Mapping Mind-Brain Development: Towards a Comprehensive Theory
Abstract
1. Introduction
- Is the architecture of mind suggested by psychological research reflected in the organization and functioning of the brain?
- What are the neuronal processes implementing mental processes and mechanisms, such as abstraction and reasoning?
- How are developmental changes in gross parameters of the brain (such as increases in neuronal volume, myelination, networking, and pruning) reflected in developmental changes of cognitive processes?
- Are there systematic individual differences in brain architecture and functioning that may be connected to individual differences in intellectual functioning and development?
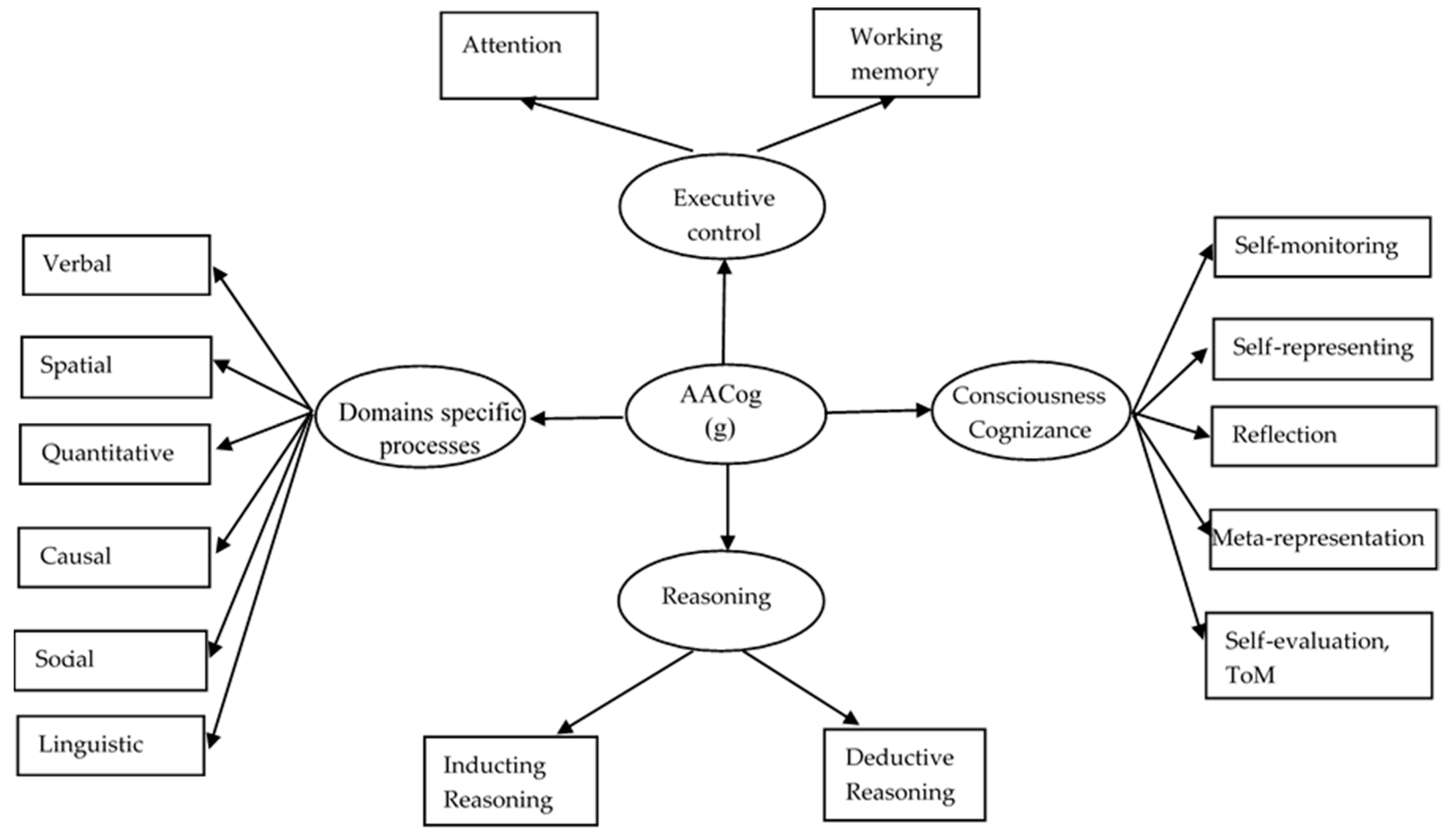
2. Architecture
2.1. Domain-Specific Mental Processes
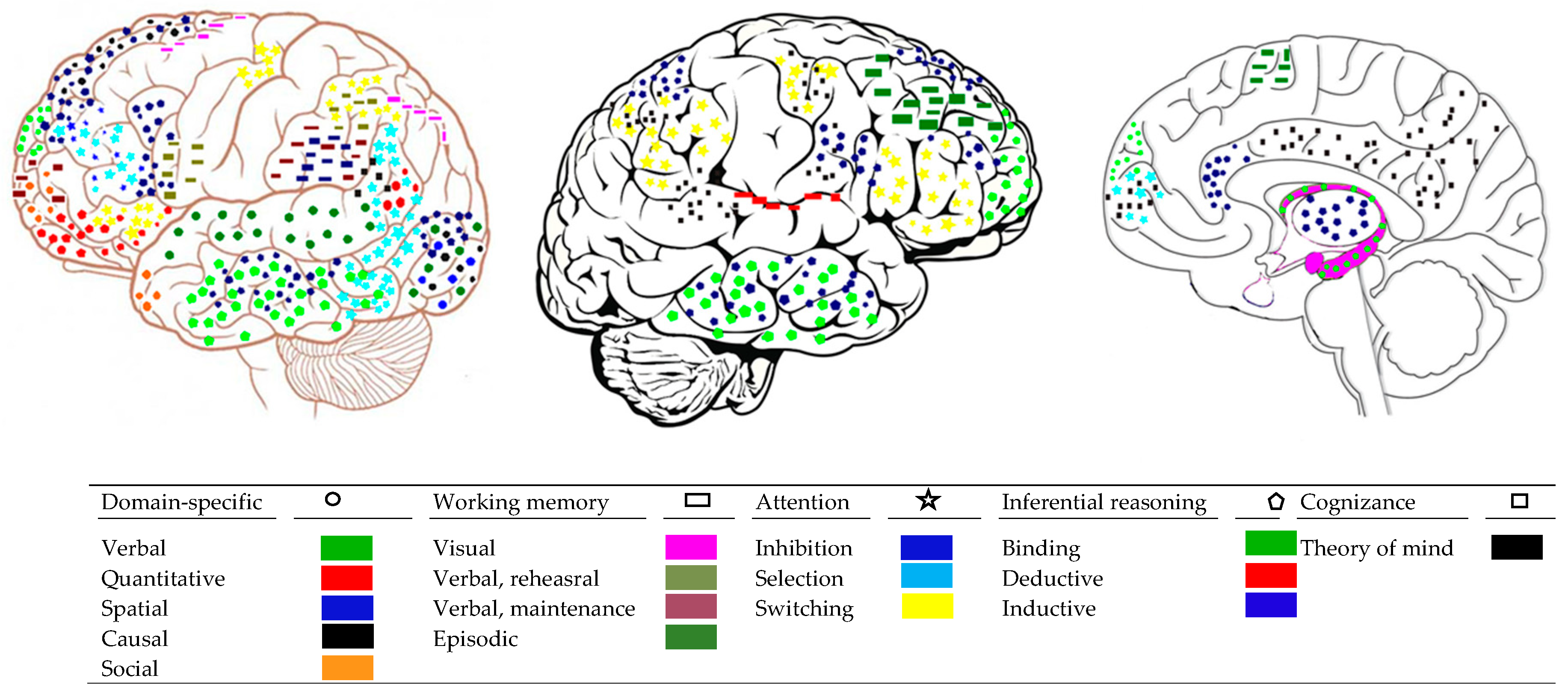
2.2. Brain Networks for Domain-Specific Mental Processes
2.2.1. Mental Systems for Attention and Working Memory
2.2.2. Brain Systems for Attention and Working Memory
Attention
Working Memory Processes
2.3. Integrative Mental Processes
2.3.1. Brain Processes Serving Intergative Mental Processes
AACog
Reasoning
Cognizance
2.3.2. Communication between Brain Networks
3. Development
3.1. Generally Accepted Assumptions about Cognitive Development
3.2. Developmental Cycles in Cognitive Development
3.3. Changes in the Integrative Processes of the Mind
3.4. The Role of Cognizance in the Development of Integrative Processes
3.5. Developmental Changes in the Brain
3.5.1. Changes in Brain Structures and Networks
3.5.2. Cycles in the Functional Aspects of Brain Development
4. Individual Differences
5. Conclusions
5.1. Mental and Brain Architecture
5.2. Mental and Neuronal Processes
5.3. Mental and Brain Development
5.4. Unanswered Questions and Predictions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, Michael L. 2014. After Phrenology: Neural Reuse and the Interactive Brain. New York: Bradford. [Google Scholar]
- Anderson, John R., and Jon M. Fincham. 2014. Extending problem-solving procedures through reflection. Cognitive Psychology 74: 1–34. [Google Scholar] [CrossRef] [PubMed]
- Anobile, Giovanni, Guido Marco Cicchini, and David C. Burr. 2016. Number as a primary perceptual attribute: A review. Perception 45: 5–31. [Google Scholar] [CrossRef] [PubMed]
- Baars, Bernard J. 1993. A Cognitive Theory of Consciousness. Cambridge: Cambridge University Press. [Google Scholar]
- Baddeley, Alan. 2012. Working memory: Theories, models, and controversies. Annual Review of Psychology 63: 1–29. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, James M. 1906. Mental Development in the Child and the Race: Methods and Processes. New York: Macmillan. [Google Scholar]
- Barbey, Aron K. 2018. Network neuroscience theory of human intelligence. Trends in Cognitive Sciences 22: 8–20. [Google Scholar] [CrossRef] [PubMed]
- Barrouillet, Pierre, and Jean-Francois Lecas. 1999. Mental models in conditional reasoning and working memory. Thinking and Reasoning 5: 289–302. [Google Scholar] [CrossRef]
- Basten, Ulrike, Kirsten Hilger, and Christian J. Fiebach. 2015. Where smart brains are different: A quantitative meta-analysis of functional and structural brain imaging studies on intelligence. Intelligence 51: 10–27. [Google Scholar] [CrossRef]
- Baumann, Oliver, Edgar Chan, and Jason B. Mattingley. 2012. Distinct neural networks underlie encoding of categorical versus coordinate spatial relations during active navigation. Neuroimage 60: 1630–37. [Google Scholar] [CrossRef]
- Benasich, April A., Zhenkun Gou, Naseem Choudhury, and Kenneth D. Harris. 2008. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research 195: 215–22. [Google Scholar] [CrossRef]
- Blair, Clancy. 2006. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral and Brain Sciences 29: 109–25. [Google Scholar] [CrossRef]
- Boly, Melanie, Anil K. Seth, Melanie Wilke, Paul Ingmundson, Bernard Baars, Steven Laureys, David Edelman, and Naotsugu Tsuchiya. 2013. Consciousness in humans and non-human animals: Recent advances and future directions. Frontiers in Psychology 4: 625. [Google Scholar] [CrossRef]
- Bosseler, Alexis, Samu Taulu, Elina Pihko, Jyrki Mäkelä, Toshiaki Imada, Antti Ahonen, and Patricia Kuhl. 2013. Theta brain rhythms index perceptual narrowing in infant speech perception. Frontiers in Psychology 4: 690. [Google Scholar] [CrossRef]
- Brodmann, Korbinian. 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien Dargestellt auf Grund des Zellenbaues. Leipzig: Barth. [Google Scholar]
- Buzsáki, György, and Brendon O. Watson. 2012. Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues in Clinical Neuroscience 14: 345. [Google Scholar] [PubMed]
- Buzsáki, György, Nikos Logothetis, and Wolf Singer. 2013. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 80: 751–64. [Google Scholar] [CrossRef] [PubMed]
- Cao, Yinan, Christopher Summerfield, Hame Park, Bruno Lucio Giordano, and Christoph Kayser. 2019. Causal inference in the multisensory brain. Neuron 102: 1076–87. [Google Scholar] [CrossRef] [PubMed]
- Carey, Susan. 2009. The Origin of Concepts. New York: Oxford University Press. [Google Scholar]
- Carlén, Marie. 2017. What constitutes the prefrontal cortex? Science 358: 478–82. [Google Scholar] [CrossRef]
- Carroll, John B. 1993. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. New York: Cambridge University Press. [Google Scholar]
- Case, Robbie. 1992. The Mind’s Staircase: Exploring the Conceptual Underpinnings of Children’s Thought and Knowledge. New York: Psychology Press. [Google Scholar]
- Case, Robbie, Andreas Demetriou, Maria Platsidou, and Smaragda Kazi. 2001. Integrating concepts and tests of intelligence from the differential and developmental traditions. Intelligence 29: 307–36. [Google Scholar] [CrossRef]
- Chevalier, Nicolas, and Agnès Blaye. 2016. Metacognitive monitoring of executive control engagement during childhood. Child Development 87: 1264–76. [Google Scholar] [CrossRef]
- Christoforides, Michael, George Spanoudis, and Andreas Demetriou. 2016. Coping with Logical Fallacies: A Developmental Training Program for Learning to Reason. Child Development 87: 1856–76. [Google Scholar] [CrossRef]
- Clarke, Alex, Brooke M. Roberts, and Charan Ranganath. 2018. Neural oscillations during conditional associative learning. NeuroImage 174: 485–93. [Google Scholar] [CrossRef]
- Conway, Martin A. 2005. Memory and the self. Journal of Memory and Language 53: 594–628. [Google Scholar] [CrossRef]
- Cowan, Nelson. 2010. The magical mystery four how is working memory capacity limited, and why? Current Directions in Psychological Science 19: 51–57. [Google Scholar] [CrossRef]
- Cowey, Carolyn M. 1996. Hippocampal sclerosis on working memory. Memory 4: 19–30. [Google Scholar] [CrossRef]
- Crick, Francis C., and Christof Koch. 2005. What is the function of the claustrum? Philosophical Transactions of the Royal Society B: Biological Sciences 360: 1271–79. [Google Scholar] [CrossRef] [PubMed]
- de Bie, Henrica M. A., Maria Boersma, Sofie Adriaanse, Dick J. Veltman, Alle Meije Wink, Stefan D. Roosendaal, Frederik Barkhof, Cornelis J. Stam, Kim J. Oostrom, Henriette A. Delemarre-van de Waal, and et al. 2012. Resting-state networks in awake five- to eight-year old children. Human Brain Mapping 33: 1189–201. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, Stanislas. 2011. The Number Sense: How the Mind Creates Mathematics. Oxford: Oxford University Press. [Google Scholar]
- Dehaene, Stanislas, Hakwan Lau, and Sid Kouider. 2017. What is consciousness, and could machines have it? Science 358: 486–92. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, Andreas, and Karin Bakracevic. 2009. Reasoning and self-awareness from adolescence to middle age: Organization and development as a function of education. Learning and Individual Differences 19: 181–94. [Google Scholar] [CrossRef]
- Demetriou, Andreas, and Smaragda Kazi. 2006. Self-awareness in g with processing efficiency and reasoning. Intelligence 34: 297–317. [Google Scholar] [CrossRef]
- Demetriou, Andreas, and George Spanoudis. 2018. Growing Minds: A Developmental Theory of Intelligence, Brain, and Education. London: Routledge. [Google Scholar]
- Demetriou, Andreas, Anastasia Efklides, Maria Platsidou, and Robert L. Campbell. 1993. The architecture and dynamics of developing mind: Experiential structuralism as a frame for unifying cognitive developmental theories. Monographs of the Society for Research in Child Development 58: 234. [Google Scholar] [CrossRef]
- Demetriou, Andreas P., Constantinos Christou, George C. Spanoudis, and Maria Platsidou. 2002. The development of mental processing: Efficiency, working memory, and thinking. Monographs of the Society of Research in Child Development 67: 268. [Google Scholar]
- Demetriou, Andreas, George Spanoudis, Michael Shayer, Antigoni Mouyi, Smaragda Kazi, and Maria Platsidou. 2013. Cycles in speed-working memory-G relations: Towards a developmental-differential theory of the mind. Intelligence 41: 34–50. [Google Scholar] [CrossRef]
- Demetriou, Andreas, George Spanoudis, Michael Shayer, Sanne Van der Ven, Christopher R. Brydges, Evelyn Kroesbergen, Gal Podjarny, and H. Lee Swanson. 2014. Relations between speed, working memory, and intelligence from preschool to adulthood: Structural equation modeling of 15 studies. Intelligence 46: 107–21. [Google Scholar] [CrossRef]
- Demetriou, Andreas, George Spanoudis, Smaragda Kazi, Antigoni Mouyi, Mislav Stjepan Žebec, Elena Kazali, Hudson Golino, Karin Bakracevic, and Michael Shayer. 2017. Developmental differentiation and binding of mental processes with g through the life-span. Journal of Intelligence 5: 23. [Google Scholar] [CrossRef]
- Demetriou, Andreas, Nikolaos Makris, George Spanoudis, Smaragda Kazi, Michael Shayer, and Elena Kazali. 2018. Mapping the Dimensions of General Intelligence: An Integrated Differential-Developmental Theory. Human Development 61: 4–42. [Google Scholar] [CrossRef]
- Demetriou, Andreas, Samuel Greiff, Nikolaos Makris, George Spanoudis, Rita Panaoura, and Smaragda Kazi. 2020. Bridging educational priorities with developmental Priorities: Towards a developmental theory of instruction. Educational Research Review. Submitted. [Google Scholar]
- Diamond, Adele. 2013. Executive functions. Annual Review of Psychology 64: 135–68. [Google Scholar] [CrossRef] [PubMed]
- Dövencioğlu, Dicle, Hiroshi Ban, Andrew J. Schofield, and Andrew E. Welchman. 2013. Perceptual integration for qualitatively different 3-D cues in the human brain. Journal of Cognitive Neuroscience 25: 1527–41. [Google Scholar] [CrossRef] [PubMed]
- Dumontheil, Iroise. 2014. Development of abstract thinking during childhood and adolescence: The role of rostrolateral prefrontal cortex. Developmental Cognitive Neuroscience 10: 57–76. [Google Scholar] [CrossRef]
- Eguchi, Akihiro, Samuel A. Neymotin, Greg D. Horwitz, and Thomas D. Albright. 2017. Representation of color. Reference Module in Neuroscience and Biobehavioral Psychology. [Google Scholar] [CrossRef]
- Epstein, Herman T. 1986. Stages in human development. Developmental Brain Research 30: 114–19. [Google Scholar] [CrossRef]
- Fairhall, Scott L. 2020. Cross recruitment of domain-selective cortical representations enables flexible semantic knowledge. The Journal of Neuroscience 19. [Google Scholar] [CrossRef]
- Fedorenko, Evelina, and Idan A. Blank. 2020. Broca’s area is not a natural kind. Trends in Cognitive Sciences. [Google Scholar] [CrossRef]
- Fleming, Stephen M., Rimona S. Weil, Zoltan Nagy, Raymond J. Dolan, and Geraint Rees. 2010. Relating Introspective Accuracy to Individual Differences in Brain Structure. Science 329: 1541–43. [Google Scholar] [CrossRef]
- Fodor, Jerry A. 1975. The Language of Thought. New York: Thomas Y. Crowell. [Google Scholar]
- Fonlupt, Pierre. 2003. Perception and judgement of physical causality involve different brain structures. Cognitive Brain Research 17: 248–54. [Google Scholar] [CrossRef]
- Fransson, Peter, Ulrika Åden, Mats Blennow, and Hugo Lagercrantz. 2011. The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral Cortex 21: 145–54. [Google Scholar] [CrossRef] [PubMed]
- Friedman, H. R., and Patricia S. Goldman-Rakic. 1988. Activation of the hippocampus and dentate gyrus by working memory: A 2-deoxyglucos study of behaving rhesus monkeys. Journal of Neuroscience 8: 4693–706. [Google Scholar] [CrossRef] [PubMed]
- Galaburda, Albert M. 2002. The neuroanatomy of categories. In The Languages of the Brain. Edited by Albert M. Galaburda, Stephen M. Kosslyn and Yves Christen. Cambridge: Harvard University Press. [Google Scholar]
- Gauthier, Isabel, Pawel Skudlarski, John C. Gore, and Adam W. Anderson. 2000. Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience 3: 191. [Google Scholar] [CrossRef] [PubMed]
- Gelman, Susan A. 2003. The Essential Child: Origins of Essentialism in Everyday Thought. New York: Oxford University Press. [Google Scholar]
- Gentner, Dedre. 2005. The development of relational category knowledge. In Building Object Categories in Developmental Time. Edited by Lisa Gershkoff-Stowe and David H. Rakison. New York: Psychology Press. [Google Scholar]
- Gignac, Gilles E., and Timothy C. Bates. 2017. Brain volume and intelligence: The moderating role of intelligence measurement quality. Intelligence 64: 18–29. [Google Scholar] [CrossRef]
- Glasser, Matthew F., Timothy S. Coalson, Emma C. Robinson, Carl D. Hacker, John Harwell, Essa Yacoub, Kamil Ugurbil, Jesper Andersson, Christian F. Beckmann, Mark Jenkinson, and et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature 536: 171–78. [Google Scholar] [CrossRef] [PubMed]
- Goel, Vinod, and Raymond J. Dolan. 2000. Anatomical segregation of component processes in an inductive inference task. Journal of Cognitive Neuroscience 12: 110–19. [Google Scholar] [CrossRef] [PubMed]
- Goel, Vinod, and Raymond J. Dolan. 2001. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia 39: 901–9. [Google Scholar] [CrossRef]
- Goel, Vinod, Christian Buchel, Chris Frith, and Raymond J. Dolan. 2000. Dissociation of mechanisms underlying syllogistic reasoning. NeuroImage 12: 504–14. [Google Scholar] [CrossRef]
- Golino, Hudson F., and Andreas Demetriou. 2017. Estimating the dimensionality of intelligence like data using Exploratory Graph Analysis. Intelligence 62: 54–70. [Google Scholar] [CrossRef]
- Gou, Zhenkun, Naseem Choudhury, and April A. Benasich. 2011. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research 220: 263–70. [Google Scholar] [CrossRef] [PubMed]
- Gruber, Oliver, and Thomas Goschke. 2004. Executive control emerging from dynamic interactions between brain systems mediating language, working memory and attentional processes. Acta Psychologica 115: 105–21. [Google Scholar] [CrossRef] [PubMed]
- Gruber, Oliver, and D. Yves von Cramon. 2003. The functional neuroanatomy of human working memory revisited Evidence from 3-T fMRI studies using classical domain-specific interference tasks. NeuroImage 19: 797–809. [Google Scholar] [CrossRef]
- Güler, O. Evren, and Kathleen M. Thomas. 2013. Developmental differences in the neural correlates of relational encoding and recall in children: An event-related fMRI study. Developmental Cognitive Neuroscience 3: 106–16. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, Jan-Eric. 1984. A unifying model for the structure of intellectual abilities. Intelligence 8: 179–203. [Google Scholar] [CrossRef]
- Haier, Richard J. 2016. The Neuroscience of Intelligence. Cambridge: Cambridge University Press. [Google Scholar]
- Halford, Graeme S., William H. Wilson, and Steven Phillips. 1998. Processing capacity defined by relational complexity: Implications for comparative, developmental, and cognitive psychology. Behavioral and Brain Sciences 21: 803–31. [Google Scholar] [CrossRef]
- Harris, Irina M., Gary F. Egan, Cynon Sonkkila, Henri J. Tochon-Danguy, George Paxinos, and John D. G. Watson. 2000. Selective right parietal lobe activation during mental rotation. Brain 123: 65–73. [Google Scholar] [CrossRef]
- Haxby, James V., Leslie G. Ungerleider, Barry Horwitz, Jose Ma Maisog, Stanley I. Rapoport, and Cheryl L. Grady. 1996. Face encoding and recognition in the human brain. Proceedings of the National Academy of Sciences 93: 922–27. [Google Scholar] [CrossRef]
- Hermer, Linda, and Elizabeth Spelke. 1996. Modularity and development: The case of spatial reorientation. Cognition 61: 195–232. [Google Scholar] [CrossRef]
- Hill, William D., Gail Davies, Andrew M. McIntosh, Catharine R. Gale, and Ian J. Deary. 2017. A combined analysis of genetically correlated traits identifies 107 loci associated with intelligence. BioRxiv, 160291. [Google Scholar] [CrossRef]
- Hoff, G. E., Martijn Van Den Heuvel, Manon J. N. L. Benders, Karina J. Kersbergen, and Linda S. de Vries. 2013. On development of functional brain connectivity in the young brain. Frontiers in Human Neuroscience, 650. [Google Scholar] [CrossRef] [PubMed]
- Holmboe, Karla, Arielle Bonneville-Roussy, Gergely Csibra, and Mark H. Johnson. 2017. Longitudinal development of attention and inhibitory control during the first year of life. Developmental Science 21: e12690. [Google Scholar] [CrossRef]
- Hudspeth, William J., and Karl H. Pribram. 1992. Psychophysiological indices of cerebral maturation. International Journal of Psychophysiology 12: 19–29. [Google Scholar] [CrossRef]
- Hwang, Kai, Michael N. Hallquist, and Beatriz Luna. 2012. The development of hub architecture in the human functional brain network. Cerebral Cortex 23: 2380–93. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Kai, Avniel S. Ghuman, Dara S. Manoach, Stephanie R. Jones, and Beatriz Luna. 2016. Frontal preparatory neural oscillations associated with cognitive control: A developmental study comparing young adults and adolescents. NeuroImage 136: 139–48. [Google Scholar] [CrossRef] [PubMed]
- Ide, Jaime S., Pradeep Shenoy, J. Yu Angela, and R. Li Chiang-Shan. 2013. Bayesian prediction and evaluation in the anterior cingulate cortex. Journal of Neuroscience 33: 2039–47. [Google Scholar] [CrossRef]
- Irrmischer, Mona, Simon-Shlomo Poil, Huibert D. Mansvelder, Francesca Sangiuliano Intra, and Klaus Linkenkaer-Hansen. 2018. Strong long-range temporal correlations of beta/gamma oscillations are associated with poor sustained visual attention performance. European Journal of Neuroscience 46: 2674–83. [Google Scholar] [CrossRef]
- Jaušovec, Norbert, and Ksenija Jaušovec. 2014. Increasing working memory capacity with theta transcranial alternating current stimulation (tACS). Biological Psychology 96: 42–47. [Google Scholar] [CrossRef]
- Jensen, Arthur R. 1998. Human Evolution, Behavior, and Intelligence. The g Factor: The Science of Mental Ability. Westport: Praeger. [Google Scholar]
- Jensen, Ole, and John E. Lisman. 2005. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends in Neurosciences 28: 67–72. [Google Scholar] [CrossRef]
- Jonides, John, Steven C. Lacey, and Derek Evan Nee. 2005. Processes of working memory in mind and brain. Current Directions of Psychological Science 14: 2–5. [Google Scholar] [CrossRef]
- Jung, Rex E., and Richard J. Haier. 2007. The Parieto-Frontal Integration Theory P-FIT of Intelligence: Converging Neuroimaging Evidence. Behavioral and Brain Sciences 30: 135–54. [Google Scholar] [CrossRef] [PubMed]
- Kail, Robert V. 2007. Longitudinal evidence that increases in processing speed and working memory enhance children’s reasoning. Psychological Science 18: 312. [Google Scholar] [CrossRef] [PubMed]
- Kanwisher, Nancy, Josh McDermott, and Marvin M. Chun. 1997. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience 17: 4302–11. [Google Scholar] [CrossRef] [PubMed]
- Kawamichi, Hiroaki, Yoshiaki Kikuchi, Madoka Noriuchi, Atsushi Senoo, and Shoogo Ueno. 2007. Distinct neural correlates underlying two- and three-dimensional mental rotations using three-dimensional objects. Brain Research 1144: 117–26. [Google Scholar] [CrossRef] [PubMed]
- Kazi, Smaragda, Elena Kazali, Nikolaos Makris, George Spanoudis, and Andreas Demetriou. 2019. Cognizance in cognitive development: A longitudinal study. Cognitive Development 52: 100805. [Google Scholar] [CrossRef]
- Khundrakpam, Budhachandra S., John D. Lewis, Andrew Reid, Sherif Karama, Lu Zhao, Francois Chouinard-Decorte, Alan C. Evans, and Brain Development Cooperative Group. 2017. Imaging structural covariance in the development of intelligence. Neuroimage 144: 227–240. [Google Scholar] [CrossRef]
- Klingberg, Torkel. 1998. Concurrent performance of two working memory tasks: Potential mechanisms of interference. Cerebral Cortex 8: 593–601. [Google Scholar] [CrossRef]
- Kovacs, Kristof, and Andrew R. A. Conway. 2016. Process overlap theory: A unified account of the general factor of intelligence. Psychological Inquiry 27: 151–77. [Google Scholar] [CrossRef]
- Kurth, Salomé, Maya Ringli, Anja Geiger, Monique LeBourgeois, Oskar G. Jenni, and Reto Huber. 2010. Mapping of cortical activity in the first two decades of life: A high-density sleep electroencephalogram study. Journal of Neuroscience 30: 13211–19. [Google Scholar] [CrossRef]
- Kyllonen, Patrick C., and Raymond E. Christal. 1990. Reasoning ability is little more than working memory capacity? Intelligence 14: 389–433. [Google Scholar] [CrossRef]
- Lisman, John. 2005. T The theta/gamma discrete phase code occuring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus 15: 913–22. [Google Scholar] [CrossRef] [PubMed]
- Lisman, John E., and Ole Jensen. 2013. The Theta-Gamma neural code. Neuron 77: 1002–16. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, Margaret, and David Hubel. 1988. Segregation of form, color, movement, and depth: Anatomy, physiology, and perception. Science 240: 740–49. [Google Scholar] [CrossRef]
- Mackey, Allyson P., Alison T. Miller Singley, and Silvia A. Bunge. 2013. Intensive reasoning training alters patterns of brain connectivity at rest. Journal of Neuroscience 33: 4796–803. [Google Scholar] [CrossRef]
- Maguire, Mandy J., Matthew R. Brier, and Thomas C. Ferree. 2010. EEG theta and alpha responses reveal qualitative differences in processing taxonomic versus thematic semantic relationships. Brain and Language 114: 16–25. [Google Scholar] [CrossRef] [PubMed]
- Mahon, Bradford Z., and Alfonso Caramazza. 2011. What drives the organization of object knowledge in the brain? Trends in Cognitive Sciences 15: 97–103. [Google Scholar] [CrossRef]
- Makris, Nikolaos, Dimitrios Tachmatzidis, Andreas Demetriou, and George Spanoudis. 2017. M Mapping the evolving core of intelligence: Changing relations between executive control, reasoning, language, and awareness. Intelligence 62: 12–30. [Google Scholar] [CrossRef]
- Marek, Scott, Kai Hwang, William Foran, Michael N. Hallquist, and Beatriz Luna. 2015. The contribution of network organization and integration to the development of cognitive control. PLoS Biology 13: e1002328. [Google Scholar] [CrossRef]
- Marshall, Peter J., Yair Bar-Haim, and Nathan A. Fox. 2002. Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology 113: 1199–208. [Google Scholar] [CrossRef]
- Martínez, Kenia, Sarah K. Madsen, Anand A. Joshi, Shantanu H. Joshi, Francisco J. Roman, Julio Villalon-Reina, Miguel Burgaleta, Sherif Karama, Joost Janssen, Eugenio Marinetto, and et al. 2015. Reproducibility of brain-cognition relationships using three cortical surface-based protocols: An exhaustive analysis based on cortical thickness. Human Brain Mapping 36: 3227–45. [Google Scholar] [CrossRef]
- Mashour, George A., Pieter Roelfsema, Jean-Pierre Changeux, and Stanislas Dehaene. 2020. Conscious processing and the Global Neuronal Workspace Hypothesis. Neuron 105. [Google Scholar] [CrossRef] [PubMed]
- Meeter, Martijn, Catherine E. Myers, and Mark A. Gluck. 2005. Integrating incremental learning and episodic memory models of the hippocampal region. Psychological Review 112: 560. [Google Scholar] [CrossRef] [PubMed]
- Menon, Vinod, and Lucina Q. Uddin. 2010. Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function 214: 655–67. [Google Scholar] [CrossRef] [PubMed]
- Millán, Ana P., J. J. Torres, S. Johnson, and J. Marro. 2018. Concurrence of form and function in developing networks and its role in synaptic pruning. Nature Communications 9: 1–10. [Google Scholar] [CrossRef]
- Miyake, Akira, and Naomi P. Friedman. 2012. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science 21: 8–14. [Google Scholar] [CrossRef]
- Moreno-Juan, Verónica, Anton Filipchuk, Noelia Antón-Bolanos, Cecilia Mezzera, Henrik Gezelius, Belen Andrés, Luis Rodríguez-Malmierca, Rafael Susín, Olivier Schaad, Takuji Iwasato, and et al. 2017. Prenatal thalamic waves regulate cortical area size prior to sensory processing. Nature Communications 8: 14172. [Google Scholar] [CrossRef]
- Moshman, David. 2004. Adolescent Rationality and Development: Cognition, Morality, Identity. New York: Psychology Press. [Google Scholar]
- Nieder, Andreas, and Stanislas Dehaene. 2009. Representation of number in the brain. Annual Review of Neuroscience 32: 185–208. [Google Scholar] [CrossRef]
- Orekhova, E. V., T. A. Stroganova, I. N. Posikera, and M. Elam. 2006. EEG theta rhythm in infants and preschool children. Clinical Neurophysiology 117: 1047–62. [Google Scholar] [CrossRef]
- Osherson, Daniel, Daniela Perani, Stefano Cappa, Tatiana Schnur, Franco Grassi, and Ferruccio Fazio. 1998. Distinct brain loci in deductive versus probabilistic reasoning. Neuropsychologia 36: 369–76. [Google Scholar] [CrossRef]
- Papageorgiou, Eleni, Constantinos Christou, George Spanoudis, and Andreas Demetriou. 2016. Augmenting intelligence: Developmental limits to learning-based cognitive change. Intelligence 56: 16–27. [Google Scholar] [CrossRef]
- Pascual-Leone, Juan. 1970. A mathematical model for the transition rule in Piaget’s developmental stages. Acta psychologica 63: 301–45. [Google Scholar] [CrossRef]
- Patterson, Karalyn, Peter J. Nestor, and Timothy T. Rogers. 2007. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience 8: 976. [Google Scholar] [CrossRef]
- Pennartz, Cyriel M. A. 2015. The Brain’s Representational Power: On Consciousness and the Integration of Modalities. London: MIT Press. [Google Scholar]
- Peters, Lien, and Bert De Smedt. 2018. Arithmetic in the developing brain: A review of brain imaging studies. Developmental Cognitive Neuroscience 30: 265–79. [Google Scholar] [CrossRef] [PubMed]
- Petersen, Steven E., and Michael I. Posner. 2012. The attention system of the human brain: 20 years after. Annual Review of Neuroscience 35: 73–89. [Google Scholar] [CrossRef] [PubMed]
- Piaget, Jean. 1970. Piaget’s theory. In Carmichael’s Handbook of Child Development. Edited by Paul H. Mussen. New York: Wiley. [Google Scholar]
- Piaget, Jean. 2014. Studies in Reflecting Abstraction. London: Psychology Press. [Google Scholar]
- Piazza, Manuela, Philippe Pinel, Denis Le Bihan, and Stanislas Dehaene. 2007. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53: 293–305. [Google Scholar] [CrossRef]
- Posner, Michael I., and Mary K. Rothbart. 2007. Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology 58: 1–23. [Google Scholar] [CrossRef]
- Protzko, John. 2015. The environment in raising early intelligence: A meta-analysis of the fadeout effect. Intelligence 53: 202–10. [Google Scholar] [CrossRef]
- Ranganath, Charan, and Mark D’Esposito. 2005. Directing the mind’s eye. Prefrontal, inferior, and medial temporal mechanisms for visual working memory. Current Opinion in Neurobiology 15: 175–82. [Google Scholar] [CrossRef]
- Reinhart, Robert M. G. 2017. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proceedings of the National Academy of Sciences of the United States of America 114: 11542–47. [Google Scholar] [CrossRef]
- Repovš, Grega, and Alan Baddeley. 2006. The multi-component model of working memory: Explorations in experimental cognitive psychology. Neuroscience 139: 5–21. [Google Scholar] [CrossRef]
- Reverberi, Carlo, Doris Pischedda, Michele Burigo, and Paolo Cherubini. 2012. Deduction without awareness. Acta Psychologica 139: 244–53. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, Elena I., Catarina I. Barriga-Paulino, María A. Rojas-Benjumea, and Carlos M. Gómez. 2015. Co-maturation of theta and low-beta rhythms during child development. Brain Topography 28: 250–60. [Google Scholar] [CrossRef]
- Rolfs, Martin, Michael Dambacher, and Patrick Cavanagh. 2013. Visual adaptation of the perception of causality. Current Biology 23: 250–54. [Google Scholar] [CrossRef]
- Roser, Matthew E., Jonathan A. Fugelsang, Kevin N. Dunbar, Paul M. Corballis, and Michael S. Gazzaniga. 2005. Dissociating processes supporting causal perception and causal inference in the brain. Neuropsychology 19: 591. [Google Scholar] [CrossRef]
- Rothbart, Mary K., and Michael I. Posner. 2015. The developing brain in a multitasking world. Developmental Review 35: 42–63. [Google Scholar] [CrossRef]
- Rounis, Elisabeth, Brian Maniscalco, John C. Rothwell, Richard E. Passingham, and Hakwan Lau. 2010. Theta-burst transcranial magnetic stimulation to the prefrontal cortex impairs metacognitive visual awareness. Cognitive Neuroscience 1: 165–75. [Google Scholar] [CrossRef]
- Rubin, Rachael, Hillary Schwarb, Heather Lucas, Michael Dulas, and Neal Cohen. 2017. Dynamic hippocampal and prefrontal contributions to memory processes and representations blur the boundaries of traditional cognitive domains. Brain Sciences 7: 82. [Google Scholar] [CrossRef]
- Rueda, M. Rosario, Michael I. Posner, and Mary K. Rothbart. 2005. The development of executive attention: Contributions to the emergence of self-regulation. Developmental Neuropsychology 28: 573–94. [Google Scholar] [CrossRef]
- Sabbagh, Mark A., Lindsay C. Bowman, Lyndsay E. Evraire, and Jennie M. B. Ito. 2009. Neurodevelopmental Correlates of Theory of Mind in Preschool Children. Child Development 80: 1147–62. [Google Scholar] [CrossRef]
- Salehi, Mehraveh, Amin Karbasi, Daniel S. Barron, Dustin Scheinost, and R. Todd Constable. 2020. Individualized functional networks reconfigure with cognitive state. NeuroImage 206: 116233. [Google Scholar] [CrossRef]
- Sauseng, Paul, Birgit Griesmayr, Roman Freunberger, and Wolfgang Klimesch. 2010. Control mechanisms in working memory: A possible function of EEG theta oscillations. Neuroscience & Biobehavioral Reviews 34: 1015–22. [Google Scholar]
- Saxe, Rebecca, and Susan Carey. 2006. The perception of causality in infancy. Acta Psychologica 123: 144–65. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K. Suzanne, John A. Sweeney, and Beatriz Luna. 2006. Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience 18: 1045–58. [Google Scholar] [CrossRef] [PubMed]
- Scott, Ryan B., Zoltan Dienes, Adam B. Barrett, Daniel Bor, and Anil K. Seth. 2014. Blind insight: Metacognitive discrimination despite chance task performance. Psychological Science 25: 2199–208. [Google Scholar] [CrossRef]
- Segneri, Marco, Hongjie Bi, Simona Olmi, and Alessandro Torcini. 2020. Theta-nested gamma oscillations in next generation neural mass models. arXiv Preprint arXiv:2003.04000. [Google Scholar]
- Sergent, Claire, and Lionel Naccache. 2012. Imaging neural signatures of consciousness: ‘What’, ‘When’, ‘Where’ and ‘How’ does it work? Archives Italiennes de Biologie 150: 91–106. [Google Scholar]
- Shipstead, Zach, Thomas S. Redick, and Randall W. Engle. 2012. Is working memory training effective? Psychological Bulletin 138: 628. [Google Scholar] [CrossRef]
- Siegler, Robert S. 2016. Continuity and change in the field of cognitive development and in the perspectives of one cognitive developmentalist. Child Development Perspectives 10: 128–33. [Google Scholar] [CrossRef]
- Simion, Francesca, Viola Macchi Cassia, Chiara Turati, and Eloisa Valenza. 2001. The origins of face perception: Specific versus non-specific mechanisms. Infant and Child Development: An International Journal of Research and Practice 10: 59–65. [Google Scholar] [CrossRef]
- Sniekers, Suzanne, Sven Stringer, Kyoko Watanabe, Philip R. Jansen, Jonathan R. I. Coleman, Eva Krapohl, Erdogan Taskesen, Anke R. Hammerschlag, Aysu Okbay, Delilah Zabaneh, and et al. 2017. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nature Genetics 49: 1107. [Google Scholar] [CrossRef]
- Somsen, Riek J. M., Ben J. van’t Klooster, Maurits W. van der Molen, Harry M. P. van Leeuwen, and Rob Licht. 1997. Growth spurts in brain maturation during middle childhood as indexed by EEG power spectra. Biological Psychology 44: 187–209. [Google Scholar] [CrossRef]
- Spanoudis, George, Andreas Demetriou, Smargada Kazi, Katerina Giorgala, and Valentina Zenonos. 2015. Embedding cognizance in intellectual development. Journal of Experimental Child Psychology 132: 32–50. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C. E. 1904. General intelligence, objectively determined and measured. American Journal of Psychology 15: 201–92. [Google Scholar] [CrossRef]
- Sporns, Olaf. 2016. Networks of the Brain. Cambridge: MIT Press. [Google Scholar]
- Sporns, Olaf, Dante R. Chialvo, Marcus Kaiser, and Claus C. Hilgetag. 2004. Organization, development and function of complex brain networks. Trends in Cognitive Sciences 8: 418–25. [Google Scholar] [CrossRef]
- Stiles, Joan, and Terry L. Jernigan. 2010. The basics of brain development. Neuropsychology Review 20: 327–48. [Google Scholar] [CrossRef]
- Striedter, George F. 2005. Principles of Brain Evolution. Sunderland: Sinauer Associates. [Google Scholar]
- Sun, Hua-Chun, Hiroshi Ban, Massimiliano Di Luca, and Andrew E. Welchman. 2015. fMRI evidence for areas that process surface gloss in the human visual cortex. Vision Research 109: 149–57. [Google Scholar] [CrossRef]
- Sun, Chen, Wannan Yang, Jared Martin, and Susumu Tonegawa. 2020. Hippocampal neurons represent events as transferable units of experience. Nature Neuroscience, 1–13. [Google Scholar] [CrossRef]
- Supekar, Kaustubh, Lucina Q. Uddin, Katherine Prater, Hitha Amin, Michael D. Greicius, and Vinod Menon. 2010. Development of functional and structural connectivity within the default mode network in young children. NeuroImage 52: 290–301. [Google Scholar] [CrossRef]
- Taylor, P., J. N. Hobbs, J. Burroni, and H. T. Siegelmann. 2015. The global landscape of cognition: Hierarchical aggregation as an organizational principle of human cortical networks and functions. Scientific Reports 5: 18112. [Google Scholar] [CrossRef]
- Tenenbaum, Joshua B., Charles Kemp, Thomas L. Griffiths, and Noah D. Goodman. 2011. How to grow a mind: Statistics, structure, and abstraction. Science 331: 1279–85. [Google Scholar] [CrossRef]
- Testolin, Alberto, Youzhi Zou, and James L. McClelland. 2020. Numerosity discrimination in deep neural networks: Initial competence, developmental refinement and experience statistics. Developmental Science. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, Robert W. 1992. Cyclic cortical reorganization during early childhood. Brain and Cognition 20: 24–50. [Google Scholar] [CrossRef]
- Thatcher, Robert W. 1994. Cyclic cortical reorganization: Origins of human cognitive development. In Human Behavior and the Developing Brain. Edited by Dawson Geraldine and Kurt W. Fischer. New York: The Guilford Press. [Google Scholar]
- Thatcher, Robert W., Duane North, and C. Biver. 2005. EEG and intelligence: Relations between EEG coherence, EEG phase delay and power. Clinical Neurophysiology 116: 2129–41. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R. W., E. Palmero-Soler, D. M. North, and C. J. Biver. 2016. Intelligence and eeg measures of information flow: Efficiency and homeostatic neuroplasticity. Scientific Reports 6: 38890. [Google Scholar] [CrossRef] [PubMed]
- Thelen, Esther, and John P. Spencer. 1998. Postural control during reaching in young infants: A dynamic systems approach. Neuroscience & Biobehavioral Reviews 22: 507–14. [Google Scholar]
- Thompson, Paul M., Jay N. Giedd, Roger P. Woods, David MacDonald, Alan C. Evans, and Arthur W. Toga. 2000. Growth patterns in the developing brain detected by using continuum tensor maps. Nature 404: 190. [Google Scholar] [CrossRef]
- Thut, Gregor, and Carlo Miniussi. 2009. New insights into rhythmic brain activity from TMS–EEG studies. Trends in Cognitive Sciences 134: 182–89. [Google Scholar] [CrossRef]
- Tran, Randy, and Harold Pashler. 2017. Learning to exploit a hidden predictor in skill acquisition: Tight linkage to conscious awareness. PLoS ONE 12: e0179386. [Google Scholar] [CrossRef]
- Van Der Maas, Han, Kees-Jan Kan, Maarten Marsman, and Claire E. Stevenson. 2017. Network models for cognitive development and intelligence. Journal of Intelligence 5: 16. [Google Scholar] [CrossRef]
- Vendetti, Michael S., and Silvia A. Bunge. 2014. Evolutionary and developmental changes in the lateral frontoparietal network: A little goes a long way for higher-level cognition. Neuron 84: 906–17. [Google Scholar] [CrossRef]
- Vergauwe, Evie, Egbert Hartstra, Pierre Barrouillet, and Marcel Brass. 2015. Domain-general involvement of the posterior frontolateral cortex in time-based resource-sharing in working memory: An fMRI study. NeuroImage 115: 104–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wellman, Henry M. 2014. Making Minds: How Theory of Mind Develops. Oxford University Press. [Google Scholar]
- Wendelken, Carter, Emilio Ferrer, Kirstie J. Whitaker, and Silvia A. Bunge. 2015. Fronto-Parietal network reconfiguration supports the development of reasoning ability. Cerebral Cortex 26: 2178–90. [Google Scholar] [CrossRef] [PubMed]
- Wendelken, Carter, Emilio Ferrer, Simona Ghetti, Stephen K. Bailey, Laurie Cutting, and Silvia A. Bunge. 2017. Frontoparietal structural connectivity in childhood predicts development of functional connectivity and reasoning ability: A large-scale longitudinal investigation. Journal of Neuroscience 37: 8549–58. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, Charlotte Grosse, Jan Schreiber, Tania Singer, Nikolaus Steinbeis, and Angela D. Friederici. 2017. White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nature Communications 8: 14692. [Google Scholar] [CrossRef]
- Wokke, Martijn E., Dalila Achoui, and Axel Cleermans. 2020. Action information contributes to metacognitive decision-making. Scientific Reports 10: 3632. [Google Scholar] [CrossRef]
- Xu, Fei. 2019. Towards a Rational Constructivist Theory of Cognitive Development. Psychological Review 126: 841–64. [Google Scholar] [CrossRef]
- Yoon, Taejib, Jeffrey Okada, Min W. Jung, and Jeansok J. Kim. 2008. Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learning & Memory 15: 97–105. [Google Scholar]
- Yue, Qiuhai, Randi C. Martin, Simon Fischer-Baum, Aurora I. Ramos-Nuñez, Fengdan Ye, and Michael W. Deem. 2017. Brain modularity mediates the relation between task complexity and performance. Journal of Cognitive Neuroscience 29: 1532–46. [Google Scholar] [CrossRef]
- Zamora-López, Gorka, Changsong Zhou, and Jürgen Kurths. 2010. Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks. Frontiers in Neuroinformatics 4: 1. [Google Scholar] [CrossRef]
- Zelazo, Philip David. 2015. Executive function: Reflection, iterative reprocessing, complexity, and the developing brain. Developmental Review 38: 55–68. [Google Scholar] [CrossRef]
- Zhen, Zonglei, Huizhen Fang, and Jia Liu. 2013. The hierarchical brain network for face recognition. PLoS ONE 8: e59886. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Xuebin, and Jagath C. Rajapakse. 2006. Learning functional structure from fMRI images. NeuroImage 31: 1601–13. [Google Scholar] [CrossRef] [PubMed]
| Domain | Core Processes | Mental Operations | Knowledge and Beliefs |
|---|---|---|---|
| Verbal | Perception according to perceptual similarity; inductive inferences based on similarity-difference relations | Specification of the semantic and logical relations between properties, classification; transformation of properties into mental objects; construction of conceptual systems | Conceptions and misconceptions about the world |
| Quantitative | Subitization; counting, pointing, bringing in, removing, sharing | Monitoring, reconstruction, execution and control of quantitative transformations, the four arithmetic operations | Factual knowledge about the quantitative aspects of the world, algebraic and statistical inference rules |
| Spatial | Perception of size, depth, and orientation; formation of mental images | Mental rotation, image integration, image reconstruction, location and direction tracking and reckoning | Stored mental images, mental maps, and scripts about objects, locations, scenes, or layouts maintained in the mind |
| Causal | Perception of overt and covert causal relations | Trial and error; combinatorial operations; hypothesis formation; systematic experimentation (isolation of variables); model construction | Knowledge, attributions and understanding of the reasons underlying physical and social events and the dynamic aspects of the world |
| Social | Recognition of conspecifics, recognition of emotionally laden facial expressions | Deciphering the mental and emotional states and intentions of others; organization of actions accordingly; imitation; decentering and taking the other’s perspective | System of social attributions about other persons, their culture, and their society |
| Linguistic | Use of the grammatical and syntactical structures of language | Identifying truth in information; abstraction of information in goal-relevant ways; differentiation of the contextual from the formal elements; elimination of biases from inferential process; securing validity of inference | Knowledge about grammar, syntax and logical reasoning; metalogical knowledge about nature and justifiability of logical inferences; metacognitive awareness, knowledge, and control of inferential processes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanoudis, G.; Demetriou, A. Mapping Mind-Brain Development: Towards a Comprehensive Theory. J. Intell. 2020, 8, 19. https://doi.org/10.3390/jintelligence8020019
Spanoudis G, Demetriou A. Mapping Mind-Brain Development: Towards a Comprehensive Theory. Journal of Intelligence. 2020; 8(2):19. https://doi.org/10.3390/jintelligence8020019
Chicago/Turabian StyleSpanoudis, George, and Andreas Demetriou. 2020. "Mapping Mind-Brain Development: Towards a Comprehensive Theory" Journal of Intelligence 8, no. 2: 19. https://doi.org/10.3390/jintelligence8020019
APA StyleSpanoudis, G., & Demetriou, A. (2020). Mapping Mind-Brain Development: Towards a Comprehensive Theory. Journal of Intelligence, 8(2), 19. https://doi.org/10.3390/jintelligence8020019






