Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PRF Matrices
2.2. Immunohistochemical Examination
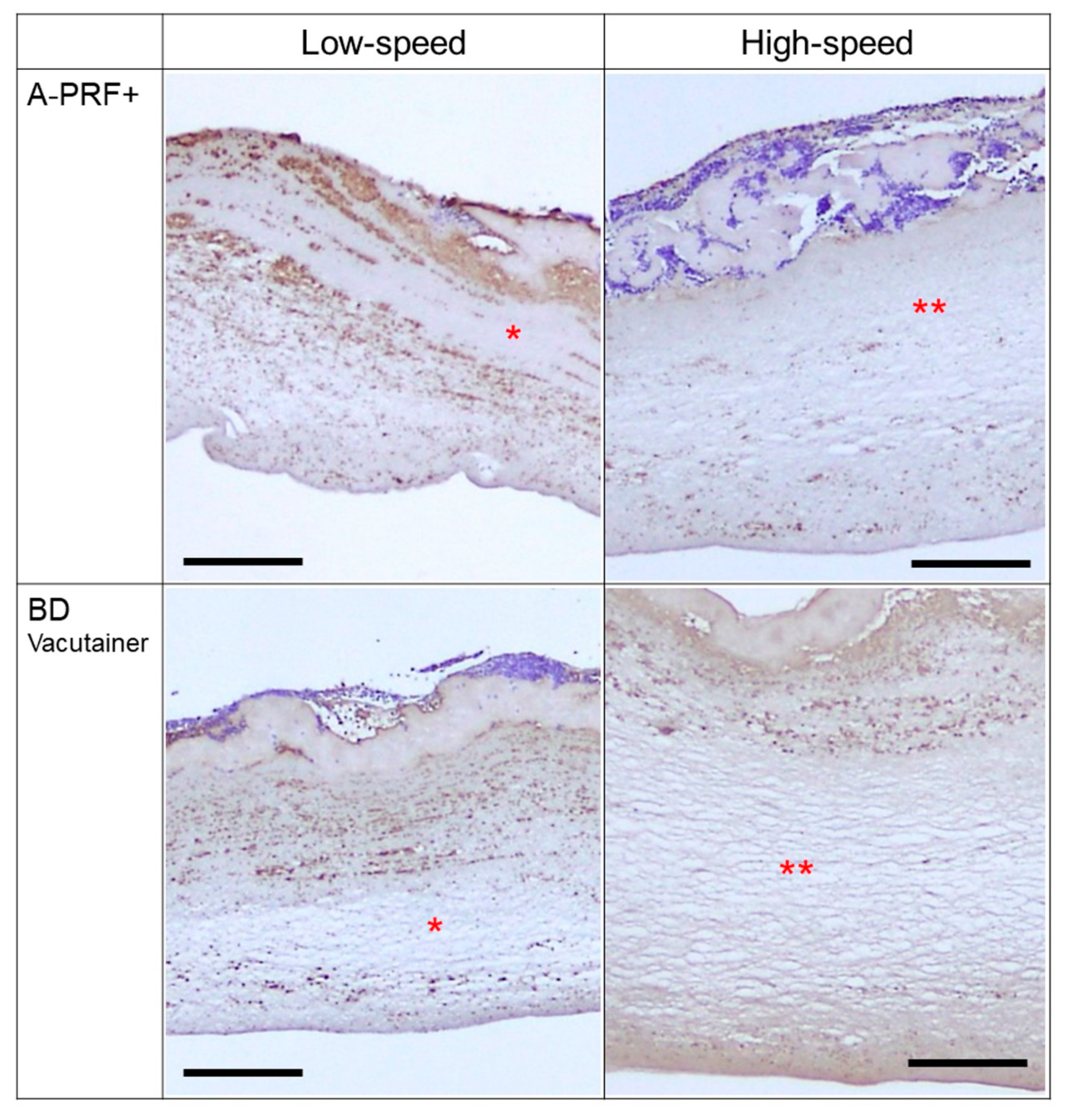
3. Results
4. Discussion
4.1. Practical Difficulty of PRF Preparation
4.2. Differences in Platelet Distribution by Tube Types
4.3. Concern with Possible Risks of Silica-Coated Tubes
4.4. Differences in Platelet Distribution by Centrifugal Speeds and Tube Types
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PRF | platelet-rich fibrin |
| A-PRF | advanced platelet-rich fibrin |
| L-PRF | leukocyte-rich PRF |
| CGF | concentrated growth factors |
| T-PBS | 0.1% Tween-20-containing PBS |
| HE | hematoxylin-eosin |
| IgG | immunoglobulin |
| HRP | horseradish peroxidase |
| DAB | 3,3’-diaminobenzidine tetrahydrochloride |
| FDA | US Food and Drug Administration |
| EMA | European Medicines Agency |
References
- Canellas, J.; Medeiros, P.J.D.; Figueredo, C.; Fischer, R.G.; Ritto, F.G. Platelet-rich fibrin in oral surgical procedures: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2019, 48, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Kawase, T. Platelet-rich plasma and its derivatives as promising bioactive materials for regenerative medicine: Basic principles and concepts underlying recent advances. Odontology 2015, 103, 126–135. [Google Scholar] [CrossRef]
- Mao, X.H.; Zhan, Y.J. The efficacy and safety of platelet-rich fibrin for rotator cuff tears: A meta-analysis. J. Orthop. Surg. Res. 2018, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng. Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.M.; Ukwuani, G.; Makhni, E.C.; Stephens, J.P.; Nho, S.J. The Effect of Platelet-Rich Fibrin Matrix at the Time of Gluteus Medius Repair: A Retrospective Comparative Study. Arthroscopy 2018, 34, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Stahli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implant. Res. 2018, 29 (Suppl. 18), 6–19. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhai, Z.; Jin, X.; Yang, X.; Qi, Z. Clinical Application of Platelet-Rich Fibrin in Plastic and Reconstructive Surgery: A Systematic Review. Aesthetic Plast. Surg. 2018, 42, 511–519. [Google Scholar] [CrossRef]
- Kawase, T.; Takahashi, A.; Watanabe, T.; Tsujino, T. Proposal for point-of-care testing of platelet-rich plasma quality. Int. J. Growth Factors Stem Cells Dent. 2019, 2, 13–17. [Google Scholar] [CrossRef]
- Kitamura, Y.; Watanabe, T.; Nakamura, M.; Isobe, K.; Kawabata, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; Kawase, T. Platelet Counts in Insoluble Platelet-Rich Fibrin Clots: A Direct Method for Accurate Determination. Front. Bioeng. Biotechnol. 2018, 6, 4. [Google Scholar] [CrossRef]
- Agostini, F.; Polesel, J.; Battiston, M.; Lombardi, E.; Zanolin, S.; Da Ponte, A.; Astori, G.; Durante, C.; Mazzucato, M. Standardization of platelet releasate products for clinical applications in cell therapy: A mathematical approach. J. Transl. Med. 2017, 15, 107. [Google Scholar] [CrossRef]
- Gomez, L.A.; Escobar, M.; Penuela, O. Standardization of a Protocol for Obtaining Platelet Rich Plasma from blood Donors; a Tool for Tissue Regeneration Procedures. Clin. Lab. 2015, 61, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Kieb, M.; Sander, F.; Prinz, C.; Adam, S.; Mau-Moller, A.; Bader, R.; Peters, K.; Tischer, T. Platelet-Rich Plasma Powder: A New Preparation Method for the Standardization of Growth Factor Concentrations. Am. J. Sports Med. 2017, 45, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Roessler, F.C.; Ohlrich, M.; Marxsen, J.H.; Stellmacher, F.; Sprenger, A.; Dempfle, C.E.; Seidel, G. The platelet-rich plasma clot: A standardized in-vitro clot formation protocol for investigations of sonothrombolysis under physiological flows. Blood Coagul. Fibrinolysis 2011, 22, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, K.; Li, Z.; Luo, T. Comparative study of different anticoagulants and coagulants in the evaluation of clinical application of platelet-rich plasma (PRP) standardization. Cell Tissue Bank. 2019, 20, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Kawase, T.; Horimizu, M.; Okuda, K.; Wolff, L.F.; Yoshie, H. A proposed protocol for the standardized preparation of PRF membranes for clinical use. Biologicals 2012, 40, 323–329. [Google Scholar] [CrossRef]
- Kawase, T.; Tanaka, T. An updated proposal for terminology and classification of platelet-rich fibrin. Regen. Ther. 2017, 7, 80–81. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jimenez, P.; Corso, M.D.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The impact of the centrifuge characteristics and centrifugation protocols on the cells, growth factors, and fibrin architecture of a leukocyte- and platelet-rich fibrin (L-PRF) clot and membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Isobe, K.; Watanebe, T.; Kawabata, H.; Kitamura, Y.; Okudera, T.; Okudera, H.; Uematsu, K.; Okuda, K.; Nakata, K.; Tanaka, T.; et al. Mechanical and degradation properties of advanced platelet-rich fibrin (A-PRF), concentrated growth factors (CGF), and platelet-poor plasma-derived fibrin (PPTF). Int. J. Implant Dent. 2017, 3, 17. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Tsujino, T.; Takahashi, A.; Yamaguchi, S.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Tanaka, T.; Nakata, K.; Kawase, T. Evidence for Contamination of Silica Microparticles in Advanced Platelet-Rich Fibrin Matrices Prepared Using Silica-Coated Plastic Tubes. Biomedicines 2019, 7, 45. [Google Scholar] [CrossRef]
- Margolis, J. Glass surface and blood coagulation. Nature 1956, 178, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Borie, E.; Olivi, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltran, V.; Fuentes, R. Platelet-rich fibrin application in dentistry: A literature review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, S.M. Safety issues associated with platelet-rich fibrin method. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 103, 587. [Google Scholar] [CrossRef] [PubMed]
- Kamikubo, Y.; Yamana, T.; Hashimoto, Y.; Sakurai, T. Induction of Oxidative Stress and Cell Death in Neural Cells by Silica Nanoparticles. ACS Chem. Neurosci. 2018, 10, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.; Hermanns, M.I.; Bantz, C.; Koshkina, O.; Lang, T.; Maskos, M.; Pohl, C.; Unger, R.E.; Kirkpatrick, C.J. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch. Toxicol. 2013, 87, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Yoshioka, Y.; Fujimura, M.; Yamashita, K.; Higashisaka, K.; Morishita, Y.; Kayamuro, H.; Nabeshi, H.; Nagano, K.; Abe, Y.; et al. Promotion of allergic immune responses by intranasally-administrated nanosilica particles in mice. Nanoscale Res. Lett. 2011, 6, 195. [Google Scholar] [CrossRef]
- Fontana, C.; Kirsch, A.; Seidel, C.; Marpeaux, L.; Darne, C.; Gate, L.; Remy, A.; Guichard, Y. In vitro cell transformation induced by synthetic amorphous silica nanoparticles. Mutat. Res. Toxicol. Environ. Mutagen. 2017, 823, 22–27. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Bielecki, T.; Jimbo, R.; Barbe, G.; Del Corso, M.; Inchingolo, F.; Sammartino, G. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF). Curr. Pharm. Biotechnol. 2012, 13, 1145–1152. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef]


| Tube Types (Manufacturer)\Centrifugation | Low-Speed (A-PRF Protocol) | High-Speed (CGF Protocol) |
|---|---|---|
| Plain glass tube (A-PRF+) | Figure S1a1 | Figure S1b |
| Plain glass tube (BD Vacutainer) | Figure S2a | Figure S2b2 |
| Plastic tube containing silica-coated film (Terumo Venoject II) | Figure S3a | Figure S3b |
| silica-coated plastic tube (Nipro Neotube) | Figure S4a | Figure S4b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsujino, T.; Masuki, H.; Nakamura, M.; Isobe, K.; Kawabata, H.; Aizawa, H.; Watanabe, T.; Kitamura, Y.; Okudera, H.; Okuda, K.; et al. Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes. J. Funct. Biomater. 2019, 10, 43. https://doi.org/10.3390/jfb10030043
Tsujino T, Masuki H, Nakamura M, Isobe K, Kawabata H, Aizawa H, Watanabe T, Kitamura Y, Okudera H, Okuda K, et al. Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes. Journal of Functional Biomaterials. 2019; 10(3):43. https://doi.org/10.3390/jfb10030043
Chicago/Turabian StyleTsujino, Tetsuhiro, Hideo Masuki, Masayuki Nakamura, Kazushige Isobe, Hideo Kawabata, Hachidai Aizawa, Taisuke Watanabe, Yutaka Kitamura, Hajime Okudera, Kazuhiro Okuda, and et al. 2019. "Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes" Journal of Functional Biomaterials 10, no. 3: 43. https://doi.org/10.3390/jfb10030043
APA StyleTsujino, T., Masuki, H., Nakamura, M., Isobe, K., Kawabata, H., Aizawa, H., Watanabe, T., Kitamura, Y., Okudera, H., Okuda, K., Nakata, K., & Kawase, T. (2019). Striking Differences in Platelet Distribution between Advanced-Platelet-Rich Fibrin and Concentrated Growth Factors: Effects of Silica-Containing Plastic Tubes. Journal of Functional Biomaterials, 10(3), 43. https://doi.org/10.3390/jfb10030043






