-processing phase and good surface; Poor mechanical properties. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morouço, P.; Fernandes, C.; Lattanzi, W. Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. J. Funct. Biomater. 2021, 12, 17. https://doi.org/10.3390/jfb12010017
Morouço P, Fernandes C, Lattanzi W. Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. Journal of Functional Biomaterials. 2021; 12(1):17. https://doi.org/10.3390/jfb12010017
Chicago/Turabian StyleMorouço, Pedro, Cristiana Fernandes, and Wanda Lattanzi. 2021. "Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies" Journal of Functional Biomaterials 12, no. 1: 17. https://doi.org/10.3390/jfb12010017
APA StyleMorouço, P., Fernandes, C., & Lattanzi, W. (2021). Challenges and Innovations in Osteochondral Regeneration: Insights from Biology and Inputs from Bioengineering toward the Optimization of Tissue Engineering Strategies. Journal of Functional Biomaterials, 12(1), 17. https://doi.org/10.3390/jfb12010017








