DFT-Guided Next-Generation Na-Ion Batteries Powered by Halogen-Tuned C12 Nanorings
Abstract
1. Introduction
2. Computational Methodology
3. Results and Discussions
3.1. Interaction of Na/Na+ with the C12 Nanoring
3.2. Electrochemical Properties of Na/Na+@ Complexes C12
3.3. Adsorption of the C12 Nanoring with Halogens (Br−, Cl−, and F−)
3.4. Adsorption of Na/Na+ on Halides@C12 Complexes
3.5. NBO Charge Analysis
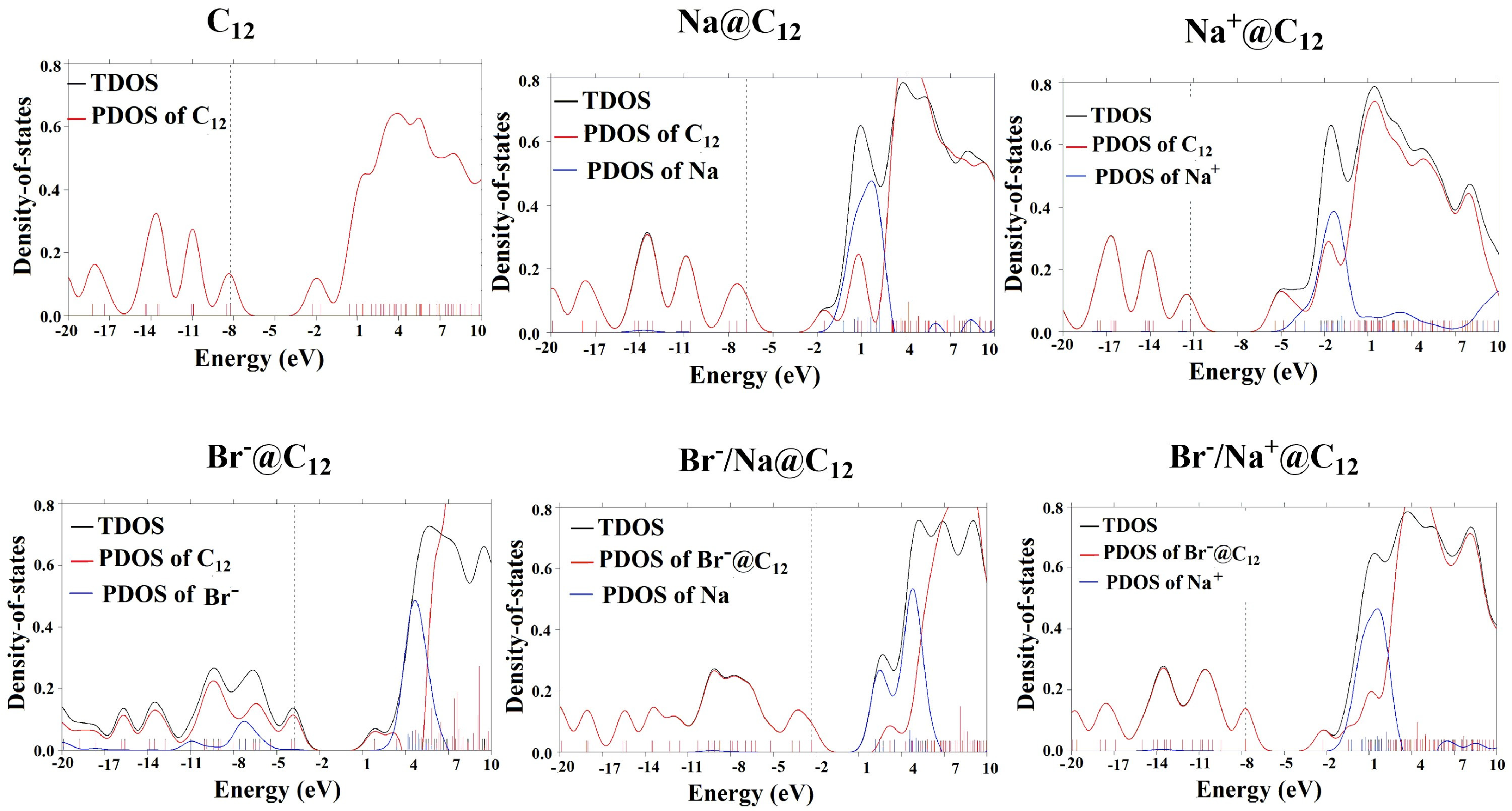
3.6. Partial Density of State (PDOS) and Total Density of State (TDOS) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Z.; Haile, S.M. A High-Performance Cathode for the next Generation of Solid-Oxide Fuel Cells. Mater. Sustain. Energy A Collect. Peer-Rev. Res. Rev. Artic. Nat. Publ. Gr. 2010, 3, 255–258. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Lithium-Air Batteries: Something from Nothing. Nat. Chem. 2012, 4, 962–963. [Google Scholar] [CrossRef] [PubMed]
- Schon, T.B.; McAllister, B.T.; Li, P.-F.; Seferos, D.S. The Rise of Organic Electrode Materials for Energy Storage. Chem. Soc. Rev. 2016, 45, 6345–6404. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Hanumandla, P.; Huang, H.-Y.; Liu, J.L. Nanostructured Materials for Advanced Energy Conversion and Storage Devices: Safety Implications at End-of-Life Disposal. In Nanostructured Materials for Next-Generation Energy Storage and Conversion; Springer: Berlin/Heidelberg, Germany, 2018; Volume 4, pp. 517–542. ISBN 9783662563649. [Google Scholar]
- Liang, Y.; Tao, Z.; Chen, J. Organic Electrode Materials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2012, 2, 742–769. [Google Scholar] [CrossRef]
- Song, Z.; Zhou, H. Towards Sustainable and Versatile Energy Storage Devices: An Overview of Organic Electrode Materials. Energy Environ. Sci. 2013, 6, 2280. [Google Scholar] [CrossRef]
- Morita, Y.; Nishida, S.; Murata, T.; Moriguchi, M.; Ueda, A.; Satoh, M.; Arifuku, K.; Sato, K.; Takui, T. Organic Tailored Batteries Materials Using Stable Open-Shell Molecules with Degenerate Frontier Orbitals. Nat. Mater. 2011, 10, 947–951. [Google Scholar] [CrossRef]
- Wu, H.; Shevlin, S.A.; Meng, Q.; Guo, W.; Meng, Y.; Lu, K.; Wei, Z.; Guo, Z. Flexible and Binder-Free Organic Cathode for High-Performance Lithium-Ion Batteries. Adv. Mater. 2014, 26, 3338–3343. [Google Scholar] [CrossRef]
- Nishide, H.; Oyaizu, K. Toward Flexible Batteries. Science (80-) 2008, 319, 737–738. [Google Scholar] [CrossRef]
- Williams, D.L.; Byrne, J.J.; Driscoll, J.S. A High Energy Density Lithium/Dichloroisocyanuric Acid Battery System. J. Electrochem. Soc. 1969, 116, 2. [Google Scholar] [CrossRef]
- Rudola, A.; Rennie, A.J.R.; Heap, R.; Meysami, S.S.; Lowbridge, A.; Mazzali, F.; Sayers, R.; Wright, C.J.; Barker, J. Commercialisation of High Energy Density Sodium-Ion Batteries: Faradion’s Journey and Outlook. J. Mater. Chem. A 2021, 9, 8279–8302. [Google Scholar] [CrossRef]
- Chen, R.; Luo, R.; Huang, Y.; Wu, F.; Li, L. Advanced High Energy Density Secondary Batteries with Multi-Electron Reaction Materials. Adv. Sci. 2016, 3, 1600051. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Kiko, T.; Hosokawa, T.; Matsumoto, K.; Nohira, T.; Hagiwara, R. Ionic Liquid Electrolytes with High Sodium Ion Fraction for High-Rate and Long-Life Sodium Secondary Batteries. J. Power Sources 2016, 332, 51–59. [Google Scholar] [CrossRef]
- Xie, F.; Niu, Y.; Zhang, Q.; Guo, Z.; Hu, Z.; Zhou, Q.; Xu, Z.; Li, Y.; Yan, R.; Lu, Y.; et al. Screening Heteroatom Configurations for Reversible Sloping Capacity Promises High-Power Na-Ion Batteries. Angew. Chem. Int. Ed. 2022, 61, e202116394. [Google Scholar] [CrossRef] [PubMed]
- Nagmani; Pahari, D.; Verma, P.; Puravankara, S. Are Na-Ion Batteries Nearing the Energy Storage Tipping Point?—Current Status of Non-Aqueous, Aqueous, and Solid-Sate Na-Ion Battery Technologies for Sustainable Energy Storage. J. Energy Storage 2022, 56, 105961. [Google Scholar] [CrossRef]
- Jian, Z.; Raju, V.; Li, Z.; Xing, Z.; Hu, Y.; Ji, X. A High-Power Symmetric Na-Ion Pseudocapacitor. Adv. Funct. Mater. 2015, 25, 5778–5785. [Google Scholar] [CrossRef]
- Nithya, C.; Gopukumar, S. Sodium Ion Batteries: A Newer Electrochemical Storage. WIREs Energy Environ. 2015, 4, 253–278. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.-M. Towards Greener and More Sustainable Batteries for Electrical Energy Storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wang, T.; Du, Y.; Cui, Y.; Liu, S.; Wang, H.; Liu, S.; Chen, M.; Zhou, J. Space-Confined Fabrication of MoS 2 @Carbon Tubes with Semienclosed Architecture Achieving Superior Cycling Capability for Sodium Ion Storage. Adv. Mater. Interfaces 2020, 7, 2000953. [Google Scholar] [CrossRef]
- Park, J.; Lee, M.; Feng, D.; Huang, Z.; Hinckley, A.C.; Yakovenko, A.; Zou, X.; Cui, Y.; Bao, Z. Stabilization of Hexaaminobenzene in a 2D Conductive Metal–Organic Framework for High Power Sodium Storage. J. Am. Chem. Soc. 2018, 140, 10315–10323. [Google Scholar] [CrossRef]
- Fang, G.; Wu, Z.; Zhou, J.; Zhu, C.; Cao, X.; Lin, T.; Chen, Y.; Wang, C.; Pan, A.; Liang, S. Observation of Pseudocapacitive Effect and Fast Ion Diffusion in Bimetallic Sulfides as an Advanced Sodium-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703155. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Lu, W.; Wang, C.; Xue, W.; Yang, F.; Zhou, J.; Suo, L.; Lin, T.; et al. Nitrogen-Doped Carbon for Sodium-Ion Battery Anode by Self-Etching and Graphitization of Bimetallic MOF-Based Composite. Chem 2017, 3, 152–163. [Google Scholar] [CrossRef]
- Ding, J.; Wang, H.; Li, Z.; Kohandehghan, A.; Cui, K.; Xu, Z.; Zahiri, B.; Tan, X.; Lotfabad, E.M.; Olsen, B.C.; et al. Carbon Nanosheet Frameworks Derived from Peat Moss as High Performance Sodium Ion Battery Anodes. ACS Nano 2013, 7, 11004–11015. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent Progress on Sodium Ion Batteries: Potential High-Performance Anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Fatima, H.; Zhong, Y.; Wu, H.; Shao, Z. Recent Advances in Functional Oxides for High Energy Density Sodium-Ion Batteries. Mater. Rep. Energy 2021, 1, 100022. [Google Scholar] [CrossRef]
- Muñoz-Márquez, M.Á.; Saurel, D.; Gómez-Cámer, J.L.; Casas-Cabanas, M.; Castillo-Martínez, E.; Rojo, T. Na-Ion Batteries for Large Scale Applications: A Review on Anode Materials and Solid Electrolyte Interphase Formation. Adv. Energy Mater. 2017, 7, 1700463. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.J.; Chang, J.H.; Seo, H.K.; Lee, J.Y.; Yuk, J.M. Atomic Visualization of a Non-Equilibrium Sodiation Pathway in Copper Sulfide. Nat. Commun. 2018, 9, 922. [Google Scholar] [CrossRef]
- Olsson, E.; Chai, G.; Dove, M.; Cai, Q. Adsorption and Migration of Alkali Metals (Li, Na, and K) on Pristine and Defective Graphene Surfaces. Nanoscale 2019, 11, 5274–5284. [Google Scholar] [CrossRef]
- Kulish, V.V.; Malyi, O.I.; Persson, C.; Wu, P. Phosphorene as an Anode Material for Na-Ion Batteries: A First-Principles Study. Phys. Chem. Chem. Phys. 2015, 17, 13921–13928. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, F.; Mu, D.; Wu, B. A Theoretical Study on Na + Solvation in Carbonate Ester and Ether Solvents for Sodium-Ion Batteries. Phys. Chem. Chem. Phys. 2020, 22, 2164–2175. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.-F.; Liu, P.; Jiao, L. Polyanion-Type Cathode Materials for Sodium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Weltner, W., Jr.; Van Zee, R.J. Carbon Molecules, Ions and Clusters. Chem. Rev. 1989, 89, 1713–1747. [Google Scholar] [CrossRef]
- Raghavachari, K.; Whiteside, R.A.; Pople, J.A. Structures of Small Carbon Clusters: Cyclic Ground State of C6. J. Chem. Phys. 1986, 85, 6623–6628. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S. Structure, Stability, and Fragmentation of Small Carbon Clusters. J. Chem. Phys. 1987, 87, 2191–2197. [Google Scholar] [CrossRef]
- Lifshitz, C. Carbon Clusters. Int. J. Mass Spectrom. 2000, 200, 423–442. [Google Scholar] [CrossRef]
- Saha, K.; Chandrasekaran, V.; Heber, O.; Iron, M.A.; Rappaport, M.L.; Zajfman, D. Ultraslow Isomerization in Photoexcited Gas-Phase Carbon Cluster -10. Nat. Commun. 2018, 9, 912. [Google Scholar] [CrossRef]
- Zhao, W.; Cao, A.; Tian, J.; Gan, L. Structural Connectivity and Formation Mechanism of Monometallic Cluster Fullerenes YCN@C n (n = 68–84). Int. J. Quantum Chem. 2018, 118, e25647. [Google Scholar] [CrossRef]
- Jäntschi, L.; Bolboacă, S.D. Conformational Study of C 24 Cyclic Polyyne Clusters. Int. J. Quantum Chem. 2018, 118, e25614. [Google Scholar] [CrossRef]
- Sheng, X.; Song, X.; Ngwenya, C.A.; Li, J.; Zhao, H. Study of Carbon Suboxide-Containing Clusters: A Potential Sink for Cumulene. Comput. Theor. Chem. 2018, 1142, 78–82. [Google Scholar] [CrossRef]
- Moreno-Armenta, M.G.; Pearce, H.R.; Winter, P.; Cooksy, A.L. Computational Search for Metastable High-Spin C5Hn (n = 4, 5, 6) Species. Comput. Theor. Chem. 2018, 1140, 1–6. [Google Scholar] [CrossRef]
- Feygelson, T.I.; Tadjer, M.J.; Hobart, K.D.; Anderson, T.J.; Pate, B.B. Reduced-Stress Nanocrystalline Diamond Films for Heat Spreading in Electronic Devices. In Thermal Management of Gallium Nitride Electronics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 275–294. [Google Scholar]
- Van Orden, A.; Saykally, R.J. Small Carbon Clusters: Spectroscopy, Structure, and Energetics. Chem. Rev. 1998, 98, 2313–2357. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W. The Spectra of Interstellar Molecules. Int. Rev. Phys. Chem. 1981, 1, 309–376. [Google Scholar] [CrossRef]
- Stevens, D.A.; Dahn, J.R. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Ma, M.; Cai, H.; Xu, C.; Huang, R.; Wang, S.; Pan, H.; Hu, Y. Engineering Solid Electrolyte Interface at Nano-Scale for High-Performance Hard Carbon in Sodium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100278. [Google Scholar] [CrossRef]
- Xie, F.; Xu, Z.; Guo, Z.; Titirici, M.-M. Hard Carbons for Sodium-Ion Batteries and Beyond. Prog. Energy 2020, 2, 042002. [Google Scholar] [CrossRef]
- Tang, K.; Fu, L.; White, R.J.; Yu, L.; Titirici, M.; Antonietti, M.; Maier, J. Hollow Carbon Nanospheres with Superior Rate Capability for Sodium-Based Batteries. Adv. Energy Mater. 2012, 2, 873–877. [Google Scholar] [CrossRef]
- Bin, D.; Li, Y.; Sun, Y.; Duan, S.; Lu, Y.; Ma, J.; Cao, A.; Hu, Y.; Wan, L. Structural Engineering of Multishelled Hollow Carbon Nanostructures for High-Performance Na-Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1800855. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Goktas, M.; Anothumakkool, B.; Sun, Y.; Schmuch, R.; Zhao, L.; Han, B.; Winter, M.; Adelhelm, P. Sodium Storage and Electrode Dynamics of Tin–Carbon Composite Electrodes from Bulk Precursors for Sodium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1900790. [Google Scholar] [CrossRef]
- Li, P.; Guo, X.; Wang, S.; Zang, R.; Li, X.; Man, Z.; Li, P.; Liu, S.; Wu, Y.; Wang, G. Two-Dimensional Sb@TiO 2−x Nanoplates as a High-Performance Anode Material for Sodium-Ion Batteries. J. Mater. Chem. A 2019, 7, 2553–2559. [Google Scholar] [CrossRef]
- Jing, W.T.; Yang, C.C.; Jiang, Q. Recent Progress on Metallic Sn- and Sb-Based Anodes for Sodium-Ion Batteries. J. Mater. Chem. A 2020, 8, 2913–2933. [Google Scholar] [CrossRef]
- Wallace, G.G.; Higgins, M.J.; Moulton, S.E.; Wang, C. Nanobionics: The Impact of Nanotechnology on Implantable Medical Bionic Devices. Nanoscale 2012, 4, 4327. [Google Scholar] [CrossRef]
- Luo, L.; Song, J.; Song, L.; Zhang, H.; Bi, Y.; Liu, L.; Yin, L.; Wang, F.; Wang, G. Flexible Conductive Anodes Based on 3D Hierarchical Sn/NS-CNFs@rGO Network for Sodium-Ion Batteries. Nano-Micro Lett. 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ni, J.; Savilov, S.V.; Li, L. Materials Based on Antimony and Bismuth for Sodium Storage. Chem. A Eur. J. 2018, 24, 13719–13727. [Google Scholar] [CrossRef]
- Bell, M.B.; Feldman, P.A.; Kwok, S.; Matthews, H.E. Detection of HC11N in IRC + 10°216. Nature 1982, 295, 389–391. [Google Scholar] [CrossRef]
- Bernath, P.F.; Hinkle, K.H.; Keady, J.J. Detection of C 5 in the Circumstellar Shell of IRC+10216. Science (80-) 1989, 244, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Čermák, I.; Förderer, M.; Čermáková, I.; Kalhofer, S.; Stopka-Ebeler, H.; Monninger, G.; Krätschmer, W. Laser-Induced Emission Spectroscopy of Matrix-Isolated Carbon Molecules: Experimental Setup and New Results on C3. J. Chem. Phys. 1998, 108, 10129–10142. [Google Scholar] [CrossRef]
- McElvany, S.W.; Ross, M.M.; Goroff, N.S.; Diederich, F. Cyclocarbon Coalescence: Mechanisms for Tailor-Made Fullerene Formation. Science (80-) 1993, 259, 1594–1596. [Google Scholar] [CrossRef]
- Arulmozhiraja, S.; Ohno, T. CCSD Calculations on C14, C18, and C22 Carbon Clusters. J. Chem. Phys. 2008, 128, 114301. [Google Scholar] [CrossRef]
- Neiss, C.; Trushin, E.; Görling, A. The Nature of One-Dimensional Carbon: Polyynic versus Cumulenic. ChemPhysChem 2014, 15, 2497–2502. [Google Scholar] [CrossRef]
- Pavliček, N.; Gawel, P.; Kohn, D.R.; Majzik, Z.; Xiong, Y.; Meyer, G.; Anderson, H.L.; Gross, L. Polyyne Formation via Skeletal Rearrangement Induced by Atomic Manipulation. Nat. Chem. 2018, 10, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R. Extended Hückel Theory—V. Tetrahedron 1966, 22, 521–538. [Google Scholar] [CrossRef]
- Narita, N.; Nagai, S.; Suzuki, S.; Nakao, K. Optimized Geometries and Electronic Structures of Graphyne and Its Family. Phys. Rev. B 1998, 58, 11009–11014. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Li, Y.; Kono, H.; Maekawa, T.; Segawa, Y.; Yagi, A.; Itami, K. Chemical Synthesis of Carbon Nanorings and Nanobelts. Acc. Mater. Res. 2021, 2, 681–691. [Google Scholar] [CrossRef]
- Yamago, S.; Watanabe, Y.; Iwamoto, T. Synthesis of [8]Cycloparaphenylene from a Square-Shaped Tetranuclear Platinum Complex. Angew. Chem. Int. Ed. 2010, 49, 757–759. [Google Scholar] [CrossRef]
- Segawa, Y.; Miyamoto, S.; Omachi, H.; Matsuura, S.; Šenel, P.; Sasamori, T.; Tokitoh, N.; Itami, K. Concise Synthesis and Crystal Structure of [12]Cycloparaphenylene. Angew. Chem. Int. Ed. 2011, 50, 3244–3248. [Google Scholar] [CrossRef]
- Friederich, R.; Nieger, M.; Vögtle, F. Auf Dem Weg Zu Makrocyclischen Para -Phenylenen. Chem. Ber. 1993, 126, 1723–1732. [Google Scholar] [CrossRef]
- Ullah, F.; Ayub, K.; Gilani, M.A.; Imran, M.; Mahmood, T. C10F as a Potential Anode Material for Alkali-Ion Batteries; a Quantum Chemical Approach. Comput. Theor. Chem. 2021, 1206, 113470. [Google Scholar] [CrossRef]
- Murtaza, T.; Kosar, N.; Amjad Gilani, M.; Ayub, K.; Hussain Shah, K.; Mahmood, T. DFT Studies on Electrochemical Properties of Halide Ions Doped GDY-28 Nanoflake for Na-Ion Battery Applications. Mater. Sci. Semicond. Process. 2022, 145, 106651. [Google Scholar] [CrossRef]
- Duraisamy, P.D.; Paul, S.P.M.; Gopalan, P.; Paranthaman, S.; Angamuthu, A. A DFT Study of Halogen (F−, Cl−, and Br−) Encapsulated Ga12X12 (X = N, P, and As) Nanocages for Sodium-Ion Batteries. J. Inorg. Organomet. Polym. Mater. 2022, 32, 4173–4185. [Google Scholar] [CrossRef]
- Chapman, N.; Borodin, O.; Yoon, T.; Nguyen, C.C.; Lucht, B.L. Spectroscopic and Density Functional Theory Characterization of Common Lithium Salt Solvates in Carbonate Electrolytes for Lithium Batteries. J. Phys. Chem. C 2017, 121, 2135–2148. [Google Scholar] [CrossRef]
- Dziedzic, J.; Bhandari, A.; Anton, L.; Peng, C.; Womack, J.C.; Famili, M.; Kramer, D.; Skylaris, C.-K. Practical Approach to Large-Scale Electronic Structure Calculations in Electrolyte Solutions via Continuum-Embedded Linear-Scaling Density Functional Theory. J. Phys. Chem. C 2020, 124, 7860–7872. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (D01); Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Dennington, K.R.; Keith, T.; Millam, J. GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Kosar, N.; Mahmood, T.; Hafeez, F.; Ayub, K. Detailed Mechanistic Study of Radical Mediated Chemoselective Phosphination of Aryl Halide. ChemistrySelect 2018, 3, 11302–11308. [Google Scholar] [CrossRef]
- Kosar, N.; Shehzadi, K.; Ayub, K.; Mahmood, T. Theoretical Study on Novel Superalkali Doped Graphdiyne Complexes: Unique Approach for the Enhancement of Electronic and Nonlinear Optical Response. J. Mol. Graph. Model. 2020, 97, 107573. [Google Scholar] [CrossRef]
- Kosar, N.; Mahmood, T.; Ayub, K. Role of Dispersion Corrected Hybrid GGA Class in Accurately Calculating the Bond Dissociation Energy of Carbon Halogen Bond: A Benchmark Study. J. Mol. Struct. 2017, 1150, 447–458. [Google Scholar] [CrossRef]
- Ullah, F.; Kosar, N.; Arshad, M.N.; Gilani, M.A.; Ayub, K.; Mahmood, T. Design of Novel Superalkali Doped Silicon Carbide Nanocages with Giant Nonlinear Optical Response. Opt. Laser Technol. 2020, 122, 105855. [Google Scholar] [CrossRef]
- Kosar, N.; Shehzadi, K.; Ayub, K.; Mahmood, T. Nonlinear Optical Response of Sodium Based Superalkalis Decorated Graphdiyne Surface: A DFT Study. Optik 2020, 218, 165033. [Google Scholar] [CrossRef]
- Kosimov, D.P.; Dzhurakhalov, A.A.; Peeters, F.M. Carbon Clusters: From Ring Structures to Nanographene. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 1–13. [Google Scholar] [CrossRef]
- Younis, U.; Qayyum, F.; Muhammad, I.; Yaseen, M.; Sun, Q. A Stable Three-Dimensional Porous Carbon as a High-Performance Anode Material for Lithium, Sodium, and Potassium Ion Batteries. Adv. Theory Simul. 2022, 5, 2200230. [Google Scholar] [CrossRef]
- Kosar, N.; Asgar, M.; Mahmood, T.; Ayub, K.; Sajid, H.; Albaqami, M.D.; Gilani, M.A. Electrochemical Properties of Lithium Metal Doped C60 Fullerene for Battery Applications. Mater. Sci. Semicond. Process. 2024, 175, 108256. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, J.; Zhang, J.; Tian, M.; Cai, J.; Wu, H.; Wei, G.; Chen, T.; Wei, X.; Dai, H. Investigation of Lithium-Ion Battery Degradation by Corrected Differential Voltage Analysis Based on Reference Electrode. Appl. Energy 2025, 389, 125735. [Google Scholar] [CrossRef]
- Zhang, K.; Jin, Z. Halogen-Enabled Rechargeable Batteries: Current Advances and Future Perspectives. Energy Storage Mater. 2022, 45, 332–369. [Google Scholar] [CrossRef]
- Ma, S.; Yan, W.; Dong, Y.; Su, Y.; Ma, L.; Li, Y.; Fang, Y.; Wang, B.; Wu, S.; Liu, C.; et al. Recent Advances in Carbon-Based Anodes for High-Performance Sodium-Ion Batteries: Mechanism, Modification and Characterizations. Mater. Today 2024, 75, 334–358. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.P.; Sougrati, M.T.; Feng, Z.; Leconte, Y.; Fisher, A.; Srinivasan, M.; Xu, Z. A Review on Design Strategies for Carbon Based Metal Oxides and Sulfides Nanocomposites for High Performance Li and Na Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601424. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Y.; Ma, Y. Salt-Assisted Synthesis of Advanced Carbon-Based Materials for Energy-Related Applications. Green Chem. 2023, 25, 10263–10303. [Google Scholar] [CrossRef]




| Complexes | Bond Distance (Å) | Eint (kcal mol−1) | ΔGcell (kcal mol−1) | Vcell (V) |
|---|---|---|---|---|
| Pure C12 | 1.30 | ----- | ----- | ---- |
| Na@C12 | C-Na = 1.46 | −50.08 | 30.57 | −1.32 |
| Na+@C12 | C-Na+ = 2.46 | −18.72 | ||
| Br−@C12 | C-Br− = 1.93 | −50.32 | ----- | |
| Br−/Na@C12 | C-Br− = 1.92 C-Na = 1.20 | −51.47 | −52.36 | 2.27 |
| Br−/Na+@C12 | C-Br− = 1.89 Na+-C = 1.43 | −103.82 | ||
| Cl−@C12 | C-Cl− = 1.78 | −47.95 | ----- | ----- |
| Cl−/Na@C12 | C-Cl− = 1.78 Na-C = 1.20 | −50.32 | −52.56 | 2.28 |
| Cl−/Na+@C12 | C-Cl− = 1.74 Na+-C = 1.49 | −103.04 | ||
| F−@C12 | C-F− = 1.37 | −84.79 | ||
| F−/Na@C12 | C-F− = 1.38 Na-C = 1.21 | −14.56 | −58.31 | 2.52 |
| F−/Na+@C12 | C-F− = 1.35 Na+-C = 1.46 | −103.64 |
| Complexes | EH eV | EL eV | EH-L | NBOs |e| | NBOm|e| | NBOx |e| |
|---|---|---|---|---|---|---|
| Pure C12 | −8.14 | −2.25 | 5.88 | 0.00 | ----- | ----- |
| Na@C12 | −6.73 | −1.51 | 5.21 | −0.96 | 0.96 | ----- |
| Na+@C12 | −11.12 | −5.35 | 5.76 | 0.02 | 0.97 | ----- |
| Br−@C12 | −3.69 | 1.82 | 5.52 | −0.98 | ----- | −0.01 |
| Br−/Na@C12 | −2.73 | 2.14 | 4.87 | −1.96 | 0.95 | 0.01 |
| Br−/Na+@C12 | −7.64 | −2.22 | 5.41 | −1.06 | 0.96 | 0.10 |
| Cl−@C12 | −3.66 | 1.86 | 5.53 | −0.92 | ----- | −0.07 |
| Cl−/Na@C12 | −2.67 | 2.15 | 4.82 | −1.89 | 0.95 | −0.05 |
| Cl−/Na+@C12 | −7.67 | −2.21 | 5.46 | −0.98 | 0.96 | 0.02 |
| F−@C12 | −3.54 | 2.03 | 5.58 | −0.62 | ----- | −0.37 |
| F−/Na@C12 | −2.53 | 2.16 | 4.69 | −1.56 | 0.95 | −0.39 |
| F−/Na+@C12 | −7.68 | −2.12 | 5.56 | −0.62 | 0.96 | −0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhammad, R.; Gulzar, A.; Kosar, N.; Mahmood, T. DFT-Guided Next-Generation Na-Ion Batteries Powered by Halogen-Tuned C12 Nanorings. Computation 2025, 13, 180. https://doi.org/10.3390/computation13080180
Muhammad R, Gulzar A, Kosar N, Mahmood T. DFT-Guided Next-Generation Na-Ion Batteries Powered by Halogen-Tuned C12 Nanorings. Computation. 2025; 13(8):180. https://doi.org/10.3390/computation13080180
Chicago/Turabian StyleMuhammad, Riaz, Anam Gulzar, Naveen Kosar, and Tariq Mahmood. 2025. "DFT-Guided Next-Generation Na-Ion Batteries Powered by Halogen-Tuned C12 Nanorings" Computation 13, no. 8: 180. https://doi.org/10.3390/computation13080180
APA StyleMuhammad, R., Gulzar, A., Kosar, N., & Mahmood, T. (2025). DFT-Guided Next-Generation Na-Ion Batteries Powered by Halogen-Tuned C12 Nanorings. Computation, 13(8), 180. https://doi.org/10.3390/computation13080180








