Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass and Adhesive Preparation
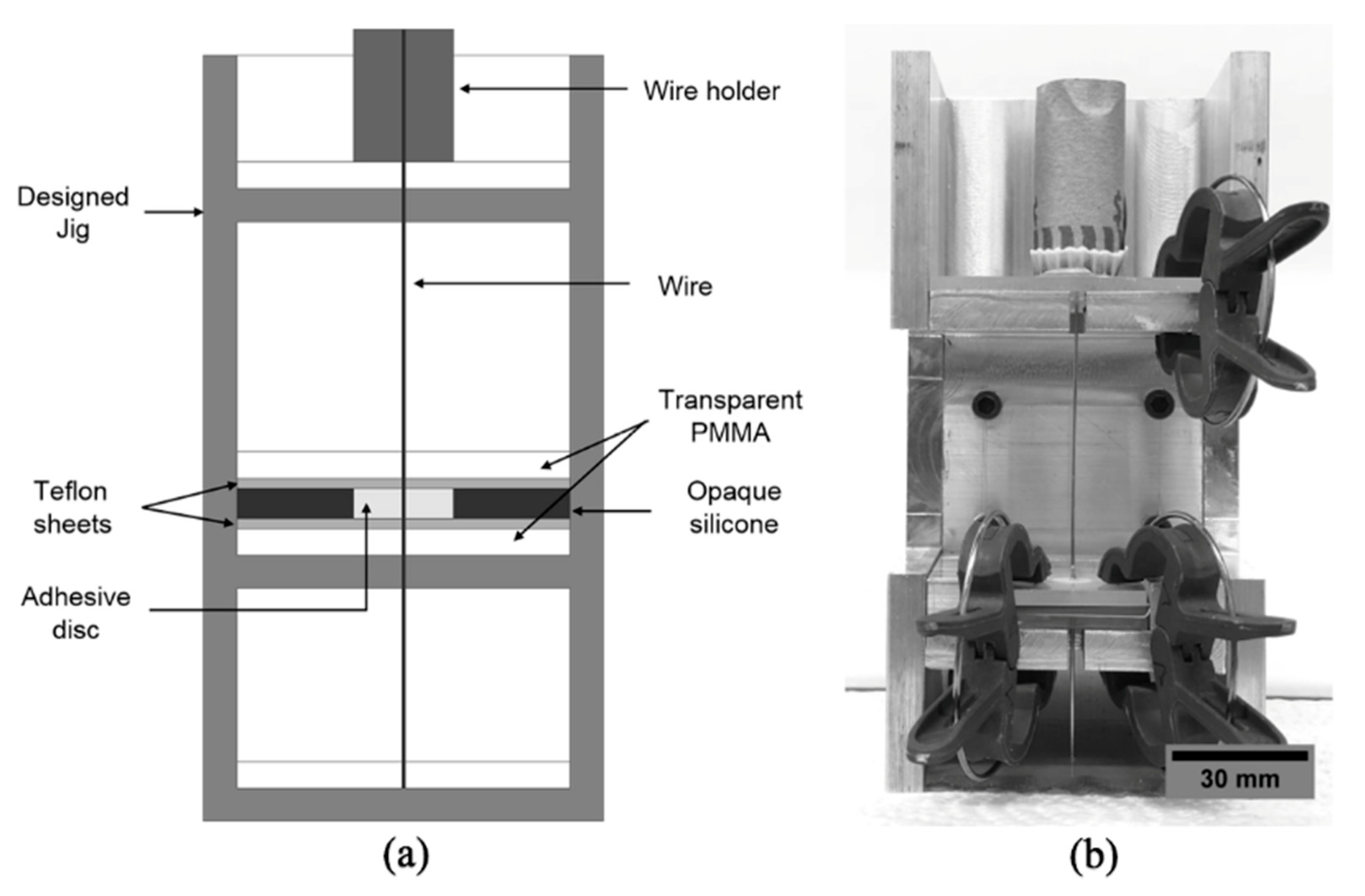
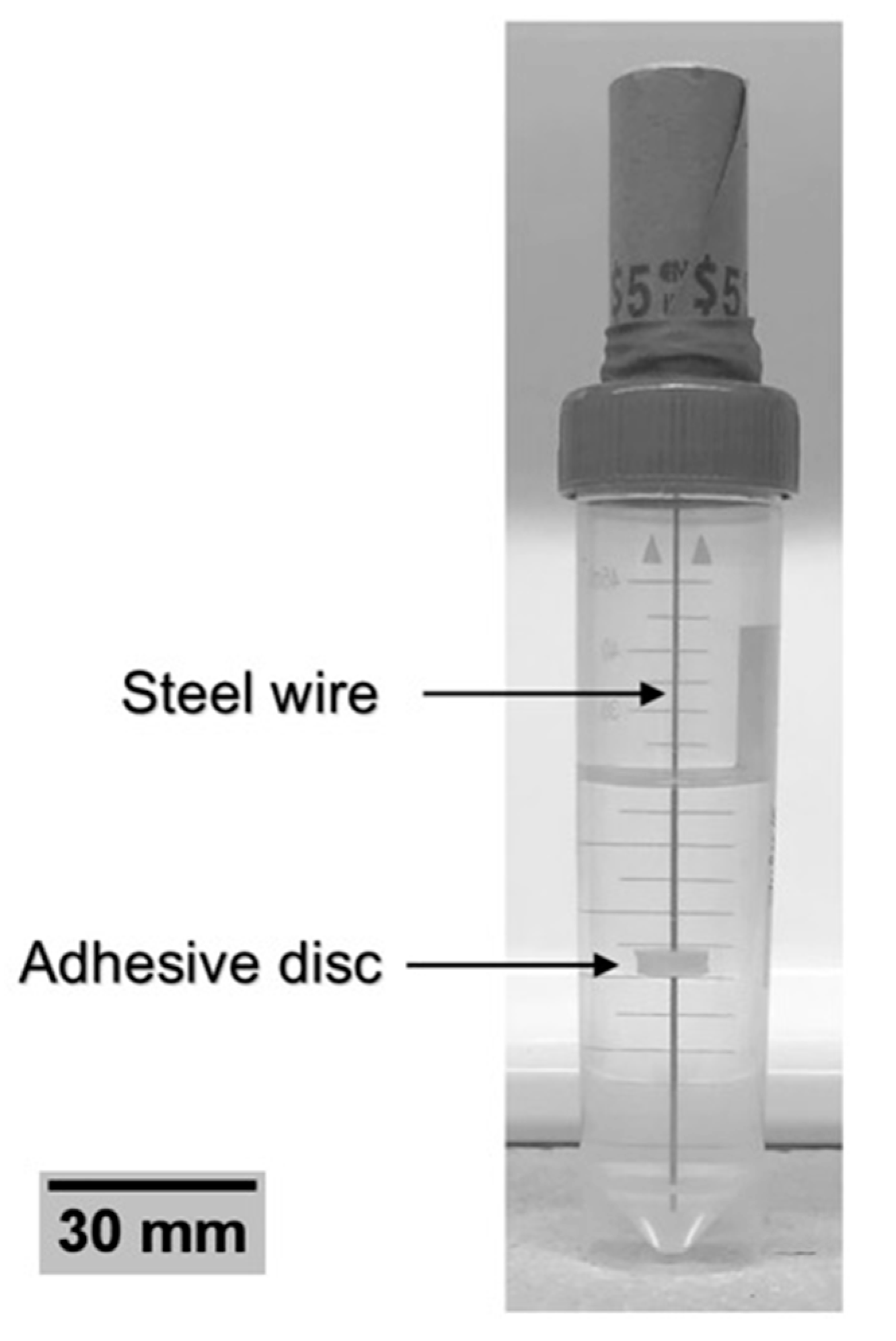
2.2. Specimen Preparation
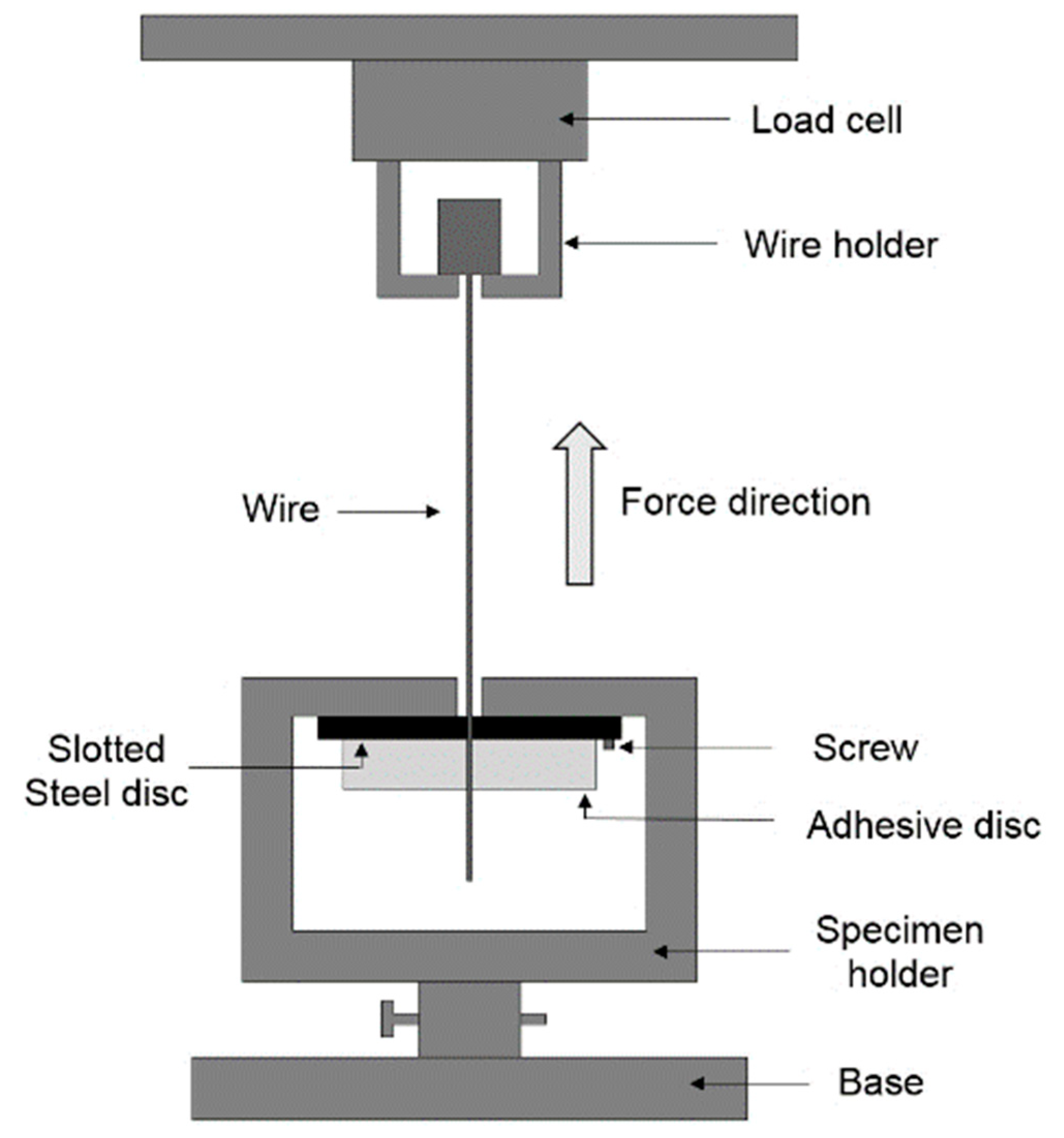
2.3. Wire Pull-Out Tests
2.4. Statistical Analysis
3. Results and Discussion
3.1. Force–Displacement Curves and Likely Mechanism for Force Generation
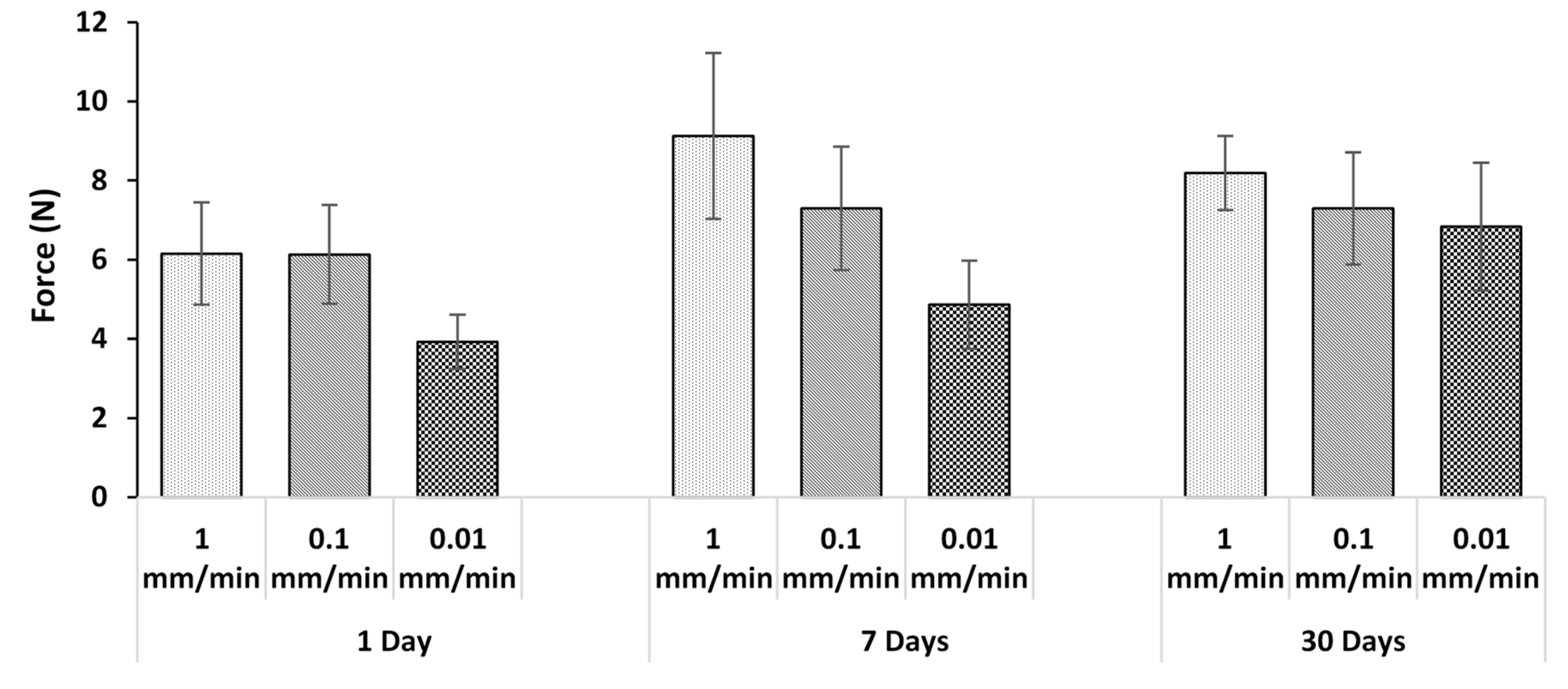
3.2. Effect of Incubation
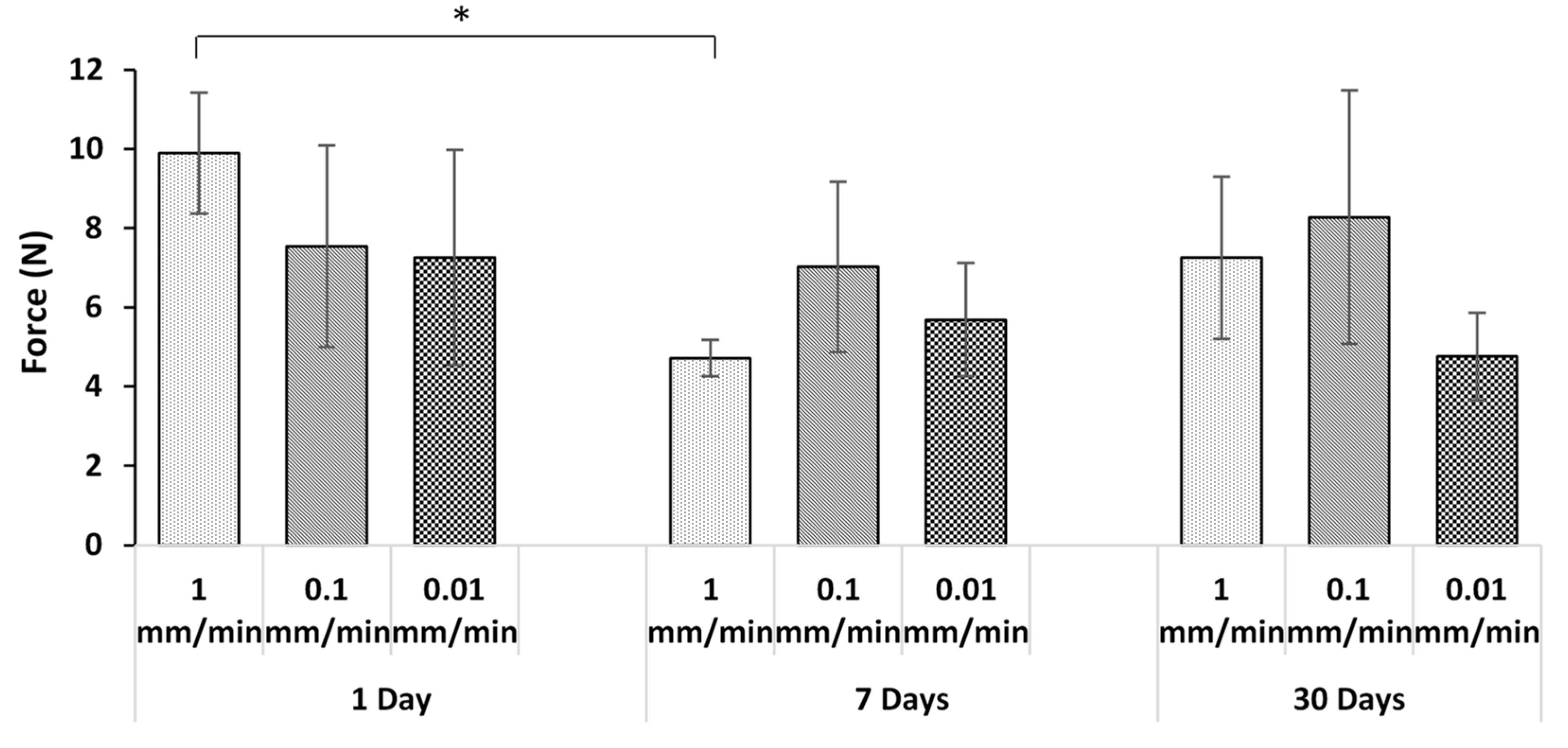
3.3. Effect of Displacement Rate
3.4. Effect of Thickness
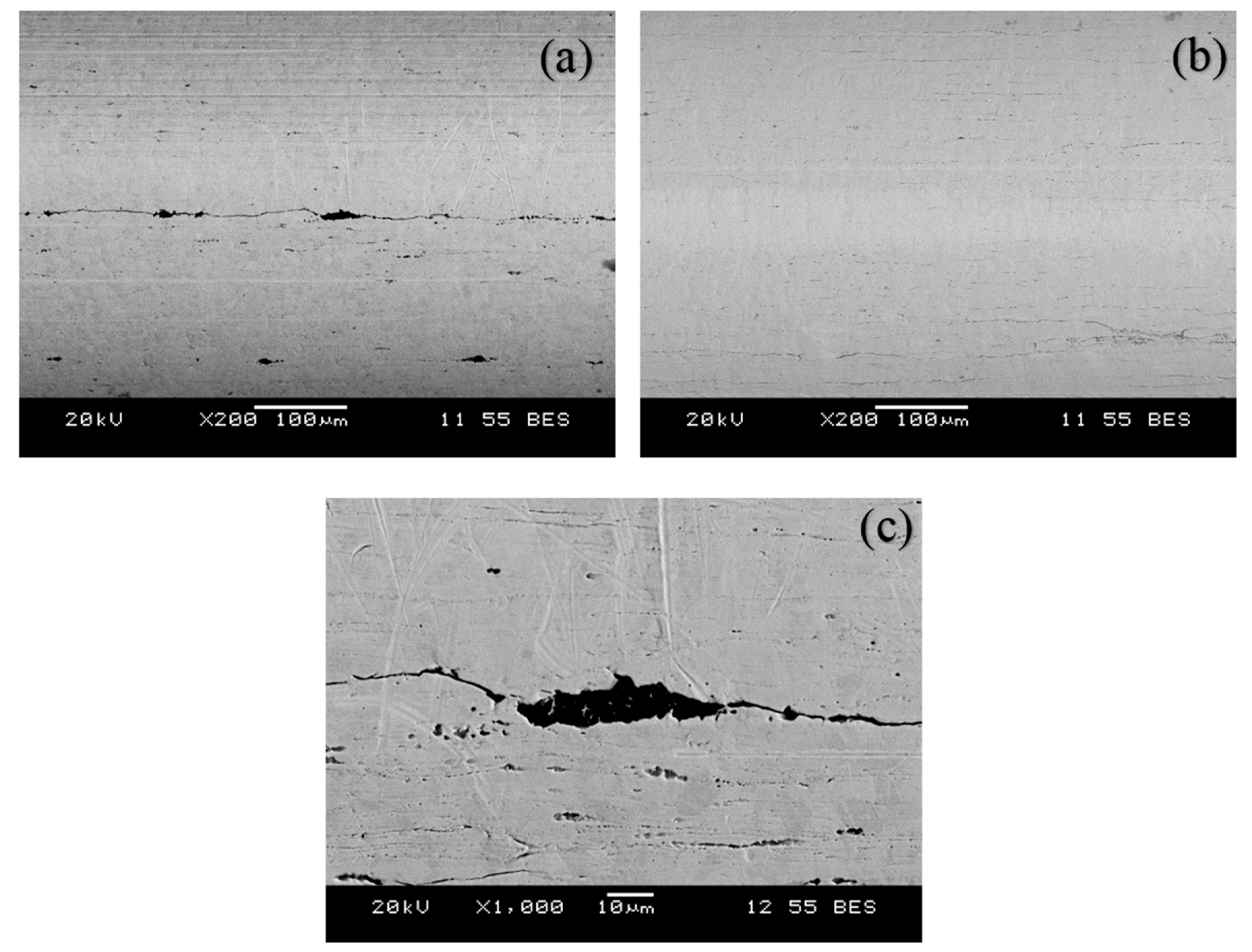
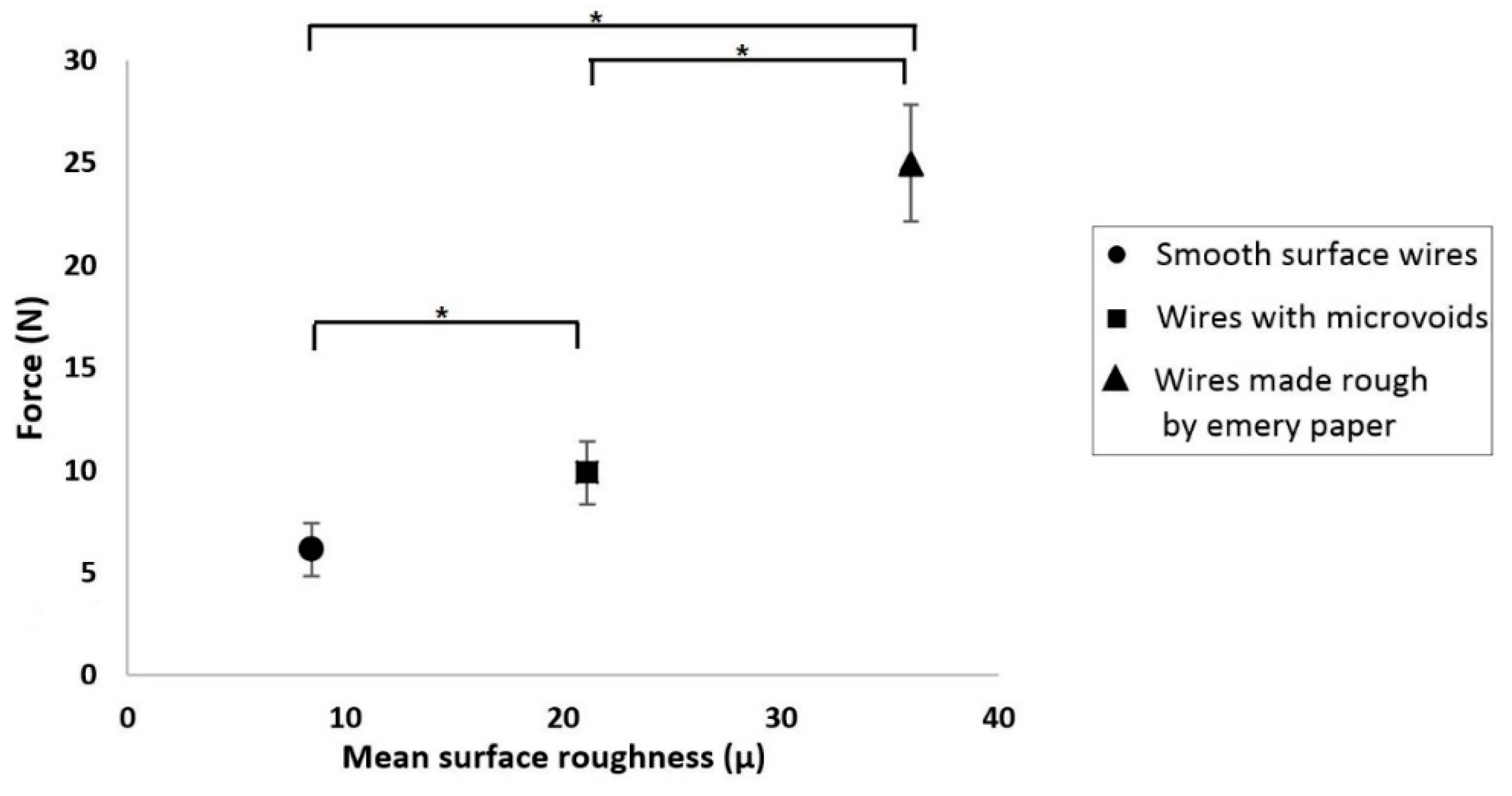
3.5. Mechanism of Generation of Pull-Out Force
4. Conclusions
- In all but one case, there was no statistically significant effect of adhesive thickness, loading rate, or incubation time on the pull-out forces. In the outlying case, it was demonstrated that the differences were due to variable wire surface roughness.
- The pull-out forces were due to friction between the adhesive and wire, rather than chemical adhesion.
- The variation of forces within the samples was due to slight differences in the mechanical interlocking of the adhesive into microvoids that existed on some of the wires. As expected, smoother wires generated less friction.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalton, M.L.; Connally, S.R.; Sealy, W.C. Julian’s re-introduction of Milton’s operation. Ann. Thorac. Surg. 1992, 53, 532–533. [Google Scholar] [CrossRef]
- Ott, D.A.; Cooley, D.A.; Solis, R.T.; Harrison, C.B. Wound complications after median sternotomy: A study of 61 patients from a consecutive series of 9,279. Cardiovasc. Dis. 1980, 7, 104–111. [Google Scholar] [PubMed]
- Doty, D.B.; DiRusso, G.B.; Doty, J.R. Full-Spectrum Cardiac Surgery through a Minimal Incision: Mini-Sternotomy (Lower Half) Technique. Ann. Thorac. Surg. 1998, 65, 573–577. [Google Scholar] [CrossRef]
- Jurkiewicz, M.J.; Bostwick, J.; Hester, T.R.; Bishop, J.B.; Craver, J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann. Surg. 1980, 191, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.M.; Kieser, T.M.; Maitland, A.M.; Holland, M.; Kasatkin, A.; Leblanc, P.; Kim, J.K.; King, K.M. Adhesive-Enhanced Sternal Closure to Improve Postoperative Functional Recovery: A Pilot, Randomized Controlled Trial. ATS 2011, 92, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Bitkover, C.Y.; Cederlund, K.; Åberg, B.; Vaage, J. Computed Tomography of the Sternum and Mediastinum After Median Sternotomy. Ann. Thoracic Surg. 1999, 68, 858–863. [Google Scholar] [CrossRef]
- Robicsek, F.; Fokin, A.; Cook, J.; Bhatia, D.; Carolina, N. Sternal Instability After Midline Sternotomy. Thorac. Cardiovasc. Surg. 2000, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mcgregor, W.E. Mechanical analysis of midline sternotomy wound closure. J. Thorac. Cardiovasc. Surg. 1999, 117, 1144–1150. [Google Scholar] [CrossRef]
- El-ansary, D.; Phty, B.; Waddington, G.; Adams, R. Measurement of Non-Physiological Movement in Sternal Instability by Ultrasound. Ann. Thorac. Surg. 2007, 83, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Cahalin, L.P.; Lapier, T.K. Sternal Precautions: Is It Time for Change ? Precautions versus Restrictions—A Review of Literature. Cardiopulm. Phys. Ther. J. 2011, 22, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Lee, A.; Denehy, L.; Lin, K.; Royse, A.; Royse, C.; El-ansary, D. Risk Factors for Sternal Complications After Cardiac Operations: A Systematic Review. Ann. Thorac. Surg. 2016, 102, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Alhalawani, A.M.; Towler, M.R. A review of sternal closure techniques. J. Biomater. Appl. 2013, 28, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Blaha, M.J.; Dai, S.; Ford, E.S.; Fox, C.S.; Franco, S.; et al. Heart disease and stroke statistics—2014 update: A report from the American Heart Association. Circulation 2014, 129, e28–e292. [Google Scholar] [CrossRef] [PubMed]
- Julian, O.C.; Lopez-Belio, M.; Dye, W.S.; Javid, H.; Grove, W.J. The median sternal incision in intracardiac surgery with extracorporeal circulation; a general evaluation of its use in heart surgery. Surgery 1957, 42, 753–761. [Google Scholar] [PubMed]
- Shih, C.-C.; Shih, C.-M.; Su, Y.-Y.; Lin, S.-J. Potential risk of sternal wires. Eur. J. Cardio Thoracic Surg. 2004, 25, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Gunja, N.J.; Dupak, E.L.; McMahon, N.L.; Roth, T.P.; Lalikos, J.F.; Dunn, R.M.; Francalancia, N.; Pins, G.D.; Billiar, K.L. In Vitro Comparison of Wire and Plate Fixation for Midline Sternotomies. Ann. Thorac. Surg. 2005, 80, 962–968. [Google Scholar] [CrossRef]
- Song, D.H.; Lohman, R.F.; Renucci, J.D.; Jeevanandam, V.; Raman, J. Primary sternal plating in high-risk patients prevents mediastinitis. Eur. J. Cardio Thorac. Surg. 2004, 26, 367–372. [Google Scholar] [CrossRef]
- Ozaki, W.; Buchman, S.R.; Iannettoni, M.D.; Frankenburg, E.P. Biomechanical study of sternal closure using rigid fixation techniques in human cadavers. Ann. Thorac. Surg. 1998, 65, 1660–1665. [Google Scholar] [CrossRef]
- Zeitani, J.; Penta de Peppo, A.; Bianco, A.; Nanni, F.; Scafuri, A.; Bertoldo, F.; Salvati, A.; Nardella, S.; Chiariello, L. Performance of a Novel Sternal Synthesis Device After Median and Faulty Sternotomy: Mechanical Test and Early Clinical Experience. Ann. Thorac. Surg. 2008, 85, 287–293. [Google Scholar] [CrossRef]
- Levin, L.S.; Miller, A.S.; Gajjar, A.H.; Bremer, K.D.; Spann, J.; Milano, C.A.; Erdmann, D. An Innovative Approach for Sternal Closure. Ann. Thorac. Surg. 2010, 89, 1995–1999. [Google Scholar] [CrossRef]
- Losanoff, J.E.; Richman, B.W.; Jones, J.W. Disruption and infection of median sternotomy: A comprehensive review. Eur. J. Cardiothorac. Surg. 2002, 21, 831–839. [Google Scholar] [CrossRef]
- Schimmer, C.; Reents, W.; Berneder, S.; Eigel, P.; Sezer, O.; Scheld, H.; Sahraoui, K.; Gansera, B.; Deppert, O.; Rubio, A.; et al. Prevention of sternal dehiscence and infection in high-risk patients: A prospective randomized multicenter trial. Ann. Thorac. Surg. 2008, 86, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Grossi, E.A.; Zakow, P.K.; Ribakove, G.; Kallenbach, K.; Ursomanno, P.; Gradek, C.E.; Baumann, F.G.; Colvin, S.B.; Galloway, A.C. Comparison of post-operative pain, stress response, and quality of life in port access vs. standard sternotomy coronary bypass patients. Eur. J. Cardiothorac. Surg. 1999, 16 (Suppl. 2), S39–S42. [Google Scholar]
- El-Ansary, D.; Waddington, G.; Adams, R. Relationship between pain and upper limb movement in patients with chronic sternal instability following cardiac surgery. Physiother. Theory Pract. 2007, 23, 273–280. [Google Scholar] [CrossRef] [PubMed]
- El Oakley, R.M.; Wright, J.E. Postoperative mediastinitis: Classification and management. Ann. Thorac. Surg. 1996, 61, 1030–1036. [Google Scholar] [CrossRef]
- Bayramoglu, Z.; Durak, Y.; Bayram, M.; Ulusoy, O.L.; Caynak, B.; Sagbas, E.; Akpınar, B. Bone cement-enhanced sternal closure technique in cardiac surgery: effects on sternal union, pain and life quality. J. Cardiothorac. Surg. 2013, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Fedak, P.W.M.; Kolb, E.; Borsato, G.; Frohlich, D.E.C.; Kasatkin, A.; Narine, K.; Akkarapaka, N.; King, K.M. Kryptonite Bone Cement Prevents Pathologic Sternal Displacement. Ann. Thorac. Surg. 2010, 90, 979–985. [Google Scholar] [CrossRef]
- Doumit, G.D.; Meisler, E.; Sidaoui, J.; Zins, J.E.; Papay, F.A. The Expansile Properties of Kryptonite Relating to Cranioplasty. J. Craniofac. Surg. 2014, 25, 880–883. [Google Scholar] [CrossRef]
- Wilson, A.D.; Kent, B.E. The glass-ionomer cement, a new translucent dental filling material. J. Appl. Chem. Biotechnol. 2007, 21, 313. [Google Scholar] [CrossRef]
- Tyas, M.J.; Burrow, M.F. Adhesive restorative materials: A review. Aust. Dent. J. 2004, 49, 112–121. [Google Scholar] [CrossRef]
- Darling, M.; Hill, R. Novel polyalkenoate (glass-ionomer) dental cements based on zinc silicate glasses. Biomaterials 1994, 15, 299–306. [Google Scholar] [CrossRef]
- Walls, A.W.G. Glass polyalkenoate (glass-ionomer) cements: A review. J. Dent. 1986, 14, 231–246. [Google Scholar] [CrossRef]
- Polizzi, S.; Pira, E.; Ferrara, M.; Bugiani, M.; Papaleo, A.; Albera, R.; Palmi, S. Neurotoxic Effects of Aluminium Among Foundry Workers and Alzheimer’s Disease. Neurotoxicology 2002, 23, 761–774. [Google Scholar] [CrossRef]
- Wren, A.; Boyd, D.; Towler, M.R. The processing, mechanical properties and bioactivity of strontium based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2008, 19, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Yamaguchi, M. Stimulatory effect of zinc on deoxyribonucleic acid synthesis in bone growth of newborn rats: enhancement with zinc and insulin-like growth factor-I. Calcif. Tissue Int. 2001, 69, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, S.; Gül, K.; Çiçek, R. The Effect of Zinc On Microbial Growth. Turk. J. Med. Sci. 1998, 28, 595–598. [Google Scholar]
- Prasad, A.S. Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Exp. Gerontol. 2008, 43, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Caverzasio, J. Strontium ranelate promotes osteoblastic cell replication through at least two different mechanisms. Bone 2008, 42, 1131–1136. [Google Scholar] [CrossRef]
- Canalis, E.; Hott, M.; Deloffre, P.; Tsouderos, Y.; Marie, P.J. The divalent strontium salt S12911 enhances bone cell replication and bone formation in vitro. Bone 1996, 18, 517–523. [Google Scholar] [CrossRef]
- Mehrvar, C.; Kuzyk, P.; Cohen, G.; Safir, O.; Zalzal, P.; Alhalawani, A.; Towler, M.R.; Papini, M. Novel adhesives for sternal fixation and stabilization: A biomechanical analysis. Clin. Biomech. 2019, 62, 66–71. [Google Scholar] [CrossRef]
- Morales, D.L.S.; Zafar, F.; Arrington, K.A.; Gonzalez, S.M.; McKenzie, E.D.; Heinle, J.S.; Fraser, C.D. Repeat Sternotomy in Congenital Heart Surgery: No Longer a Risk Factor. Ann. Thorac. Surg. 2008, 86, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, E.; Bolcal, C.; Cingoz, F.; Günay, C.; Yildirim, V.; Kilic, S.; Özal, E.; Demirkilic, U.; Arslan, M.; Tatar, H. Cardiac reoperation by Carpentier bicaval femoral venous cannula: GATA experience. Ann. Thorac. Surg. 2004, 77, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.; Desouza, A.; Magee, P.; Walesby, R.; Wright, J.; Uppal, R. Re-do cardiac surgery in patients over 70 years old. Eur. J. Cardio Thoracic Surg. 1997, 12, 40–46. [Google Scholar] [CrossRef][Green Version]
- Morales, D.; Williams, E.; John, R. Is resternotomy in cardiac surgery still a problem? Interact. Cardiovasc. Thorac. Surg. 2010, 11, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, A.; Ooi, A.; Kitcat, J.; Nashef, S. Outcomes in emergency redo cardiac surgery: Cost, benefit and risk assessment. Interact. Cardiovasc. Thorac. Surg. 2003, 2, 227–230. [Google Scholar] [CrossRef]
- Boyd, D.; Li, H.; Tanner, D.A.; Towler, M.R.; Wall, J.G. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J. Mater. Sci. Mater. Med. 2006, 17, 489–494. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, J.; Xu, P. Investigation of interfacial debonding between steel wire and adhesive resin. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Iliadi, A.; Eliades, T.; Silikas, N.; Eliades, G. Development and testing of novel bisphenol A-free adhesives for lingual fixed retainer bonding. Eur. J. Orthod. 2017, 39, 1–8. [Google Scholar] [CrossRef]
- Kotha, S.P.; Lieberman, M.; Vickers, A.; Schmid, S.R.; Mason, J.J. Adhesion enhancement of steel fibers to acrylic bone cement through a silane coupling agent. J. Biomed. Mater. Res. Part A 2006, 76A, 111–119. [Google Scholar] [CrossRef]
- Mann, K.A.; Bartel, D.L.; Wright, T.M.; Ingraffea, A.R. Mechanical characteristics of the stem-cement interface. J. Orthop. Res. 1991, 9, 798–808. [Google Scholar] [CrossRef]
- Homola, A.M.; Israelachvili, J.N.; McGuiggan, P.M.; Gee, M.L. Fundamental experimental studies in tribology: The transition from “interfacial” friction of undamaged molecularly smooth surfaces to “normal” friction with wear. Wear 1990, 136, 65–83. [Google Scholar] [CrossRef]
- Ringlein, J.; Robbins, M.O. Understanding and illustrating the atomic origins of friction. Am. J. Phys. 2004, 72, 884–891. [Google Scholar] [CrossRef]

| Glass Composition (Mole Percentage) | Glass Particle Size (μm) | Adhesive Formulation | |||||
|---|---|---|---|---|---|---|---|
| SiO2 | ZnO | CaO | SrO | P2O5 | Ta2O5 | Glass (g):PAA (g):Water (mL):TSC (g) | |
| 48 | 35.5 | 6 | 8 | 2 | 0.5 | <45 | 1:0.4:0.6:0.075 |
| Adhesive Disc Thickness | 2 mm | 3 mm | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incubation Days | 1 | 7 | 30 | 1 | 7 | 30 | ||||||||||||
| Loading Rates (mm/min) | 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 | 1 | 0.1 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, V.P.S.; Towler, M.R.; Papini, M. Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive. J. Funct. Biomater. 2019, 10, 37. https://doi.org/10.3390/jfb10030037
Sidhu VPS, Towler MR, Papini M. Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive. Journal of Functional Biomaterials. 2019; 10(3):37. https://doi.org/10.3390/jfb10030037
Chicago/Turabian StyleSidhu, Varinder Pal Singh, Mark R. Towler, and Marcello Papini. 2019. "Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive" Journal of Functional Biomaterials 10, no. 3: 37. https://doi.org/10.3390/jfb10030037
APA StyleSidhu, V. P. S., Towler, M. R., & Papini, M. (2019). Measurement of Adhesion of Sternal Wires to a Novel Bioactive Glass-Based Adhesive. Journal of Functional Biomaterials, 10(3), 37. https://doi.org/10.3390/jfb10030037




