A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
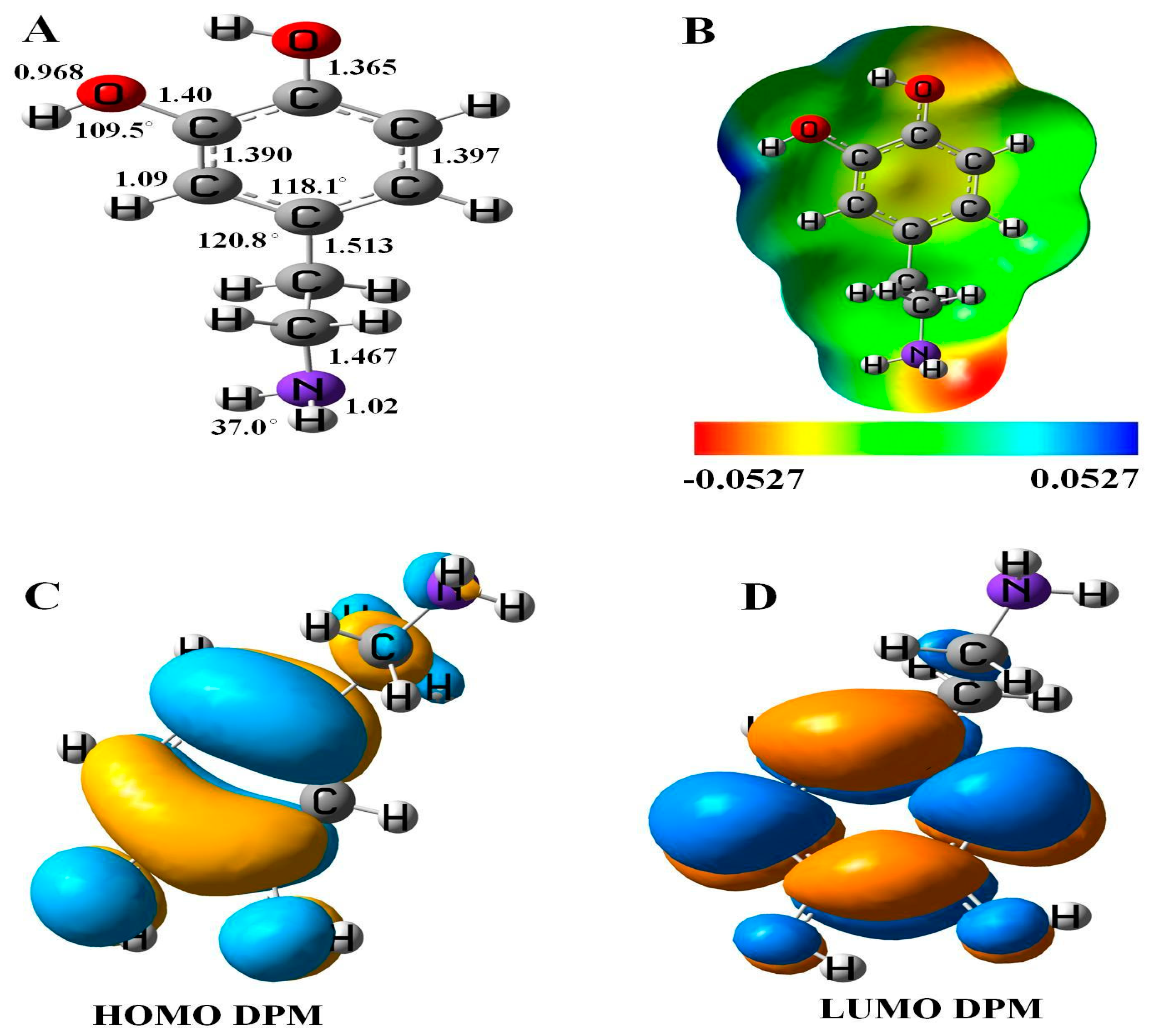
3.1. Characteristics of DPM
3.2. DPM Adsorption Phenomena upon the BN Nano-Cages
3.3. Adsorption of the Second DPM upon B12N12
3.4. DPM Adsorption Phenomena upon the BN Nanotubes
3.5. Adsorption of the DPM on the Al- and Ga-Doped B12N12
3.6. The Solvent Effect on the Adsorption Phenomenon
4. Conclusions
- The DPM shows different binding characteristics on various BN nanostructures.
- The adsorption of DPM on BN nano-cages is stronger than other BN nanotubes.
- The most stable adsorption configuration relates to a single DPM–B12N12 system with adsorption energy of −1.41 eV.
- The doped BN nano-cages with Al and Ga atoms exhibit dramatic changes in adsorption and electronic properties with respect to their pristine counterparts.
- The adsorption of DPM on the Ga-doped B12N12 systems is stronger than that of the other studied systems.
- The adsorption of DPM on the studied systems in water phase is stronger than the gas phase.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Kam, N.W.S.; Shim, M.; Li, Y.M.; Kim, W.; Utz, P.J.; Dai, H.J. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Zhang, Y.G.; Wang, D.W.; Dai, H.J. Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. J. Am. Chem. Soc. 2001, 123, 3838. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Liu, Y.C.; Wang, Q.; Shen, J.W.; Wu, T.; Guan, W.J. On the spontaneous encapsulation of proteins in carbon nanotubes. Biomaterials 2009, 30, 2807. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Partidos, C.D.; Graff, R.; Hoebeke, J.; Briand, J.P.; Prato, M.; Bianco, A.J. Synthesis, structural characterization, and immunological properties of carbon nanotubes functionalized with peptides. Am. Chem. Soc 2003, 125, 6160. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Joselevich, E.; Woolley, A.T.; Cheung, C.L.; Lieber, C.M. Covalently functionalized nanotubes as nanometre- sized probes in chemistry and biology. Nature 1998, 394, 52. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.Q.; Park, H.K.; Lieber, C.M. Nanowire Nanosensors for Highly Sensitive and Selective Detection of Biological and Chemical Species. Science 2001, 293, 1289. [Google Scholar] [CrossRef]
- Benyamini, H.; Shulman-Peleg, A.; Wolfson, H.J.; Belgorodsky, B.; Fadeev, L.; Gozin, M. Interaction of C60-Fullerene and Carboxyfullerene with Proteins: Docking and Binding Site Alignment. Bioconjugate Chem 2006, 17, 378. [Google Scholar] [CrossRef]
- Hong, R.; Fischer, N.O.; Verma, A.; Goodman, C.M.; Emrick, T.; Rotello, V.M. Control of Protein Structure and Function through Surface Recognition by Tailored Nanoparticle Scaffolds. J. Am. Chem. Soc 2004, 126, 739. [Google Scholar] [CrossRef]
- You, C.C.; Agasti, S.S.; De, M.; Knapp, M.J.; Rotello, V.M. Modulation of the Catalytic Behavior of α-Chymotrypsin at Monolayer-Protected Nanoparticle Surfaces. J. Am. Chem. Soc 2006, 128, 14612. [Google Scholar] [CrossRef]
- Kia, M.; Golzar, M.; Mahjoub, K.; Soltani, A. A first-principles study of functionalized clusters and carbon nanotubes or fullerenes with 5-Aminolevulinic acid as vehicles for drug delivery. Superlattices Microstruct 2013, 62, 251–259. [Google Scholar] [CrossRef]
- Gallo, M.; Favila, A.; Glossman-Mitnik, D. DFT studies of functionalized carbon nanotubes and fullerenes as nanovectors for drug delivery of antitubercular compounds. Chem. Phys. Lett 2007, 447, 105–109. [Google Scholar] [CrossRef]
- Shukla, M.K.; Dubey, M.; Zakar, E.; Namburu, R.; Leszczynski, J. Density functional theory investigation of interaction of zigzag (7,0) single-walled carbon nanotube with Watson–Crick DNA base pairs. Chem. Phys. Lett 2010, 496, 128–132. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, R.; Zhang, D. Adsorption of formaldehyde molecule on the pristine and silicon-doped boron nitride nanotubes. Chem. Phys. Lett 2008, 467, 131–135. [Google Scholar] [CrossRef]
- Choi, H.; Park, Y.C.; Kim, Y.-H.; Lee, Y.S. Ambient Carbon Dioxide Capture by Boron-Rich Boron Nitride Nanotube. J. Am. Chem. Soc 2011, 133, 2084–2087. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.-Y.; Yang, B.-S.; Wu, H.-S. Ab initio investigation of hydrogenation of (BN)16: A comparison with that of (BN)12. J. Mol. Struct. THEOCHEM 2010, 941, 144–149. [Google Scholar] [CrossRef]
- Mirzaei, M.; Yousefi, M. Boron nitride nanotubes with quadrangular cross sections: Density functional studies. Superlattices Microstruct 2012, 52, 648–652. [Google Scholar] [CrossRef]
- Soltani, A.; Ahmadian, N.; Amirazami, A.; Masoodi, A.; Lemeski, E.T.; Moradi, A.V. Theoretical investigation of OCN− adsorption onto boron nitride nanotubes. Appl. Surf. Sci 2012, 261, 262–267. [Google Scholar] [CrossRef]
- Soltani, A.; Ahmadian, N.; Kanani, Y.; Dehno khalaji, A.; Mighani, H. Ab initio investigation of the SCN− chemisorption of single-walled boron nitride nanotubes. Appl. Surf. Sci 2012, 258, 9536–9543. [Google Scholar] [CrossRef]
- Beheshtian, J.; Peyghan, A.A.; Bagheri, Z. Detection of phosgene by Sc-doped BN nanotubes: A DFT study. Sens. Actuators B Chem 2012, 171–172, 846–852. [Google Scholar] [CrossRef]
- Ribeiroa, F.A.D.; Tarley, C.R.T.; Borges, K.B.; Pereira, A.C. Development of a square wave voltammetric method for dopamine determination using a biosensor based on multiwall carbon nanotubes paste and crude extract of Cucurbita pepo L. Sens. Actuators B Chem 2013, 185, 743–754. [Google Scholar] [CrossRef]
- Njagi, J.; Chernov, M.M.; Leiter, J.C.; Silvana, A. Amperometric Detection of Dopamine in Vivo with an Enzyme Based Carbon Fiber Microbiosensor. Anal. Chem 2010, 82, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Fei, J.; Hu, S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem 2003, 318, 100–106. [Google Scholar] [CrossRef]
- Saikia, N.; Pati, S.K.; Deka, R.C. First principles calculation on the structure and electronic properties of BNNTs functionalized with isoniazid drug molecule. Appl. Nanosci 2012, 2, 389–400. [Google Scholar] [CrossRef]
- Juárez, A.R.; Anota, E.C.; Cocoletzi, H.H.; Riveros, A.F. Adsorption of chitosan on BN nanotubes: A DFT investigation. Appl. Surf. Sci 2013, 268, 259–264. [Google Scholar]
- Mukhopadhyay, S.; Scheicher, R.H.; Pandey, R.; Karna, S.P. Sensitivity of Boron Nitride Nanotubes toward Biomolecules of Different Polarities. J. Phys. Chem. Lett 2011, 2, 2442–2447. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Gowtham, S.; Scheicher, R.H.; Pandey, R.; Karna, S.P. Theoretical study of physisorption of nucleobases on boron nitride nanotubes: a new class of hybrid nano-biomaterials. Nanotechnology 2010, 21, 165703. [Google Scholar] [CrossRef]
- Baei, M.T.; Taghartapeh, M.R.; Lemeski, E.T.; Soltani, A. A computational study of adenine, uracil, and cytosine adsorption upon AlN and BN nano-cages. Physica B 2014, 444, 6–13. [Google Scholar] [CrossRef]
- Soltani, A.; Baei, M.T.; Lemeski, E.T.; Shahini, M. Sensitivity of BN nano-cages to caffeine and nicotine molecules. Superlattices Microstruct 2014, 76, 315–325. [Google Scholar] [CrossRef]
- Baei, M.T.; Taghartapeh, M.R.; Lemeski, E.T.; Soltani, A. Computational study of OCN− chemisorption over AlN nanostructures. Superlattices Microstruct 2014, 72, 370–382. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02-SMP; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry, III. The role of exact exchange. J. Chem. Phys 1993, 98, 5648–5654. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Anota, E.C.; Cocoletzi, G.H. GGA-based analysis of the metformin adsorption on BN nanotubes. Physica E 2014, 56, 134–140. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.; Liu, S.J. Electrophilicity Index. Am. Chem. Soc 1999, 121, 1922. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: companion parameter to absolute electronegativity. J. Am. Chem. Soc 1983, 105, 7512. [Google Scholar] [CrossRef]
- Mohammad-Shiri, H.; Ghaemi, M.; Riahi, S.; Akbari-Sehat, A. Computational and electrochemical studies on the redox reaction of dopamine in aqueous solution. Int. J. Electrochem. Sci 2011, 6, 317–336. [Google Scholar]
- Ge, B.; Tan, Y.; Xie, Q.; Ma, M.; Yao, S. Preparation of chitosan–dopamine-multiwalled carbon nanotubes nanocomposite for electrocatalytic oxidation and sensitive electroanalysis of NADH. Sens. Actuators B 2009, 137, 547–554. [Google Scholar] [CrossRef]
- Xuan, Y.; Jiang, G.; Li, Y.; Wang, J.; Geng, H. Inhibiting effect of dopamine adsorption and polymerization on hydrated swelling of montmorillonite. Colloids Surf. A Physicochem. Eng. Asp 2013, 422, 50–60. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A.E. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian Inc.: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Lewars, E. Computational Chemistry - Introduction to the Theory and Applications of Molecular and Quantum Mechanics; Kluwer Academic: Norwell, MA, USA, 2003. [Google Scholar]
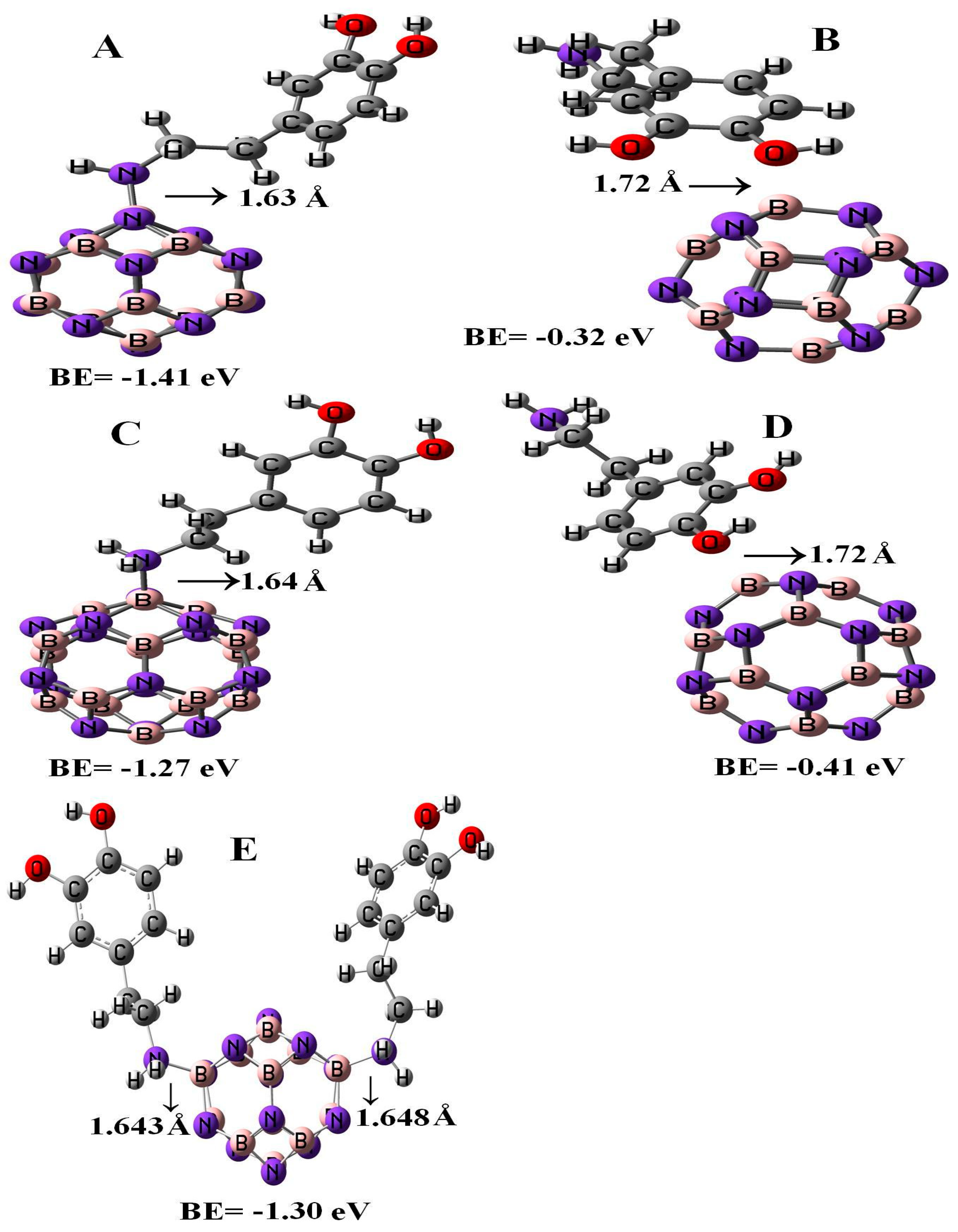




| System | B12N12 | DPM/B12N12 | B16N16 | DPM/ B16N16 | Two DPM/B12N12 |
|---|---|---|---|---|---|
| RB-N/Å | 1.486 | 1.569 | 1.473 | 1.557 | 1.567 |
| RB-N-B/¼ | 80.5 | 84.0 | 78.5 | 82.4 | 83.78 |
| RN-B-N/¼ | 98.2 | 91.7 | 99.3 | 92.9 | 92.0 |
| D/Å | - | 1.635 | - | 1.645 | 1.643 |
| Ead/eV | - | −1.41 | - | −1.27 | −1.30 |
| QB/e | 0.440 | 0.619 | 0.437 | 0.665 | 0.630 |
| QN/e | −0.440 | −0.512 | −0.437 | −0.514 | −0.511 |
| ΔN/e | - | −0.11 | - | −0.15 | −0.12 |
| EHOMO/eV | −7.71 | −5.98 | −7.38 | −5.98 | −5.73 |
| ELUMO/eV | −0.87 | −0.27 | −1.01 | −0.29 | −0.01 |
| Eg/eV | 6.84 | 5.71 | 6.37 | 5.69 | 5.72 |
| ∆Eg (%) | - | −19.8 | - | −10.7 | −16.37 |
| EFL/eV | −4.29 | −3.12 | −4.20 | −3.13 | −2.87 |
| μ/eV | −4.29 | −3.13 | −4.19 | −3.13 | −2.87 |
| η/eV | 3.42 | 2.86 | 3.19 | 2.84 | 2.86 |
| ω/eV | 2.69 | 1.71 | 2.76 | 1.73 | 1.44 |
| S/eV | 0.15 | 0.18 | 0.16 | 0.18 | 0.17 |
| DM/Debye | 0.0 | 9.69 | 0.0 | 9.78 | 7.58 |
| System | (6,0) BNNT | (5,5) BNNT | (8,0) BNNT | DPM/(6,0) BNNT | DPM/(5,5) BNNT | DPM/(8,0) BNNT |
|---|---|---|---|---|---|---|
| RB-N/Å | 1.488 | 1.450 | 1.449 | 1.511 | 1.463 | 1.510 |
| RB-N-B/¼ | 117.77 | 115.24 | 118.79 | 117.06 | 118.58 | 120.62 |
| RN-B-N/¼ | 119.85 | 119.62 | 119.97 | 114.26 | 119.23 | 114.54 |
| D/Å | - | - | - | 1.682 | 2.890 | 1.714 |
| Ead/eV | - | - | - | −0.77 | −0.12 | −0.51 |
| QB/e | 0.490 | 0.467 | 0.476 | 0.793 | 0.601 | 0.772 |
| QN/e | −0.490 | −0.467 | −0.476 | −0.564 | −0.459 | −0.553 |
| ΔN/e | - | - | - | −0.23 | −0.14 | −0.22 |
| EHOMO/eV | −6.62 | −6.40 | −6.47 | −5.94 | −5.51 | −5.94 |
| ELUMO/eV | −1.92 | −0.09 | −1.08 | −1.62 | 0.0 | −0.85 |
| Eg/eV | 4.70 | 6.31 | 5.39 | 4.32 | 5.51 | 5.09 |
| ∆Eg (%) | - | - | - | −8.08 | −12.68 | −5.56 |
| EFL/eV | −4.27 | −3.25 | −3.78 | −3.78 | −2.76 | −3.40 |
| μ/eV | −4.27 | −3.25 | −3.78 | −3.78 | −2.76 | −3.40 |
| η/eV | 2.35 | 3.20 | 2.70 | 2.16 | 2.76 | 2.54 |
| ω/eV | 3.88 | 1.67 | 2.64 | 3.31 | 1.38 | 2.26 |
| S/eV | 0.21 | 0.16 | 0.19 | 0.23 | 0.18 | 0.20 |
| DM/Debye | 7.96 | 0.00 | 11.87 | 10.06 | 3.98 | 12.89 |
| System | AlB11N12 | DPM/AlB11N12 | GaB11N12 | DPM/GaB11N12 |
|---|---|---|---|---|
| RAl-N/Å | 1.835 | 1.864 | - | - |
| RGa-N/Å | - | - | 1.905 | 1.923 |
| RAl-N-B/¼ | 83.6 | 84.9 | - | - |
| RN-Al-N/¼ | 83.2 | 81.1 | - | - |
| RGa-N-B/¼ | - | - | 84.9 | 85.9 |
| RN-Ga-N/¼ | - | - | 79.8 | 78.4 |
| D/Å | - | 1.998 | - | 2.05 |
| Ead/eV | - | –2.29 | - | –2.36 |
| QAl/e | 0.613 | 0.538 | - | - |
| QGa/e | - | - | 0.497 | 0.417 |
| QN/e | −0.548 | −0.575 | −0.613 | −0.592 |
| ΔN/e | - | −0.08 | - | −0.08 |
| EHOMO/eV | –7.31 | –6.14 | –7.34 | –6.10 |
| ELUMO/eV | –3.05 | −0.47 | –3.58 | −0.52 |
| Eg/eV | 4.26 | 5.67 | 3.76 | 5.58 |
| ∆Eg (%) | - | 33.1 | - | 48.4 |
| EFL/eV | –5.18 | –3.30 | –5.46 | –3.31 |
| μ/eV | –5.18 | –3.30 | –5.46 | –3.31 |
| η/eV | 2.13 | 2.83 | 1.88 | 2.79 |
| ω/eV | 6.30 | 1.92 | 7.93 | 1.96 |
| S/eV | 0.23 | 0.18 | 0.26 | 0.18 |
| DM/Debye | 3.24 | 12.49 | 2.76 | 12.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soltani, A.R.; Baei, M.T. A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine. Computation 2019, 7, 61. https://doi.org/10.3390/computation7040061
Soltani AR, Baei MT. A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine. Computation. 2019; 7(4):61. https://doi.org/10.3390/computation7040061
Chicago/Turabian StyleSoltani, Ali Reza, and Mohammad T. Baei. 2019. "A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine" Computation 7, no. 4: 61. https://doi.org/10.3390/computation7040061
APA StyleSoltani, A. R., & Baei, M. T. (2019). A DFT Study on Structure and Electronic Properties of BN Nanostructures Adsorbed with Dopamine. Computation, 7(4), 61. https://doi.org/10.3390/computation7040061





