Disentangling the Effects of Processing Speed on the Association between Age Differences and Fluid Intelligence
Abstract
1. Introduction
1.1. The Role of Processing Speed in Cognitive Aging
1.2. A Neurocognitive Psychometrics Account of Mental Speed
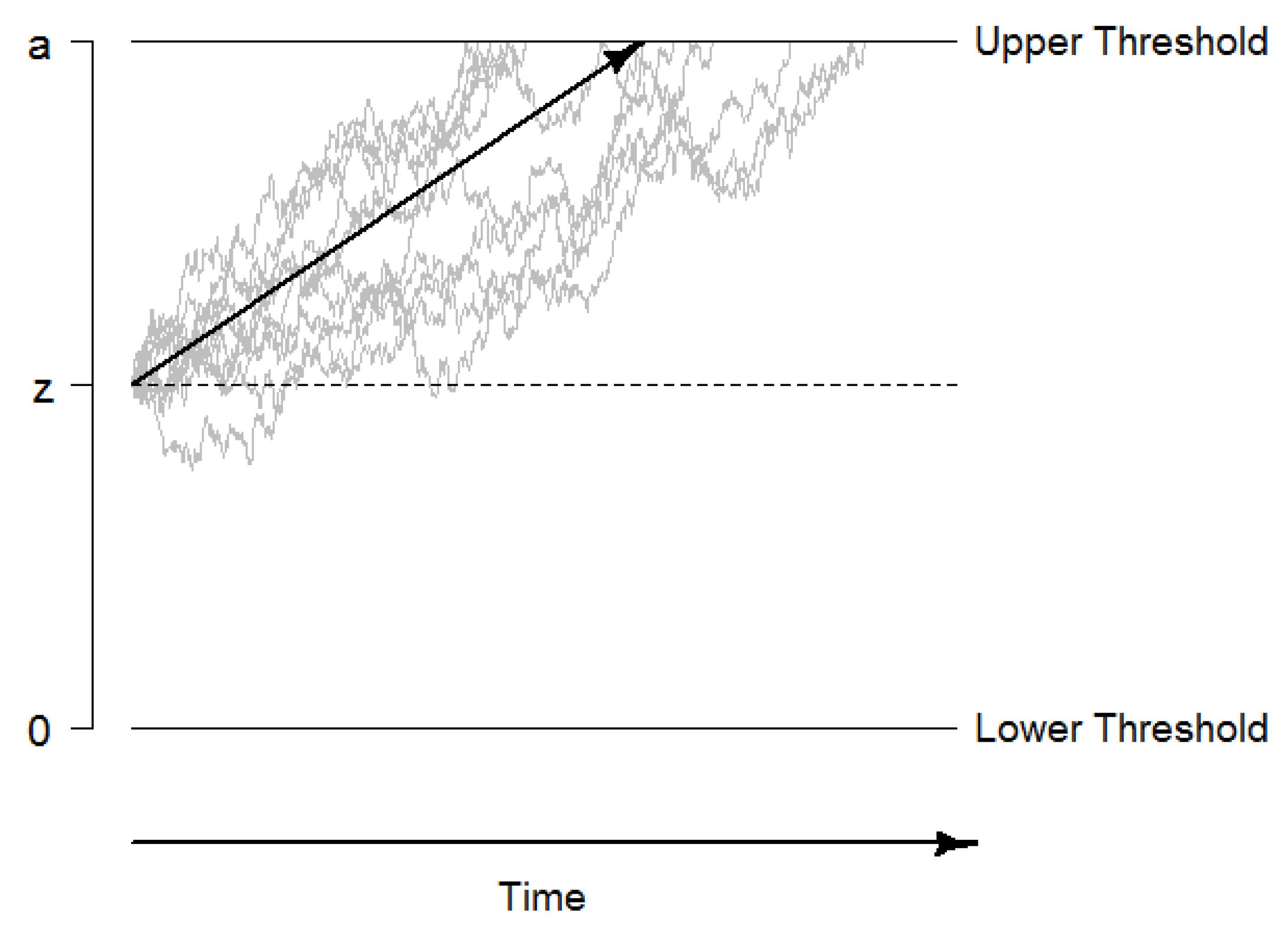
1.2.1. Diffusion Modeling
1.2.2. Chronometric Analyses of the ERP
1.3. The Present Study
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Measures
2.3.1. Experimental Tasks
2.3.2. Intelligence Test
2.3.3. EEG Recording
2.4. Data Analysis
2.4.1. Reaction Time Data
2.4.2. Electrophysiological Data
2.4.3. Statistical Analyses
3. Results
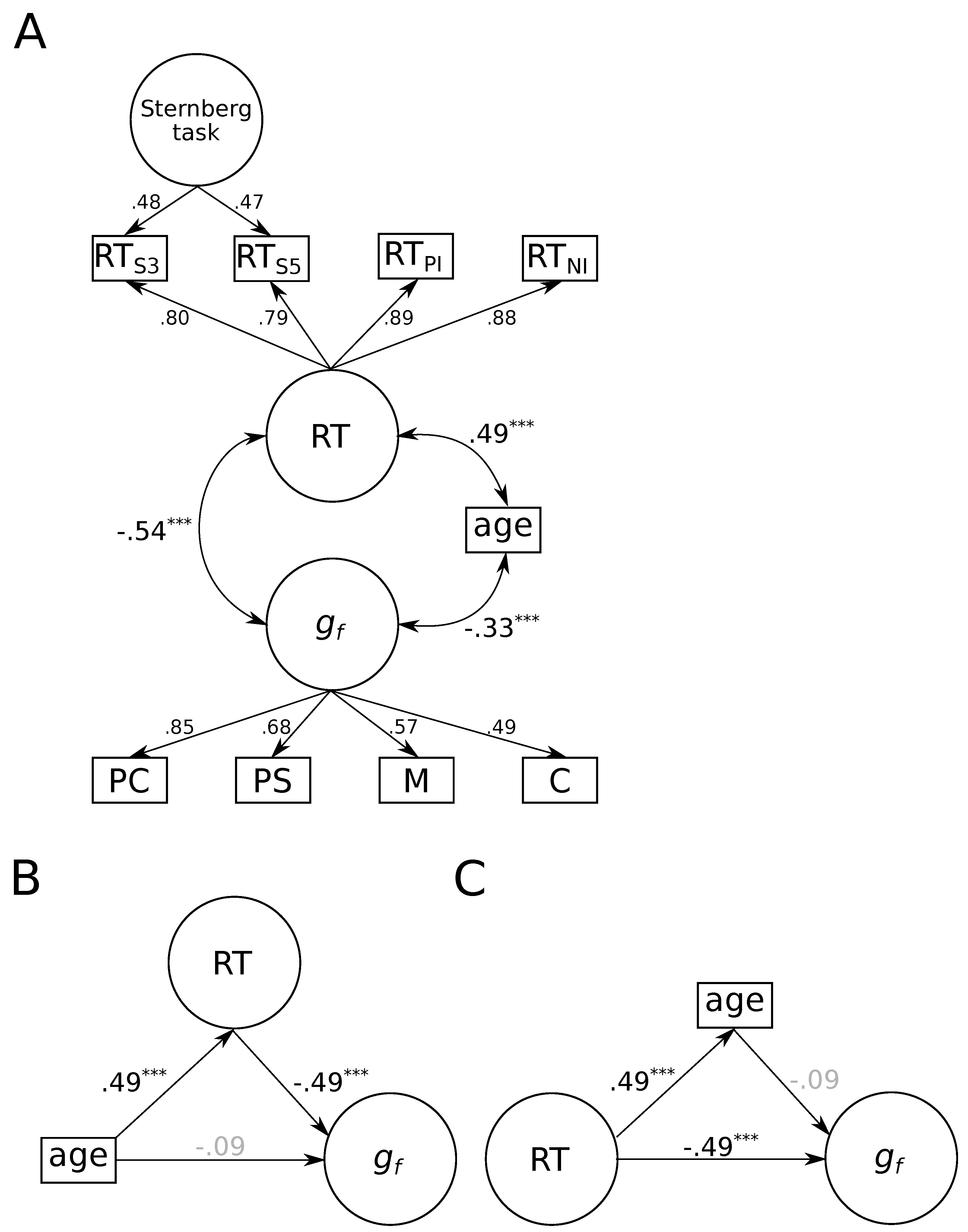
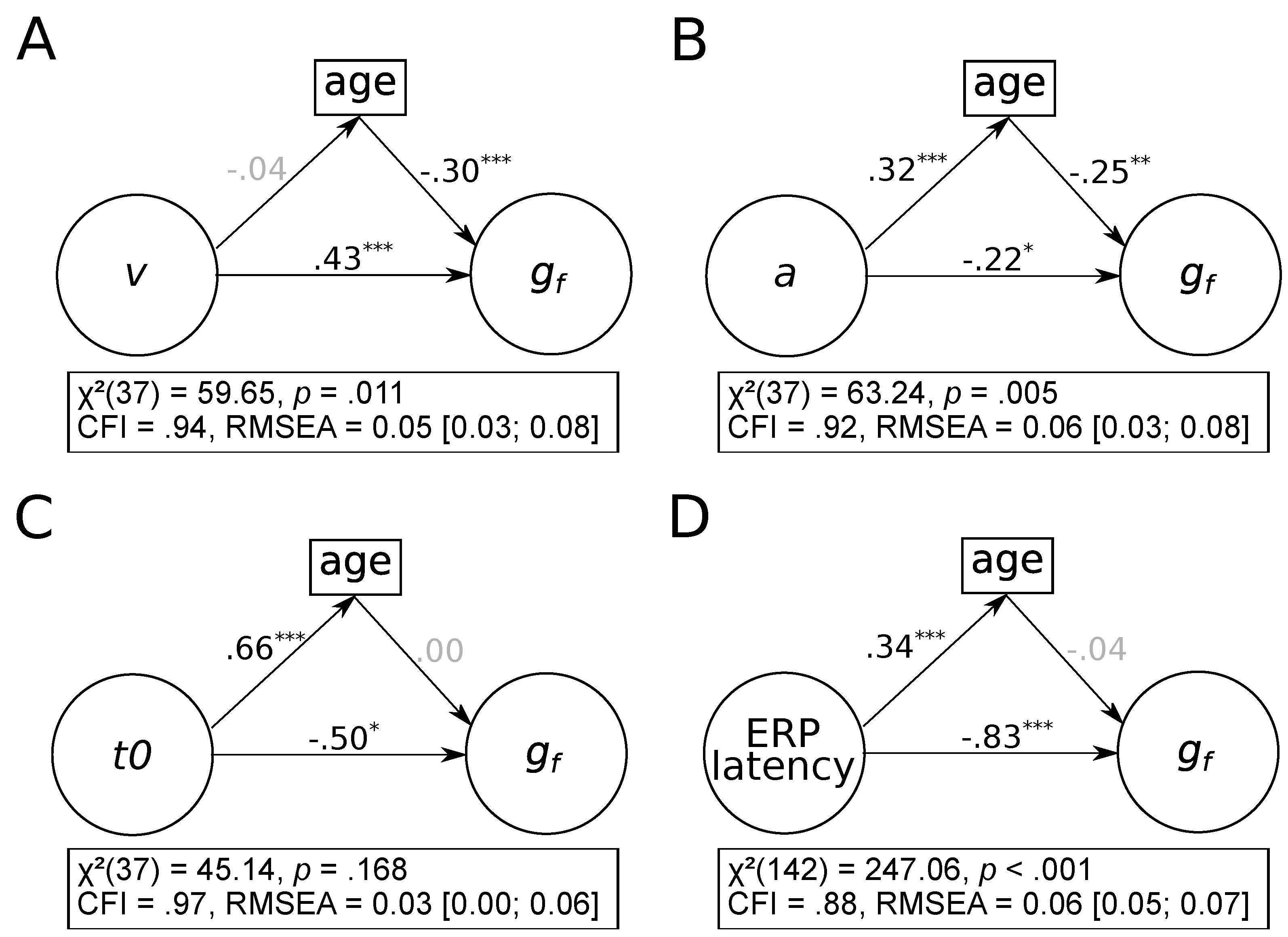
3.1. Associations between aGe Differences, Fluid Intelligence, and Reaction Times
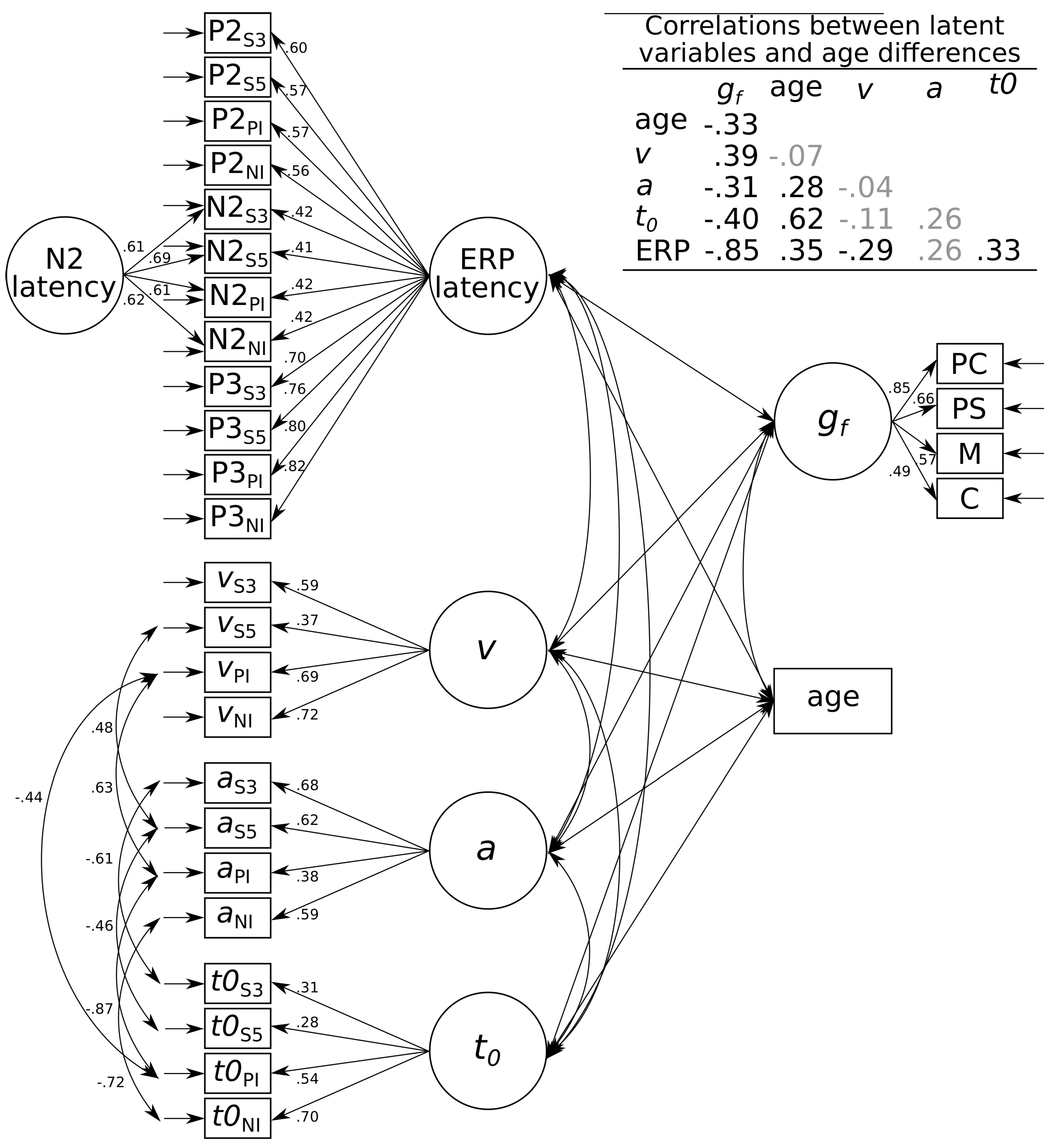
3.2. Which Process-Related Parameter Accounts for the Association between Age Differences and Fluid Intelligence?
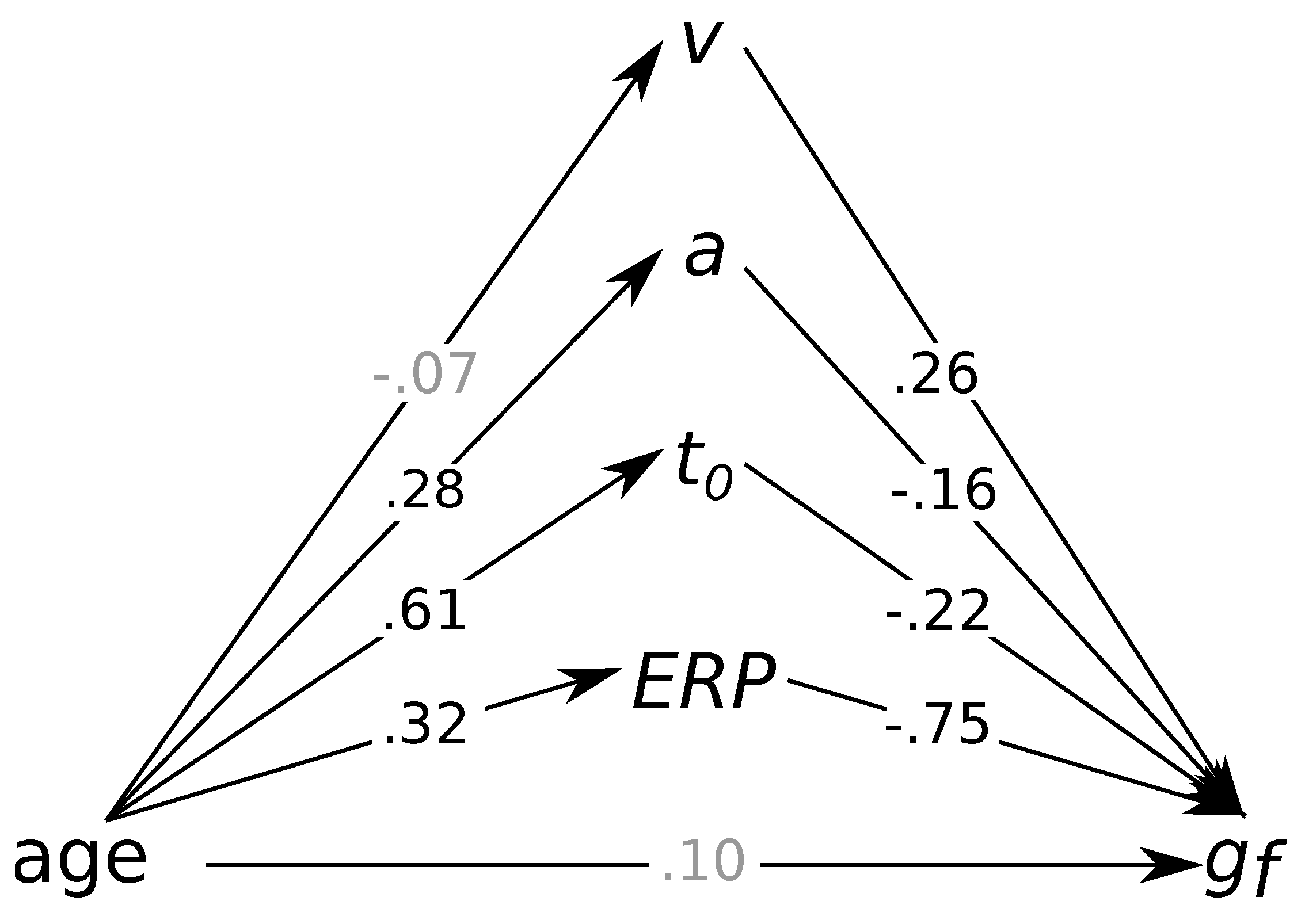
3.3. Do Age Differences Account for the Association between Process-Related Parameters and Fluid Intelligence?
4. Discussion
4.1. Insights from a Neurocognitive Psychometrics Account of Mental Speed
4.2. The Association between Processing Speed and Fluid Intelligence Cannot Be Accounted for by Age Differences
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anstey, Kaarin J., Mary A. Luszcz, and Linnett Sanchez. 2001. A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences 56: P3–P11. [Google Scholar] [CrossRef] [PubMed]
- Baltes, Paul B. 1987. Theoretical propositions of life-span developmental psychology: On the dynamics between growth and decline. Developmental Psychology 23: 611–26. [Google Scholar] [CrossRef]
- Bazana, P. Gordon, and Robert Stelmack. 2002. Intelligence and information processing during an auditory discrimination task with backward masking: An event-related potential analysis. Journal of Personality and Social Psychology 83: 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Bentler, Peter. 1990. Comparative fit indexes in structural models. Psychological Bulletin 107: 238–46. [Google Scholar] [CrossRef]
- Birren, James E. 1974. Translations in gerontology–from lab to life. Psychophysiology and speed of response. The American Psychologist 29: 808–15. [Google Scholar] [CrossRef]
- Birren, James E., and Laurel M. Fisher. 1995. Aging and speed of behavior: possible consequences for psychological functioning. Annual Review of Psychology 46: 329–53. [Google Scholar] [CrossRef]
- Bors, Douglas A., and Bert Forrin. 1995. Age, speed of information processing, recall, and fluid intelligence. Intelligence 20: 229–48. [Google Scholar] [CrossRef]
- Browne, Michael W., and Robert Cudeck. 1992. Alternative Ways of Assessing Model Fit. Sociological Methods & Research 21: 230–58. [Google Scholar] [CrossRef]
- Bucik, Valentin, and Aljoscha C. Neubauer. 1996. Bimodality in the Berlin model of intelligence structure (BIS): A replication study. Personality and Individual Differences 21: 987–1005. [Google Scholar] [CrossRef]
- Deary, Ian J., Michael Allerhand, and Geoff Der. 2009. Smarter in middle age, faster in old age: A cross-lagged panel analysis of reaction time and cognitive ability over 13 years in the West of Scotland Twenty-07 Study. Psychology and Aging 24: 40–47. [Google Scholar] [CrossRef]
- De Sanctis, Pierfilippo, Richard Katz, Glenn R.Wylie, Pejman Sehatpour, George S. Alexopoulos, and John J. Foxe. 2008. Enhanced and bilateralized visual sensory processing in the ventral stream may be a feature of normal aging. Neurobiology of Aging 29: 1576–86. [Google Scholar] [CrossRef] [PubMed]
- Dirk, Judith, Gesa Katharina Kratzsch, John P. Prindle, Ulf Kröhne, Frank Goldhammer, and Florian Schmiedek. 2017. Paper-Based Assessment of the Effects of Aging on Response Time: A Diffusion Model Analysis. Journal of Intelligence 5: 12. [Google Scholar] [CrossRef] [PubMed]
- Dully, Jessica, David P. McGovern, and Redmond G. O’Connell. 2018. The impact of natural aging on computational and neural indices of perceptual decision making: A review. Behavioural Brain Research 355: 48–55. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Deborah, Chandra Reynolds, John J. Mcardle, and Nancy L. Pedersen. 2007. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging 22: 558–68. [Google Scholar] [CrossRef] [PubMed]
- Folstein, Jonathan, and Cyma Van Petten. 2008. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 45: 152–70. [Google Scholar] [CrossRef] [PubMed]
- Friedman, David. 2012. The Components of Aging. In The Oxford Handbook of Event-Related Potential Components. Edited by Emily S. Kappenman and Steven J. Luck. New York: Oxford University Press. [Google Scholar]
- Frischkorn, Gidon T., and Anna-Lena Schubert. 2018. Cognitive Models in Intelligence Research: Advantages and Recommendations for Their Application. Journal of Intelligence 6: 34. [Google Scholar] [CrossRef]
- Frischkorn, Gidon T., Anna-Lena Schubert, and Dirk Hagemann. 2019. Processing speed, working memory, and executive functions: Independent or inter-related predictors of general intelligence. Intelligence 75: 95–110. [Google Scholar] [CrossRef]
- Gajewski, Patrick D., Nicola K. Ferdinand, Jutta Kray, and Michael Falkenstein. 2018. Understanding sources of adult age differences in task switching: Evidence from behavioral and ERP studies. Neuroscience & Biobehavioral Reviews 92: 255–75. [Google Scholar] [CrossRef]
- Gratton, Gabriele, Michael G. H. Coles, and Emanuel Donchin. 1983. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology 55: 468–84. [Google Scholar] [CrossRef]
- Hämmerer, Dorothea, Viktor Müller, and Shu-Chen Li. 2014. Performance monitoring across the lifespan: Still maturing post-conflict regulation in children and declining task-set monitoring in older adults. Neuroscience and Biobehavioral Reviews 46 Pt 1: 105–23. [Google Scholar] [CrossRef]
- Hartley, Alan. 2006. Changing Role of the Speed of Processing Construct in the Cognitive Psychology of Human Aging. In Handbook of the Psychology of Aging, 6th ed. Edited by James E. Birren and K. Warner Schaie. Amsterdam: Elsevier. [Google Scholar]
- Hartshorne, Joshua K., and Laura Germine. 2015. When Does Cognitive Functioning Peak? The Asynchronous Rise and Fall of Different Cognitive Abilities Across the Life Span. Psychological Science 26: 433–43. [Google Scholar] [CrossRef] [PubMed]
- Hedden, Trey, Gary Lautenschlager, and Denise C. Park. 2005. Contributions of processing ability and knowledge to verbal memory tasks across the adult life-span. The Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology 58: 169–90. [Google Scholar] [CrossRef]
- Hedden, Trey, and John D. E. Gabrieli. 2004. Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience 5: 87–96. [Google Scholar] [CrossRef] [PubMed]
- Heywood, H. B. 1931. On finite sequences of real numbers. Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character 134: 486–501. [Google Scholar] [CrossRef]
- Horn, John L., and Raymond B. Cattell. 1967. Age differences in fluid and crystallized intelligence. Acta Psychologica 26: 107–29. [Google Scholar] [CrossRef]
- Hu, Li-tze, and Peter M. Bentler. 1999. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling 6: 1–55. [Google Scholar] [CrossRef]
- Huff, Mark J., and Andrew J. Aschenbrenner. 2018. Item-specific processing reduces false recognition in older and younger adults: Separating encoding and retrieval using signal detection and the diffusion model. Memory & Cognition 46: 1287–301. [Google Scholar] [CrossRef]
- Jäger, Adolf O., and Heinz-Martin Süß. 1997. Berlinger Intelligenzstruktur-Test. Form 4. Göttingen: Hogrefe. [Google Scholar]
- Jensen, Arthur R. 2006. Clocking the Mind: Mental Chronometry and Individual Differences, 1st ed.Amsterdam, Boston and London: Elsevier Science. [Google Scholar]
- Lee, Kyoung-Min, Kee-Hyun Chang, and Jae-Kyu Roh. 1999. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. NeuroImage 9: 117–23. [Google Scholar] [CrossRef]
- Lerche, Veronika, and Andreas Voss. 2016. Model Complexity in Diffusion Modeling: Benefits of Making the Model More Parsimonious. Frontiers in Psychology 7: 1324. [Google Scholar] [CrossRef]
- Lerche, Veronika, and Andreas Voss. 2019. Experimental validation of the diffusion model based on a slow response time paradigm. Psychological Research 83: 1194–209. [Google Scholar] [CrossRef]
- Lindenberger, Ulman, Timo von Oertzen, Paolo Ghisletta, and Christopher Hertzog. 2011. Cross-sectional age variance extraction: what’s change got to do with it? Psychology and Aging 26: 34–47. [Google Scholar] [CrossRef]
- McGarry-Roberts, Patricia A., Robert M. Stelmack, and Kenneth B. Campbell. 1992. Intelligence, reaction time, and event-related potentials. Intelligence 16: 289–313. [Google Scholar] [CrossRef]
- Nettelbeck, Ted, and Nicholas R. Burns. 2010. Processing speed, working memory and reasoning ability from childhood to old age. Personality and Individual Differences 48: 379–84. [Google Scholar] [CrossRef]
- Nunez, Michael D., Ramesh Srinivasan, and Joachim Vandekerckhove. 2015. Individual differences in attention influence perceptual decision making. Frontiers in Psychology 6. [Google Scholar] [CrossRef]
- Oschwald, Jessica, Sabrina Guye, Franziskus Liem, Philippe Rast, Sherry Willis, Christina Röcke, Lutz Jäncke, Mike Martin, and Susan Mérillat. 2019. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Reviews in the Neurosciences. [Google Scholar] [CrossRef]
- Picard, Nathalie, and Peter L. Strick. 1996. Motor areas of the medial wall: A review of their location and functional activation. Cerebral Cortex 6: 342–53. [Google Scholar] [CrossRef] [PubMed]
- Polich, John. 1996. Meta-analysis of P300 normative aging studies. Psychophysiology 33: 334–53. [Google Scholar] [CrossRef] [PubMed]
- Polich, John. 2007. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology 118: 2128–48. [Google Scholar] [CrossRef] [PubMed]
- Price, Darren, Lorraine K. Tyler, Rafael Neto Henriques, Karen L. Campbell, Nitin Williams, Matthias S. Treder, Jason R. Taylor, amCAN, and Richard N. A. Henson. 2017. Age-related delay in visual and auditory evoked responses is mediated by white- and grey-matter differences. Nature Communications 8: 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ratcliff, Roger. 1978. A theory of memory retrieval. Psychological Review 85: 59–108. [Google Scholar] [CrossRef]
- Ratcliff, Roger, Anjali Thapar, and Gail Mckoon. 2003. A diffusion model analysis of the effects of aging on brightness discrimination. Perception & Psychophysics 65: 523–35. [Google Scholar]
- Ratcliff, Roger, Anjali Thapar, Pablo Gómez, and Gail McKoon. 2004. A Diffusion Model Analysis of the Effects of Aging in the Lexical-Decision Task. Psychology and Aging 19: 278–89. [Google Scholar] [CrossRef]
- Ratcliff, Roger, Anjali Thapar, and Gail McKoon. 2010. Individual differences, aging, and IQ in two-choice tasks. Cognitive Psychology 60: 127–57. [Google Scholar] [CrossRef]
- Ratcliff, Roger, Anjali Thapar, and Gail McKoon. 2011. Effects of aging and IQ on item and associative memory. Journal of Experimental Psychology: General 140: 464–87. [Google Scholar] [CrossRef]
- Rhemtulla, Mijke, and Elliot Tucker-Drob. 2011. Correlated longitudinal changes across linguistic, achievement, and psychomotor domains in early childhood: Evidence for a global dimension of development. Developmental Science 14: 1245–54. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, Annie, Andrea M. Piccinin, Graciela Muniz-Terrera, Lesa Hoffman, Boo Johansson, Dorly J. H. Deeg, Marja J. Aartsen, Hannie C. Comijs, and Scott Hofer. 2013. Longitudinal mediation of processing speed on age-related change in memory and fluid intelligence. Psychology and Aging 28: 887–901. [Google Scholar] [CrossRef] [PubMed]
- Rosseel, Yves. 2012. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software 48: 1–36. [Google Scholar] [CrossRef]
- Salthouse, Timothy A. 1985. Speed of behavior and its implications for cognition. In Handbook of the Psychology of Aging, 2nd ed. Edited by James E. Birren and K. Warner Schaie. New York: Van Nostrand Reinhold. [Google Scholar]
- Salthouse, Timothy A. 1996. The processing-speed theory of adult age differences in cognition. Psychological Review 103: 403–28. [Google Scholar] [CrossRef]
- Salthouse, Timothy A. 2001. Structural models of the relations between age and measures of cognitive functioning. Intelligence 29: 93–115. [Google Scholar] [CrossRef]
- Salthouse, Timothy, and Emilio Ferrer-Caja. 2003. What needs to be explained to account for age-related effects on multiple cognitive variables? Psychology and Aging 18: 91–110. [Google Scholar] [CrossRef]
- Salthouse, Timothy A. 2004. Localizing age-related individual differences in a hierarchical structure. Intelligence 32: 541–61. [Google Scholar] [CrossRef]
- Salthouse, Timothy A. 2009. When does age-related cognitive decline begin? Neurobiology of Aging 30: 507–14. [Google Scholar] [CrossRef]
- Schaie, K. Warner. 2005. What Can We Learn From Longitudinal Studies of Adult Development? Research in Human Development 2: 133–58. [Google Scholar] [CrossRef]
- Schapkin, Sergei A., Patrick D. Gajewski, and Gabriele Freude. 2014. Age Differences in Memory-Based Task Switching With and Without Cues. Journal of Psychophysiology 28: 187–201. [Google Scholar] [CrossRef]
- Schmiedek, Florian, Klaus Oberauer, Oliver Wilhelm, Heinz-Martin Süß, and Werner W. Wittmann. 2007. Individual differences in components of reaction time distributions and their relations to working memory and intelligence. Journal of Experimental Psychology: General 136: 414–29. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, Florian, and Oliver Wilhelm. 2016. Modeling Mental Speed: Decomposing Response Time Distributions in Elementary Cognitive Tasks and Correlations with Working Memory Capacity and Fluid Intelligence. Journal of Intelligence 4: 13. [Google Scholar] [CrossRef]
- Schmitz, Florian, Dominik Rotter, and Oliver Wilhelm. 2018. Scoring Alternatives for Mental Speed Tests: Measurement Issues and Validity for Working Memory Capacity and the Attentional Blink Effect. Journal of Intelligence 6: 47. [Google Scholar] [CrossRef] [PubMed]
- Schubert, Anna-Lena, Dirk Hagemann, Andreas Voss, Andrea Schankin, and Katharina Bergmann. 2015. Decomposing the relationship between mental speed and mental abilities. Intelligence 51: 28–46. [Google Scholar] [CrossRef]
- Schubert, Anna-Lena, Gidon T. Frischkorn, Dirk Hagemann, and Andreas Voss. 2016. Trait Characteristics of Diffusion Model Parameters. Journal of Intelligence 4: 7. [Google Scholar] [CrossRef]
- Schubert, Anna-Lena, Dirk Hagemann, and Gidon T. Frischkorn. 2017. Is general intelligence little more than the speed of higher-order processing? Journal of Experimental Psychology: General 146: 1498–512. [Google Scholar] [CrossRef]
- Schubert, Anna-Lena, Michael D. Nunez, Dirk Hagemann, and Joachim Vandekerckhove. 2019. Individual Differences in Cortical Processing Speed Predict Cognitive Abilities: A Model-Based Cognitive Neuroscience Account. Computational Brain & Behavior 2: 64–84. [Google Scholar] [CrossRef]
- Sowell, Elizabeth R., Bradley Peterson, Paul Thompson, Suzanne E. Welcome, Amy L. Henkenius, and Arthur W. Toga. 2004. Mapping Changes in the Human Cortex throughout the Span of Life. The Neuroscientist 10: 372–92. [Google Scholar] [CrossRef]
- Spaniol, Julia, Andreas Voss, and Cheryl L. Grady. 2008. Aging and emotional memory: cognitive mechanisms underlying the positivity effect. Psychology and Aging 23: 859–72. [Google Scholar] [CrossRef]
- Sternberg, Robert J. 1977. Intelligence, Information Processing, and Analogical Reasoning: The Componential Analysis of Human Abilities. Oxford: Lawrence Erlbaum. [Google Scholar]
- Theisen, Maximilian, Veronika Lerche, Mischa von Krause, and AndreasVoss. 2019. Slower, but why? A meta-analysis on age differences in diffusion model parameters. Presented at the 2019 Meeting of the European Mathematical Psychology Group (EMPG), Heidelberg, Germany, August 5–7. [Google Scholar]
- Troche, Stefan Johannes, Michael E. Houlihan, Robert M. Stelmack, and Thomas H. Rammsayer. 2009. Mental ability, P300, and mismatch negativity: Analysis of frequency and duration discrimination. Intelligence 37: 365–73. [Google Scholar] [CrossRef]
- Troche, Stefan Johannes, Rebekka Indermühle, Hartmut Leuthold, and Thomas H. Rammsayer. 2015. Intelligence and the psychological refractory period: A lateralized readiness potential study. Intelligence 53: 138–44. [Google Scholar] [CrossRef]
- Tucker-Drob, Elliot. 2011. Global and Domain-Specific Changes in Cognition throughout Adulthood. Developmental Psychology 47: 331–43. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Drob, Elliot, Daniel A Briley, John M. Starr, and Ian J. Deary. 2014. Structure and correlates of cognitive aging in a narrow age cohort. Psychology and Aging 29: 236–49. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghen, Paul, and Timothy A. Salthouse. 1997. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychological Bulletin 122: 231–49. [Google Scholar] [CrossRef]
- Voss, Voss, Klaus Rothermund, and Jochen Voss. 2004. Interpreting the parameters of the diffusion model: An empirical validation. Memory & Cognition 32: 1206–20. [Google Scholar] [CrossRef]
- Voss, Andreas, and Jochen Voss. 2007. Fast-dm: A free program for efficient diffusion model analysis. Behavior Research Methods 39: 767–75. [Google Scholar] [CrossRef]
- Yap, Melvin J., David A. Balota, Daragh E. Sibley, and Roger Ratcliff. 2012. Individual differences in visual word recognition: Insights from the English Lexicon Project. Journal of Experimental Psychology: Human Perception and Performance 38: 53–79. [Google Scholar] [CrossRef]
- Zimprich, Daniel, and Mike Martin. 2002. Can longitudinal changes in processing speed explain longitudinal age changes in fluid intelligence? Psychology and Aging 17: 690–95. [Google Scholar] [CrossRef]
| ACC | RT | v | a | t0 | P2 Latency | N2 latency | P3 Latency | |
|---|---|---|---|---|---|---|---|---|
| Sternberg task | ||||||||
| S3 | 0.98 (0.02) | 799.30 (203.15) | 2.93 (0.91) | 1.85 (0.74) | 0.46 (0.12) | 237.41 (40.13) | 253.49 (50.21) | 362.62 (91.23) |
| S5 | 0.97 (0.03) | 961.99 (279.98) | 2.44 (0.98) | 2.00 (0.89) | 0.53 (0.16) | 237.54 (35.41) | 253.02 (50.27) | 387.50 (94.89) |
| Posner task | ||||||||
| PI | 0.99 (0.02) | 629.79 (100.31) | 4.25 (1.50) | 1.59 (0.77) | 0.43 (0.08) | 220.63 (37.97) | 246.80 (42.80) | 411.03 (94.46) |
| NI | 0.98 (0.02) | 707.39 (113.15) | 3.26 (1.07) | 1.52 (0.47) | 0.46 (0.07) | 225.03 (30.51) | 244.78 (36.63) | 418.36 (88.49) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, A.-L.; Hagemann, D.; Löffler, C.; Frischkorn, G.T. Disentangling the Effects of Processing Speed on the Association between Age Differences and Fluid Intelligence. J. Intell. 2020, 8, 1. https://doi.org/10.3390/jintelligence8010001
Schubert A-L, Hagemann D, Löffler C, Frischkorn GT. Disentangling the Effects of Processing Speed on the Association between Age Differences and Fluid Intelligence. Journal of Intelligence. 2020; 8(1):1. https://doi.org/10.3390/jintelligence8010001
Chicago/Turabian StyleSchubert, Anna-Lena, Dirk Hagemann, Christoph Löffler, and Gidon T. Frischkorn. 2020. "Disentangling the Effects of Processing Speed on the Association between Age Differences and Fluid Intelligence" Journal of Intelligence 8, no. 1: 1. https://doi.org/10.3390/jintelligence8010001
APA StyleSchubert, A.-L., Hagemann, D., Löffler, C., & Frischkorn, G. T. (2020). Disentangling the Effects of Processing Speed on the Association between Age Differences and Fluid Intelligence. Journal of Intelligence, 8(1), 1. https://doi.org/10.3390/jintelligence8010001






