Power Conversion Efficiency of Arylamine Organic Dyes for Dye-Sensitized Solar Cells (DSSCs) Explicit to Cobalt Electrolyte: Understanding the Structural Attributes Using a Direct QSPR Approach
Abstract
:1. Introduction
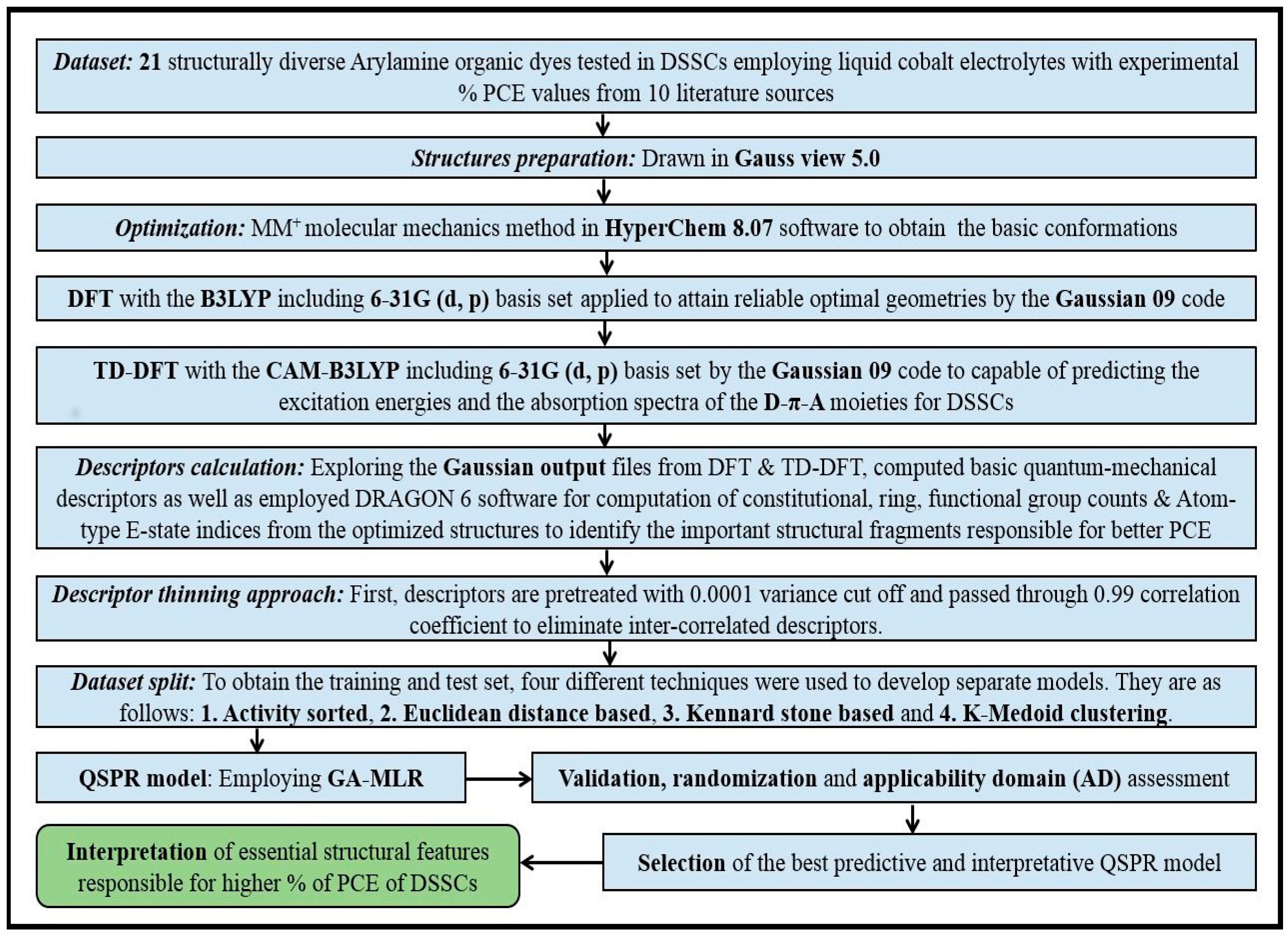
2. Methods and Materials
2.1. Dataset
2.2. Structure Preparation, Molecular and Quantum Chemical Calculations (DFT/TD-DFT)
2.3. Descriptor Selection
2.4. Data Pre-Processing
2.5. Dataset Splitting
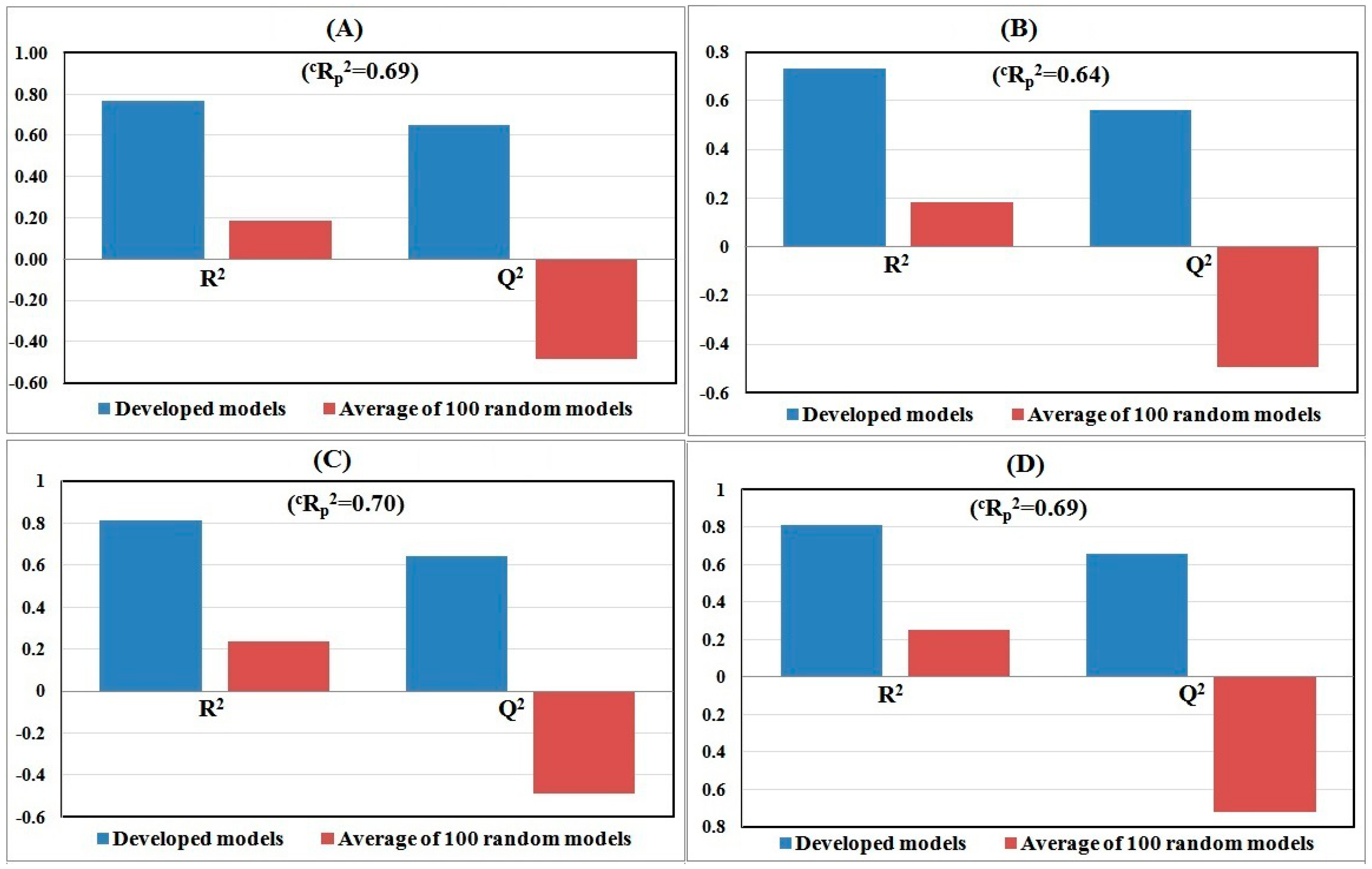
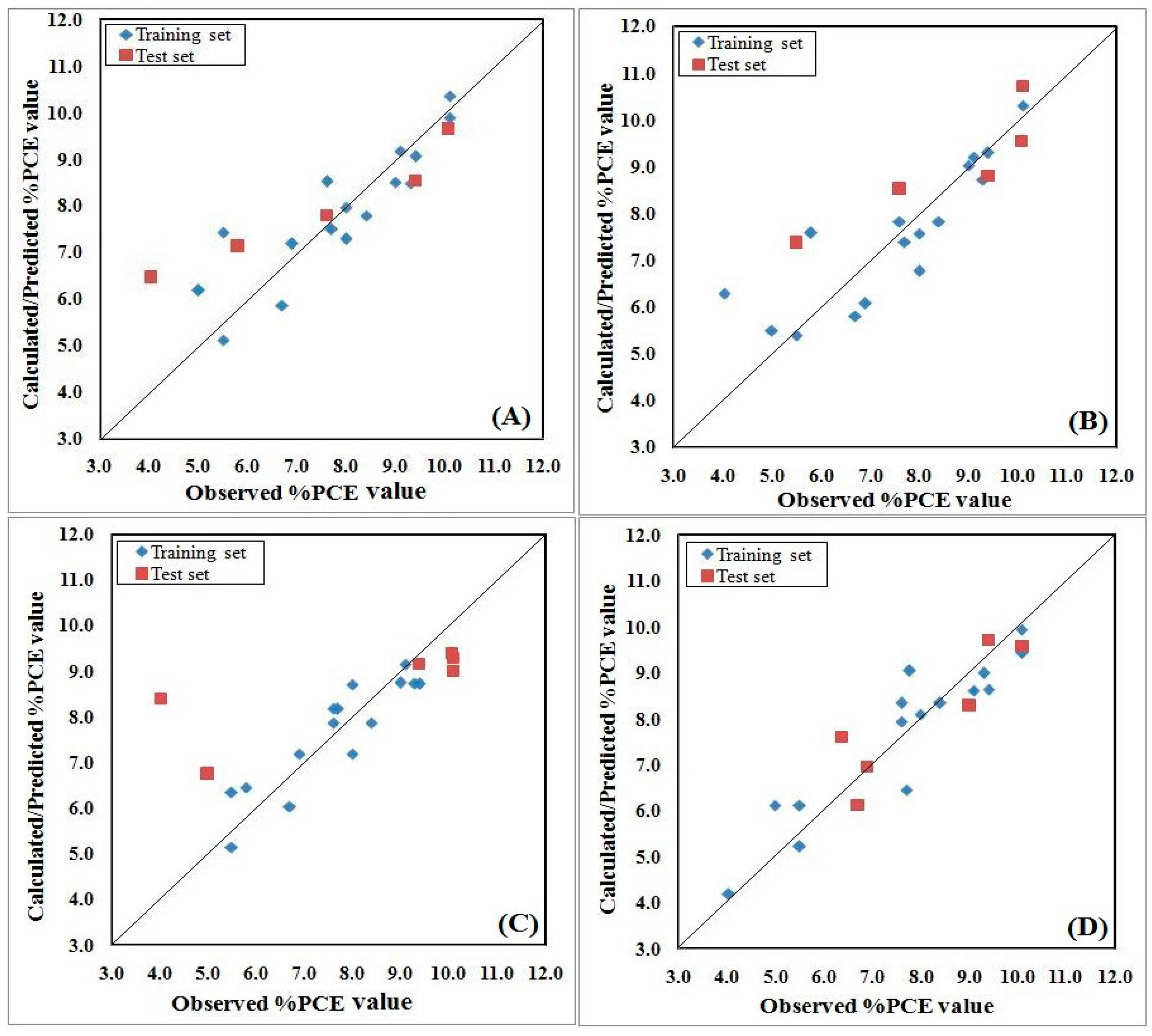
2.6. Model Development and Validation
2.7. Model Validation and Metrics
2.8. Y-Randomization
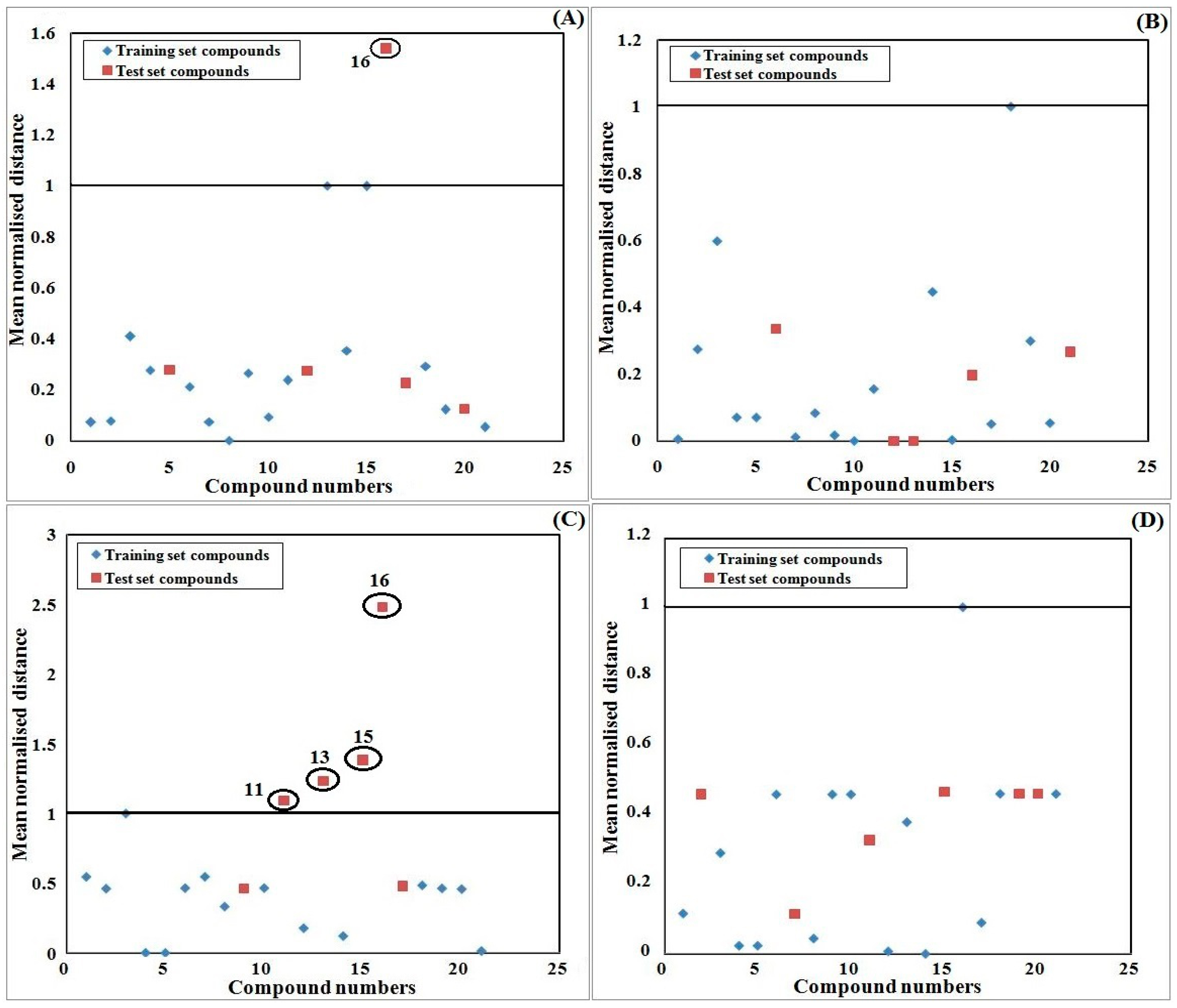
2.9. Applicability Domain Study
3. Results and Discussion
3.1. Computational Results
3.2. Interpretation of the Developed Model
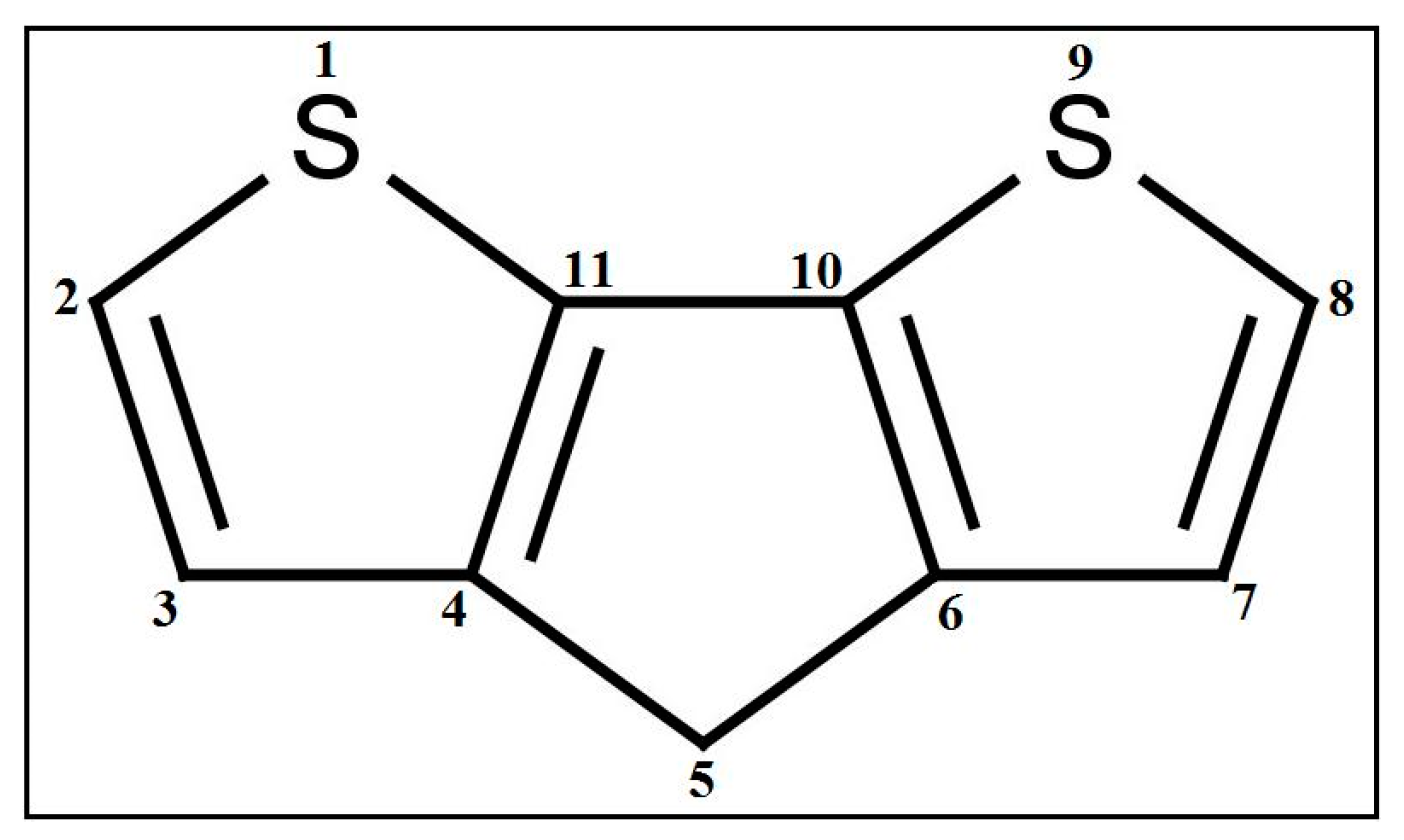
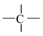 fragments in a molecule, and is a functional group count related to the number of total quaternary C(sp3). Although they are different descriptors, their impact on PCE is the same. A higher number of alkyl substitutions results in a high E-state () value along with the increment of count of sp3 carbon atoms. Structure and the orientation of dyes mostly dictate the efficiency of the solar cell via electron injection dynamics. In this regard, carboxyl plays the role of an anchor group and the longer alkyl chain is one of the most important entities in the dyes. The literature suggests [46] that J-aggregates formation becomes easier with the increasing length of the alkyl chain. Not only that, but the intrinsic sensitization or quantum efficiency is increased with the length of the alkyl chain due to the bathochromic shift of the absorption band [47]. Moreover, longer alkyl chains attached to the sensitizer dyes allow the dye to bind normally to the TiO2 surface [48]. Therefore, our findings are completely in agreement with the literature.
fragments in a molecule, and is a functional group count related to the number of total quaternary C(sp3). Although they are different descriptors, their impact on PCE is the same. A higher number of alkyl substitutions results in a high E-state () value along with the increment of count of sp3 carbon atoms. Structure and the orientation of dyes mostly dictate the efficiency of the solar cell via electron injection dynamics. In this regard, carboxyl plays the role of an anchor group and the longer alkyl chain is one of the most important entities in the dyes. The literature suggests [46] that J-aggregates formation becomes easier with the increasing length of the alkyl chain. Not only that, but the intrinsic sensitization or quantum efficiency is increased with the length of the alkyl chain due to the bathochromic shift of the absorption band [47]. Moreover, longer alkyl chains attached to the sensitizer dyes allow the dye to bind normally to the TiO2 surface [48]. Therefore, our findings are completely in agreement with the literature.4. Conclusions
- The QSPR model enables identification of the essential structural attributes necessary for quantifying the prime molecular prerequisites of a diverse AOD system that could guide the design and synthesis of more efficient dyes in the near future. The interpretation of the model revealed that a higher number of alkyl substitutions, along with the increment of count of sp3 carbon atoms and the combination of a distance/detour ring index of order 11 fragment, enable rapid electron injection into the semiconductor. This dynamic step allows efficient regeneration of the oxidized dye and helps to achieve a higher PCE value for an arylamine dye-sensitized solar cell explicit to cobalt electrolytes.
- The QSPR model, developed from a set of 21 diverse AOD, is an efficient tool to screen a wide range of AOD, allowing for the identification of dyes with high PCE in a time- and cost-effective manner.
- The developed QSPR model is particularly valuable for predicting and characterizing the nature of the donor:π-bridge:acceptor relationships critical for photoconversion.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kohn, W.; Heeger, A. The Power of the Sun. 2005. Available online: http:/powerofthesun.ucsb.edu (accessed on 10 September 2016).
- Kammen, D.M. The rise of renewable energy. Sci. Am. 2006, 295, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-Sensitized Solar Cells with Conversion Efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638–L640. [Google Scholar] [CrossRef]
- Regan, B.O.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt(II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Lo, Y.S. Highly Efficient Quantum-Dot-Sensitized Solar Cell Based on Co-Sensitization of CdS/CdSe. Adv. Funct. Mater. 2009, 19, 604–609. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Yang, S.H.; Hsu, C.S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, H.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Islam, A.; Chen, H.; Malapaka, C.; Chiranjeevi, B.; Zhang, S.; Yang, X.; Yanagida, M. High-efficiency dye-sensitized solar cell with a novel Co-adsorbent. Energy Environ. Sci. 2012, 5, 6057–6060. [Google Scholar] [CrossRef]
- Wang, M.; Chamberland, N.; Breau, L.; Moser, J.E.; Baker, R.H.; Marsan, B.; Zakeeruddin, S.M.; Grätzel, M. An organic redox electrolyte to rival triiodide/iodide in dye-sensitized solar cells. Nat. Chem. 2010, 2, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Nusbaumer, H.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M. An Alternative Efficient Redox Couple for the Dye-Sensitized Solar Cell System. Chem. Eur. J. 2003, 9, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, E.; Yum, J.-H.; Kessler, F.; Gómez García, C.J.; Zuccaccia, C.; Cinti, A.; Nazeeruddin, M.K.; Grätzel, M.; De Angelis, F. Cobalt Electrolyte/Dye Interactions in Dye-Sensitized Solar Cells: A Combined Computational and Experimental Study. J. Am. Chem. Soc. 2012, 134, 19438–19453. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.J.; Amick, T.J.; Elliott, C.M. Mass Transport of Polypyridyl Cobalt Complexes in Dye-Sensitized Solar Cells with Mesoporous TiO2 Photoanodes. J. Phys. Chem. C 2008, 112, 18255–18263. [Google Scholar] [CrossRef]
- Zhang, J.; Kan, Y.-H.; Li, H.-B.; Geng, Y.; Wu, Y.; Su, Z.-M. How to design proper π-spacer order of the D-π-A dyes for DSSCs? A density functional response. Dyes Pigments 2012, 95, 313–321. [Google Scholar] [CrossRef]
- Le Bahers, T.; Labat, F.; Pauporté, T.; Lainé, P.P.; Ciofini, I. Theoretical Procedure for Optimizing Dye-Sensitized Solar Cells: From Electronic Structure to Photovoltaic Efficiency. J. Am. Chem. Soc. 2011, 133, 8005–8013. [Google Scholar] [CrossRef] [PubMed]
- Labat, F.; Le Bahers, T.; Ciofini, I.; Adamo, C. First-principles modeling of dye-sensitized solar cells: Challenges and perspectives. Acc. Chem. Res. 2012, 45, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Le Bahers, T.; Pauporté, T.; Lainé, P.P.; Labat, F.; Adamo, C.; Ciofini, I. Modeling Dye-Sensitized Solar Cells: From Theory to Experiment. J. Phys. Chem. Lett. 2013, 4, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kar, S.; Das, R.N. Understanding the Basics of QSAR for Applications in Pharmaceutical Sciences and Risk Assessment; Academic Press: San Diego, CA, USA, 2015. [Google Scholar]
- Kar, S.; Sizochenko, N.; Ahmed, L.; Batista, V.S.; Leszczynski, J. Quantitative structure-property relationship model leading to virtual screening of fullerene derivatives: Exploring structural attributes critical for photoconversion efficiency of polymer solar cell acceptors. Nano Energy 2016, 26, 677–691. [Google Scholar] [CrossRef]
- Venkatraman, V.; Åstrand, P.-O.; Alsberg, B.K. Quantitative Structure-Property Relationship Modeling of Grätzel Solar Cell Dyes. J. Comput. Chem. 2014, 35, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Abburu, S.; Alsberg, B.K. Artificial evolution of coumarin dyes for dye sensitized solar cells. Phys. Chem. Chem. Phys. 2015, 17, 27672–27682. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, V.; Alsberg, B.K. A quantitative structure-property relationship study of the photovoltaic performance of phenothiazine dyes. Dyes Pigments 2015, 114, 69–77. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.; Li, L.; Gao, R.; Cui, J.; Gao, T.; Hu, L.H.; Lu, Y.; Su, Z.M.; Li, H. A Cascaded QSAR Model for Efficient Prediction of Overall Power Conversion Efficiency of All-Organic Dye-Sensitized Solar Cells. J. Comput. Chem. 2015, 36, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Feldt, S.M.; Gibson, E.A.; Gabrielsson, E.; Sun, L.; Boschloo, G.; Hagfeldt, A. Design of organic dyes and cobalt polypyridine redox mediators for high-efficiency dye-sensitized solar cells. J. Am. Chem. Soc. 2010, 132, 16714–16724. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhou, D.; Cai, N.; Liu, J.; Li, R.; Wang, P. Electrical and photophysical analyses on the impacts of arylamine electron donors in cyclopentadithiophene dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 4735–4742. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, M.; Pastore, M.; Li, R.Z.; Angelis, F.D.; Wang, P. Joint electrical, photophysical and computational studies on D-π-A dye sensitized solar cells: The impacts of dithiophene rigidification. Chem. Sci. 2012, 3, 976–983. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, J.Y.; Wang, Y.H.; Zhou, D.F.; Wang, P. Redox couple related influences of π-conjugation extension in organic dye-sensitized mesoscopic solar cells. Chem. Sci. 2011, 2, 1401–1406. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, J.; Zhou, D.; Wang, Y.; Zhang, M.; Wang, P. Engineering Organic Sensitizers for Iodine-Free Dye-Sensitized Solar Cells: Red-Shifted Current Response Concomitant with Attenuated Charge Recombination. J. Am. Chem. Soc. 2011, 133, 11442–11445. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.M.; Cai, N.; Wang, Y.L.; Li, R.Z.; Yuan, Y.; Wang, P. Modulating the assembly of organic dye molecules on titania nanocrystals via alkyl chain elongation for efficient mesoscopic cobalt solar cells. Phys. Chem. Chem. Phys. 2012, 14, 8282–8286. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.H.; Baranoff, E.; Kessler, F.; Mmoehl, T.; Ahmad, S.; Bessho, T.; Mmarchioro, A.; Ghadiri, E.; Mmoser, J.E.; Yi, C.Y.; et al. A cobalt complex redox shuttle for dye-sensitized solar cells with high open-circuit potentials. Nat. Commun. 2012, 3, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Peng, W.; Zhang, K.; Islam, A.; Han, L. A novel organic sensitizer combined with a cobalt complex redox shuttle for dye-sensitized solar cells. Org. Lett. 2012, 14, 2532–2535. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.P.; Liang, M.; Fan, C.R.; Tang, K.; Li, G.; Sun, Z.; Xue, S. Design of Truxene-Based Organic Dyes for High-Efficiency Dye-Sensitized Solar Cells Employing Cobalt Redox Shuttle. J. Phys. Chem. C 2012, 116, 11241–11250. [Google Scholar] [CrossRef]
- Zong, X.P.; Liang, M.; Chen, T.; Jia, J.N.; Wang, L.N.; Sun, Z.; Xue, S. Efficient iodine-free dye-sensitized solar cells employing truxene-based organic dyes. Chem. Commun. 2012, 48, 6645–6647. [Google Scholar] [CrossRef] [PubMed]
- HyperChem (TM), Hypercube, Inc.: Gainesville, FL, USA.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Preat, J. Photoinduced Energy-Transfer and Electron-Transfer Processes in Dye-Sensitized Solar Cells: TDDFT Insights for Triphenylamine Dyes. J. Phys. Chem. C 2010, 114, 16716–16725. [Google Scholar] [CrossRef]
- Irfan, A.; Jin, R.; Al-Sehemi, A.G.; Asiri, A.M. Quantum Chemical Study of the Donor-Bridge-Acceptor Triphenylamine Based Sensitizers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 110, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Magyar, R.J.; Tretiak, S. Dependence of Spurious Charge-Transfer Excited States on Orbital Exchange in TDDFT: Large Molecules and Clusters. J. Chem. Theory Comput. 2007, 3, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Dragon Molecular Descriptors. Available online: http://www.talete.mi.it/products/dragon_molecular_descriptors.htm (accessed on 20 December 2016).
- Software Tools. Available online: http://teqip.jdvu.ac.in/QSAR_Tools/ (accessed on 20 December 2016).
- Roy, K.; Mitra, I.; Kar, S.; Ojha, P.; Das, R.N.; Kabir, H. Comparative studies on some metrics for external validation of QSPR models. J. Chem. Inf. Model. 2012, 52, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kar, S. How to Judge Predictive Quality of Classification and Regression Based QSAR Models? In Frontiers of Computational Chemistry; Haq, Z.U., Madura, J., Eds.; Bentham Science Publishers: Sharjah, UAE, 2015; pp. 71–120. [Google Scholar]
- Roy, K.; Kar, S.; Ambure, P. On a simple approach for determining applicability domain of QSAR models. Chemom. Intell. Lab. Syst. 2015, 145, 22–29. [Google Scholar] [CrossRef]
- Roy, K.; Das, R.N.; Ambure, P.; Aher, R.B. Be aware of error measures. Further studies on validation of predictive QSAR models. Chemom. Intell. Lab. Syst. 2016, 152, 18–33. [Google Scholar] [CrossRef]
- Iriyama, K.; Mizutani, F.; Yoshiura, M. Enhancement of Photocurrent at a Merocyanine-Coated Electrode due to Chromophore Aggregation. Chem. Lett. 1980, 11, 1399–1402. [Google Scholar] [CrossRef]
- Sayama, K.; Hara, K.; Sugihara, H.; Arakawa, H.; Mori, N.; Satsuki, M.; Suga, S.; Tsukagoshi, S.; Abe, Y.; O’Regan, B.; et al. Photosensitization of a Porous TiO2 Electrode with Merocyanine Dyes Containing a Carboxyl Group and a Long Alkyl Chain. Chem. Commun. 2000, 353, 1173–1174. [Google Scholar] [CrossRef]
- Kroeze, J.E.; Hirata, N.; Koops, S.; Nazeeruddin, M.K.; Schmidt-Mende, L.; Grätzel, M.; Durrant, J.R. Alkyl Chain Barriers for Kinetic Optimization in Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2006, 128, 16376–16383. [Google Scholar] [CrossRef] [PubMed]
| ID | JSC/mA·cm−2 | VOC/mV | FF | %PCE (Experimental) | %PCE (Predicted) | Structure |
|---|---|---|---|---|---|---|
| 1 | 13.3 | 950 | 0.74 | 9.3 | 9.0 |  |
| 2* | 10.7 | 920 | 0.68 | 6.7 | 6.2 |  |
| 3 | 10.54 | 950 | 0.77 | 7.7 | 9.2 | 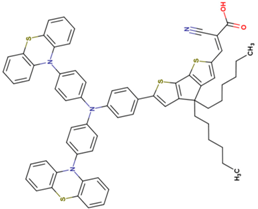 |
| 4 | 12.05 | 930 | 0.75 | 8.4 | 8.3 | 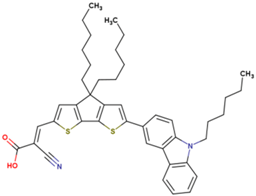 |
| 5 | 11.41 | 870 | 0.77 | 7.6 | 8.3 | 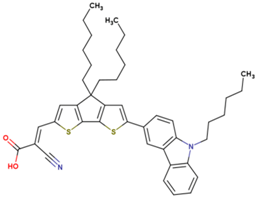 |
| 6 | 6.91 | 1050 | 0.76 | 5.5 | 6.1 | 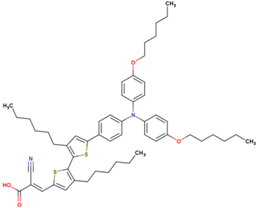 |
| 7* | 12.17 | 990 | 0.75 | 9.0 | 8.3 | 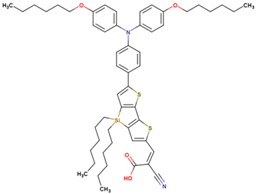 |
| 8 | 12.92 | 860 | 0.72 | 8.0 | 8.1 | 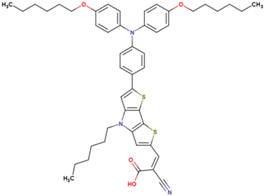 |
| 9 | 7.99 | 815 | 0.76 | 5.0 | 6.1 | 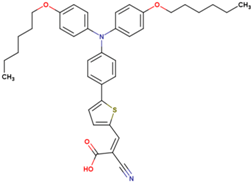 |
| 10 | 12.98 | 837 | 0.74 | 8.0 | 6.1 | 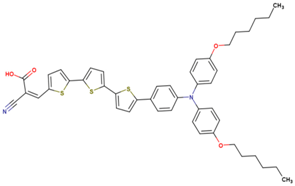 |
| 11* | 15.31 | 850 | 0.73 | 9.4 | 9.7 | 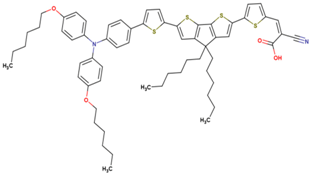 |
| 12 | 14.86 | 840 | 0.753 | 9.4 | 8.6 | 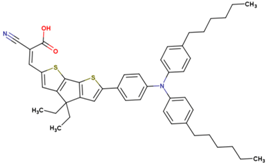 |
| 13 | 14.81 | 930 | 0.73 | 10.1 | 9.4 | 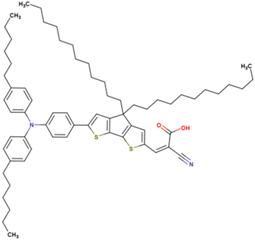 |
| 14 | 14.67 | 830 | 0.75 | 9.1 | 8.6 | 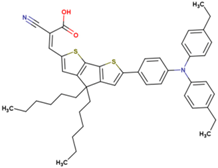 |
| 15* | 14.55 | 930 | 0.743 | 10.1 | 9.6 | 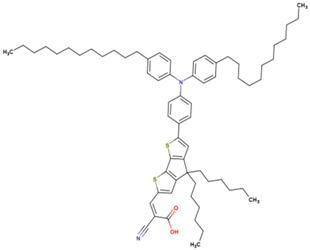 |
| 16 | 13.06 | 998 | 0.774 | 10.1 | 10.0 | 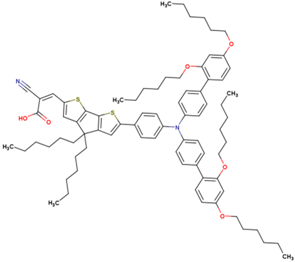 |
| 17 | 8.38 | 747 | 0.648 | 4.1 | 4.2 | 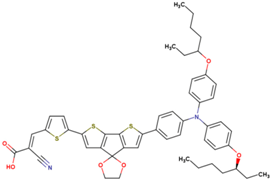 |
| 18 | 9 | 865 | 0.71 | 5.5 | 5.2 | 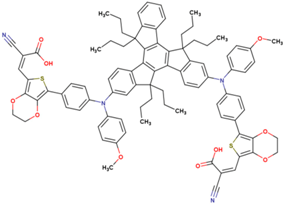 |
| 19* | 11.2 | 870 | 0.71 | 6.9 | 6.9 |  |
| 20* | 9.6 | 870 | 0.7 | 5.8 | 7.9 |  |
| 21 | 11.9 | 900 | 0.71 | 7.6 | 7.9 |  |
| Validation | Metrics | Division Tool | Threshold | |||
|---|---|---|---|---|---|---|
| Activity Sorted | Euclidean Distance Based | Kennard-Stone Based | K-Medoid Clustering | |||
| Model No. | Model 1 | Model 2 | Model 3 | Model 4 | ||
| Internal | NTraining | 16 | 16 | 15 | 15 | - |
| R2 | 0.77 | 0.73 | 0.81 | 0.81 | >0.5 | |
| R2adjusted | 0.71 | 0.66 | 0.76 | 0.76 | >0.5 | |
| SEE | 0.86 | 1.02 | 0.66 | 0.92 | - | |
| F | 13.46 (DF:3,12) | 10.59 (DF:3,12) | 15.44 (DF:3,11) | 15.71 (DF:3,11) | - | |
| Q2LOO | 0.65 | 0.56 | 0.64 | 0.66 | >0.5 | |
| PRESS | 9.01 | 12.58 | 4.73 | 9.44 | - | |
| 0.55 | 0.44 | 0.56 | 0.55 | >0.5 | ||
| 0.08 | 0.15 | 0.03 | 0.14 | <0.2 | ||
| External | NTest | 5 | 5 | 6 | 6 | - |
| RMSEP | 1.31 | 1.04 | 2.02 | 0.98 | - | |
| 0.67 | 0.74 | 0.41 | 0.63 | >0.5 | ||
| 0.66 | 0.65 | 0.39 | 0.62 | >0.5 | ||
| 0.17 | 0.33 | −0.15 | 0.50 | >0.5 | ||
| 0.31 | 0.39 | 0.96 | 0.20 | <0.2 | ||
| Descriptors* | DEx RBN O% | DEx EH-1 Me | DEx D/Dtr08 SssO | D/Dtr11 nCq SssssC | - | |
| Pearson Correlation (R2) | D/Dtr11 | nCq | SssssC |
|---|---|---|---|
| D/Dtr11 | 0 | 0.03 | 0.01 |
| nCq | 0.03 | 0 | 0.27 |
| SssssC | 0.01 | 0.27 | 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kar, S.; Roy, J.K.; Leszczynska, D.; Leszczynski, J. Power Conversion Efficiency of Arylamine Organic Dyes for Dye-Sensitized Solar Cells (DSSCs) Explicit to Cobalt Electrolyte: Understanding the Structural Attributes Using a Direct QSPR Approach. Computation 2017, 5, 2. https://doi.org/10.3390/computation5010002
Kar S, Roy JK, Leszczynska D, Leszczynski J. Power Conversion Efficiency of Arylamine Organic Dyes for Dye-Sensitized Solar Cells (DSSCs) Explicit to Cobalt Electrolyte: Understanding the Structural Attributes Using a Direct QSPR Approach. Computation. 2017; 5(1):2. https://doi.org/10.3390/computation5010002
Chicago/Turabian StyleKar, Supratik, Juganta K. Roy, Danuta Leszczynska, and Jerzy Leszczynski. 2017. "Power Conversion Efficiency of Arylamine Organic Dyes for Dye-Sensitized Solar Cells (DSSCs) Explicit to Cobalt Electrolyte: Understanding the Structural Attributes Using a Direct QSPR Approach" Computation 5, no. 1: 2. https://doi.org/10.3390/computation5010002
APA StyleKar, S., Roy, J. K., Leszczynska, D., & Leszczynski, J. (2017). Power Conversion Efficiency of Arylamine Organic Dyes for Dye-Sensitized Solar Cells (DSSCs) Explicit to Cobalt Electrolyte: Understanding the Structural Attributes Using a Direct QSPR Approach. Computation, 5(1), 2. https://doi.org/10.3390/computation5010002







