Deep Learning Segmentation Techniques for Atherosclerotic Plaque on Ultrasound Imaging: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Analysis
3. Results
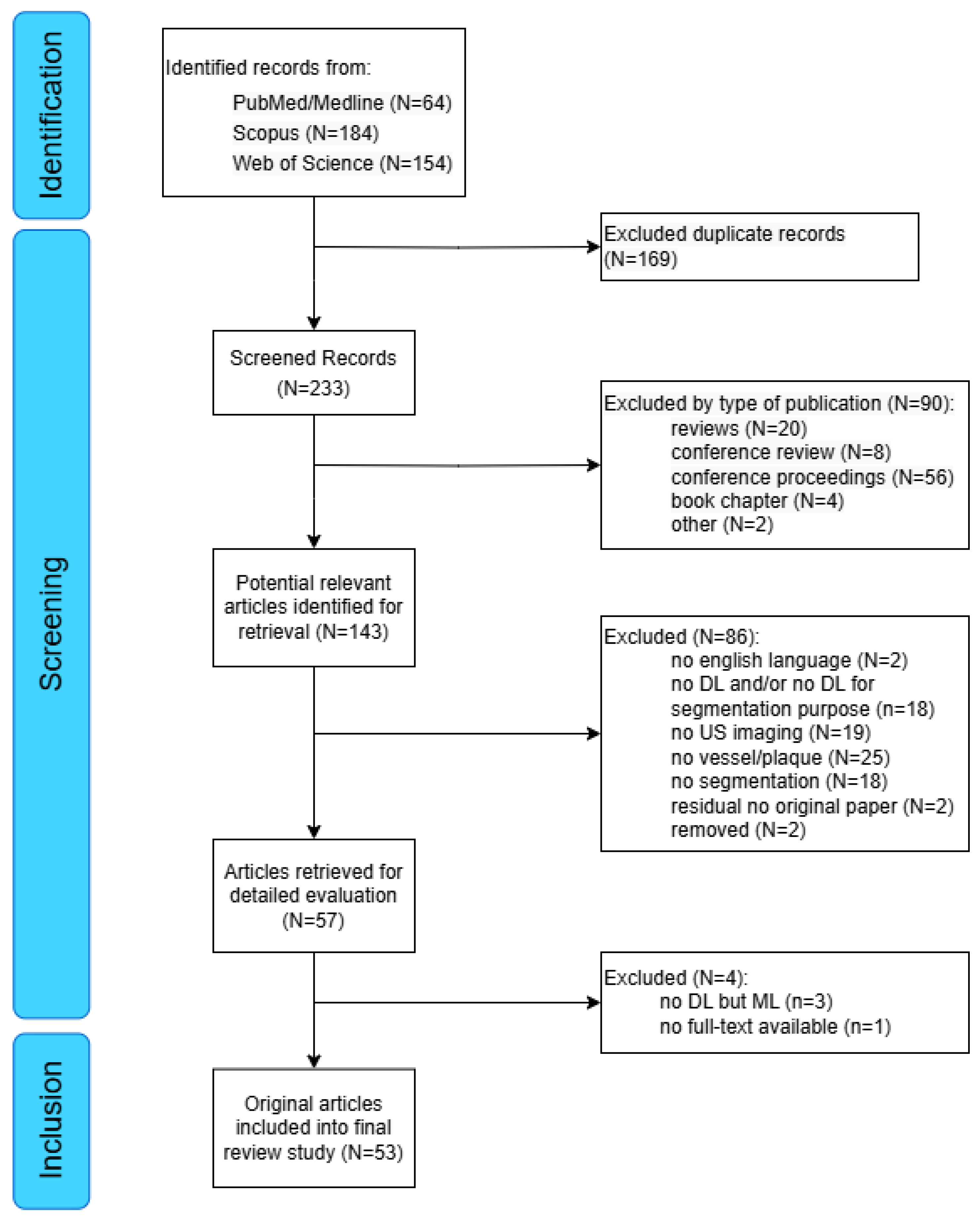
3.1. Search Results
3.2. Carotid US
3.3. Coronary Intravascular US
3.4. Quantification of Plaques
3.5. Plaque Characterization/Classification
3.6. Direct DL Plaque Segmentation
4. Discussion
Limitation of the Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| US | Ultrasound |
| DL | Deep learning |
| CV | Cardiovascular |
| IMT | Intima–media thickness |
| IVUS | Intravascular ultrasound |
| ML | Machine learning |
| TL | Transfer learning |
| SegNet | Semantic segmentation network |
| PSPNet | Pyramid scene parsing network |
| CUS | Conventional ultrasound |
| AI | Artificial intelligence |
| WoS | Web of Science |
| PRISMA | Preferred Reporting for Systematic Reviews and Meta-Analysis |
| CCA | Common carotid artery |
| SSI | Stenosis severity index |
| CNN | Convolutional neural network |
| DCNN | Dynamic convolutional neural network |
| CASM | Convolutional Attention Shrinkage Module |
| VGG | Visual Geometry Group |
| WAL-Net | Weakly supervised auxiliary task learning network model |
| AMPTS | Automatic Multi-Plaque Tracking and Segmentation |
| EEM | External elastic membrane |
| CSA | Cross-sectional area |
| PB | Plaque burden |
| TPA | Total plaque area |
| ICA | Internal carotid artery |
| OP | Operator |
| ResNet | Residual network |
| ROI | Region of interest |
| DSC | Dice similarity coefficient |
References
- Stone, N.J.; Smith, S.C.; Orringer, C.E.; Rigotti, N.A.; Navar, A.M.; Khan, S.S.; Jones, D.W.; Goldberg, R.; Mora, S.; Blaha, M.; et al. Managing Atherosclerotic Cardiovascular Risk in Young Adults. J. Am. Coll. Cardiol. 2022, 79, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction—From Atherosclerosis to Thrombosis. Arter. Thromb. Vasc. Biol. 2019, 39, e176–e185. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.V.; Fuster, V.; Bundgaard, H.; Fuster, J.J.; Johri, A.M.; Kofoed, K.F.; Douglas, P.S.; Diederichsen, A.; Shapiro, M.D.; Nicholls, S.J.; et al. Personalized Intervention Based on Early Detection of Atherosclerosis. J. Am. Coll. Cardiol. 2024, 83, 2112–2127. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Eremin, I.I.; Nadelyaeva, I.I.; Orekhov, A.N. Diagnostics of Atherosclerosis: Overview of the Existing Methods. Front. Cardiovasc. Med. 2023, 10, 1134097. [Google Scholar] [CrossRef]
- Faita, F.; Gemignani, V.; Bianchini, E.; Giannarelli, C.; Ghiadoni, L.; Demi, M. Real-Time Measurement System for Evaluation of the Carotid Intima-Media Thickness with a Robust Edge Operator. J. Ultrasound Med. 2008, 27, 1353–1361. [Google Scholar] [CrossRef]
- Molinari, F.; Pattichis, C.S.; Zeng, G.; Saba, L.; Acharya, U.R.; Sanfilippo, R.; Nicolaides, A.; Suri, J.S. Completely Automated Multiresolution Edge Snapper—A New Technique for an Accurate Carotid Ultrasound IMT Measurement: Clinical Validation and Benchmarking on a Multi-Institutional Database. IEEE Trans. Image Process. 2012, 21, 1211–1222. [Google Scholar] [CrossRef]
- Bianchini, E.; Giannarelli, C.; Maria Bruno, R.; Armenia, S.; Landini, L.; Faita, F.; Gemignani, V.; Taddei, S.; Ghiadoni, L. Functional and Structural Alterations of Large Arteries: Methodological Issues. Curr. Pharm. Des. 2013, 19, 2390–2400. [Google Scholar] [CrossRef]
- de Groot, E.; Hovingh, G.K.; Wiegman, A.; Duriez, P.; Smit, A.J.; Fruchart, J.-C.; Kastelein, J.J.P. Measurement of Arterial Wall Thickness as a Surrogate Marker for Atherosclerosis. Circulation 2004, 109, III-33-38. [Google Scholar] [CrossRef]
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef]
- Brinjikji, W.; Huston, J.; Rabinstein, A.A.; Kim, G.-M.; Lerman, A.; Lanzino, G. Contemporary Carotid Imaging: From Degree of Stenosis to Plaque Vulnerability. J. Neurosurg. 2016, 124, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, E.; Bozec, E.; Gemignani, V.; Faita, F.; Giannarelli, C.; Ghiadoni, L.; Demi, M.; Boutouyrie, P.; Laurent, S. Assessment of Carotid Stiffness and Intima-Media Thickness From Ultrasound Data. J. Ultrasound Med. 2010, 29, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Molinari, F.; Meiburger, K.M.; Saba, L.; Zeng, G.; Acharya, U.R.; Ledda, M.; Nicolaides, A.; Suri, J.S. Fully Automated Dual-Snake Formulation for Carotid Intima-Media Thickness Measurement. J. Ultrasound Med. 2012, 31, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Shah, M. A Fast Algorithm for Active Contours and Curvature Estimation. CVGIP Image Underst. 1992, 55, 14–26. [Google Scholar] [CrossRef]
- Xia, M.; Yan, W.; Huang, Y.; Guo, Y.; Zhou, G.; Wang, Y. IVUS Image Segmentation Using Superpixel-Wise Fuzzy Clustering and Level Set Evolution. Appl. Sci. 2019, 9, 4967. [Google Scholar] [CrossRef]
- Xia, M.; Yan, W.; Huang, Y.; Guo, Y.; Zhou, G.; Wang, Y. IVUS Images Segmentation Using Spatial Fuzzy Clustering and Hierarchical Level Set Evolution. Comput. Biol. Med. 2019, 109, 207–217. [Google Scholar] [CrossRef]
- Qian, C.; Yang, X. An Integrated Method for Atherosclerotic Carotid Plaque Segmentation in Ultrasound Image. Comput. Methods Programs Biomed. 2018, 153, 19–32. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Changaai Mangalote, I.A.; Meher, P.K.; Aboumarzouk, O.; Al-Ansari, A.; Halabi, O.; Dakua, S.P. Advancements in Deep Learning for B-Mode Ultrasound Segmentation: A Comprehensive Review. IEEE Trans. Emerg. Top. Comput. Intell. 2024, 8, 2126–2149. [Google Scholar] [CrossRef]
- Shen, D.; Wu, G.; Suk, H.-I. Deep Learning in Medical Image Analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Peñalvo, J.L.; Fernández-Ortiz, A.; Ibañez, B.; López-Melgar, B.; Laclaustra, M.; Oliva, B.; Mocoroa, A.; Mendiguren, J.; Martínez de Vega, V.; et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort. Circulation 2015, 131, 2104–2113. [Google Scholar] [CrossRef] [PubMed]
- Baber, U.; Mehran, R.; Sartori, S.; Schoos, M.M.; Sillesen, H.; Muntendam, P.; Garcia, M.J.; Gregson, J.; Pocock, S.; Falk, E.; et al. Prevalence, Impact, and Predictive Value of Detecting Subclinical Coronary and Carotid Atherosclerosis in Asymptomatic Adults. J. Am. Coll. Cardiol. 2015, 65, 1065–1074. [Google Scholar] [CrossRef]
- Stefanadis, C.; Antoniou, C.; Tsiachris, D.; Pietri, P. Coronary Atherosclerotic Vulnerable Plaque: Current Perspectives. J. Am. Heart Assoc. 2017, 6, e005543. [Google Scholar] [CrossRef]
- Ottakath, N.; Al-Maadeed, S.; Zughaier, S.M.; Elharrouss, O.; Mohammed, H.H.; Chowdhury, M.E.H.; Bouridane, A. Ultrasound-Based Image Analysis for Predicting Carotid Artery Stenosis Risk: A Comprehensive Review of the Problem, Techniques, Datasets, and Future Directions. Diagnostics 2023, 13, 2614. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2015: 18th International Conference, Munich, Germany, 5–9 October 2015; Lecture Notes in Computer Science. Springer: Cham, Switzderland, 2015; Volume 9351, pp. 234–241. [Google Scholar] [CrossRef]
- Chen, L.-C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. DeepLab: Semantic Image Segmentation with Deep Convolutional Nets, Atrous Convolution, and Fully Connected CRFs. IEEE Trans. Pattern Anal. Mach. Intell. 2018, 40, 834–848. [Google Scholar] [CrossRef]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. SegNet: A Deep Convolutional Encoder-Decoder Architecture for Image Segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef]
- Zhao, H.; Shi, J.; Qi, X.; Wang, X.; Jia, J. Pyramid Scene Parsing Network. In Proceedings of the 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Honolulu, HI, USA, 21–26 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 6230–6239. [Google Scholar] [CrossRef]
- Chen, L.-C.; Zhu, Y.; Papandreou, G.; Schroff, F.; Adam, H. Encoder-Decoder with Atrous Separable Convolution for Semantic Image Segmentation. In Proceedings of the European Conference on Computer Vision (ECCV), Munich, Germany, 8–14 September 2018; Lecture Notes in Computer Science. Springer: Cham, Switzerland, 2018; Volume 11211, pp. 833–851. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Behnam, H.; Gifani, P. Ultrasound Image Analysis with Vision Transformers—Review. Diagnostics 2024, 14, 542. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Gan, H.; Zhou, R.; Ou, Y.; Wang, F.; Cheng, X.; Fu, L.; Fenster, A. A Region and Category Confidence-Based Multi-Task Network for Carotid Ultrasound Image Segmentation and Classification. IEEE J. Biomed. Health Inform. 2025, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; An, H.; Zhang, J.; Huang, D.; Wen, J. DPSMUNet: A New Network Based on a Dual-Pooling Self-Attention Module for Carotid Artery Plaque Segmentation in Ultrasound Images. J. Supercomput. 2025, 81, 267. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Song, S.; Chen, H.; Shi, J.; Xu, D.; Zhang, H.; Chen, M.; Zheng, R. Automatic Diagnosis of Carotid Atherosclerosis Using a Portable Freehand 3-D Ultrasound Imaging System. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2024, 71, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Fu, L.; Zhou, R.; Gan, W.; Wang, F.; Wu, X.; Yang, Z.; Huang, Z. WAL-Net: Weakly Supervised Auxiliary Task Learning Network for Carotid Plaques Classification. Eng. Appl. Artif. Intell. 2024, 137, 109144. [Google Scholar] [CrossRef]
- Gao, W.; Liu, M.; Xu, J.; Hong, S.; Chen, J.; Cui, C.; Shi, S.; Dong, Y.; Song, D.; Dong, F. A Video-Based Automated Tracking and Analysis System of Plaque Burden in Carotid Artery Using Deep Learning: A Comparison with Senior Sonographers. Curr. Med. Imaging Rev. 2024, 20, e15734056296233. [Google Scholar] [CrossRef] [PubMed]
- Kybic, J.; Pakizer, D.; Kozel, J.; Michalčová, P.; Charvát, F.; Školoudík, D. Carotid Atherosclerotic Plaque Stability Prediction from Transversal Ultrasound Images Using Deep Learning. Česká A Slov. Neurol. A Neurochir. 2024, 87, 255–263. [Google Scholar] [CrossRef]
- Liapi, G.D.; Loizou, C.P.; Pattichis, C.S.; Pattichis, M.S.; Nicolaides, A.N.; Griffin, M.; Kyriacou, E. Assessing the Impact of Ultrasound Image Standardization in Deep Learning-Based Segmentation of Carotid Plaque Types. Comput. Methods Programs Biomed. 2024, 257, 108460. [Google Scholar] [CrossRef]
- Liu, M.; Gao, W.; Song, D.; Dong, Y.; Hong, S.; Cui, C.; Shi, S.; Wu, K.; Chen, J.; Xu, J.; et al. A Deep Learning-Based Calculation System for Plaque Stenosis Severity on Common Carotid Artery of Ultrasound Images. Vascular 2024, 33, 349–356. [Google Scholar] [CrossRef]
- Xie, X.; Liu, P.; Lang, Y.; Guo, Z.; Yang, Z.; Zhao, Y. US-Net: U-Shaped Network with Convolutional Attention Mechanism for Ultrasound Medical Images. Comput. Graph. 2024, 124, 104054. [Google Scholar] [CrossRef]
- Biswas, M.; Saba, L.; Kalra, M.; Singh, R.; Fernandes e Fernandes, J.; Viswanathan, V.; Laird, J.R.; Mantella, L.E.; Johri, A.M.; Fouda, M.M.; et al. MultiNet 2.0: A Lightweight Attention-Based Deep Learning Network for Stenosis Measurement in Carotid Ultrasound Scans and Cardiovascular Risk Assessment. Comput. Med. Imaging Graph. 2024, 117, 102437. [Google Scholar] [CrossRef]
- Ding, J.; Zhou, R.; Fang, X.; Wang, F.; Wang, J.; Gan, H.; Fenster, A. An Image Registration-Based Self-Supervised Su-Net for Carotid Plaque Ultrasound Image Segmentation. Comput. Methods Programs Biomed. 2024, 244, 107957. [Google Scholar] [CrossRef]
- Deng, C.; Adu, J.; Xie, S.; Li, Z.; Meng, Q.; Zhang, Q.; Yin, L.; Peng, B. Automatic Segmentation of Ultrasound Images of Carotid Atherosclerotic Plaque Based on Dense-UNet. Technol. Health Care 2023, 31, 165–179. [Google Scholar] [CrossRef]
- Hu, X.; Cao, Y.; Hu, W.; Zhang, W.; Li, J.; Wang, C.; Mukhopadhyay, S.C.; Li, Y.; Liu, Z.; Li, S. Refined Feature-Based Multi-Frame and Multi-Scale Fusing Gate Network for Accurate Segmentation of Plaques in Ultrasound Videos. Comput. Biol. Med. 2023, 163, 107091. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, M.; Chan, W.S.; Chiu, B. Development of a Three-Dimensional Carotid Ultrasound Image Segmentation Workflow for Improved Efficiency, Reproducibility and Accuracy in Measuring Vessel Wall and Plaque Volume and Thickness. Bioengineering 2023, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, F.; Fang, X.; Fenster, A.; Gan, H. An Adaptively Weighted Ensemble of Multiple CNNs for Carotid Ultrasound Image Segmentation. Biomed. Signal Process. Control 2023, 83, 104673. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, T.; He, Y.; Xie, S.; Luo, A.; Peng, B.; Yin, L. Contrast U-Net Driven by Sufficient Texture Extraction for Carotid Plaque Detection. Math. Biosci. Eng. 2023, 20, 15623–15640. [Google Scholar] [CrossRef]
- Chen, X.; Kong, Z.; Wei, S.; Liang, F.; Feng, T.; Wang, S.; Gao, J. Ultrasound Lmaging-Vulnerable Plaque Diagnostics: Automatic Carotid Plaque Segmentation Based on Deep Learning. J. Radiat. Res. Appl. Sci. 2023, 16, 100598. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Huang, Y.; Zhu, W.; Zhao, C.; Wang, Y.; Chen, M.; Yu, J. BP-Net: Boundary and Perfusion Feature Guided Dual-Modality Ultrasound Video Analysis Network for Fibrous Cap Integrity Assessment. Comput. Med. Imaging Graph. 2023, 107, 102246. [Google Scholar] [CrossRef]
- Zhou, R.; Ou, Y.; Fang, X.; Azarpazhooh, M.R.; Gan, H.; Ye, Z.; Spence, J.D.; Xu, X.; Fenster, A. Ultrasound Carotid Plaque Segmentation via Image Reconstruction-Based Self-Supervised Learning with Limited Training Labels. Math. Biosci. Eng. 2023, 20, 1617–1636. [Google Scholar] [CrossRef]
- Gago, L.; del Mar Vila, M.; Grau, M.; Remeseiro, B.; Igual, L. An End-to-End Framework for Intima Media Measurement and Atherosclerotic Plaque Detection in the Carotid Artery. Comput. Methods Programs Biomed. 2022, 223, 106954. [Google Scholar] [CrossRef]
- Jain, P.K.; Dubey, A.; Saba, L.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Fouda, M.M.; Suri, J.S.; Sharma, N. Attention-Based UNet Deep Learning Model for Plaque Segmentation in Carotid Ultrasound for Stroke Risk Stratification: An Artificial Intelligence Paradigm. J. Cardiovasc. Dev. Dis. 2022, 9, 326. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Kalra, M.K.; Johri, A.; Saba, L.; Suri, J.S. Far Wall Plaque Segmentation and Area Measurement in Common and Internal Carotid Artery Ultrasound Using U-Series Architectures: An Unseen Artificial Intelligence Paradigm for Stroke Risk Assessment. Comput. Biol. Med. 2022, 149, 106017. [Google Scholar] [CrossRef]
- Li, Y.; Zou, L.; Xiong, L.; Yu, F.; Jiang, H.; Fan, C.; Cheng, M.; Li, Q. FRDD-Net: Automated Carotid Plaque Ultrasound Images Segmentation Using Feature Remapping and Dense Decoding. Sensors 2022, 22, 887. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, C.; Zhang, K.; Hua, Y.; Zhang, J. HRU-Net: A Transfer Learning Method for Carotid Artery Plaque Segmentation in Ultrasound Images. Diagnostics 2022, 12, 2852. [Google Scholar] [CrossRef] [PubMed]
- Dašić, L.; Radovanović, N.; Šušteršič, T.; Blagojević, A.; Benolić, L.; Filipović, N. Patch-Based Convolutional Neural Network for Atherosclerotic Carotid Plaque Semantic Segmentation. Ipsi Trans. Internet. Res. 2022, 18, 56–61. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Nicolaides, A.N.; Suri, J.S. Automated Deep Learning-Based Paradigm for High-Risk Plaque Detection in B-Mode Common Carotid Ultrasound Scans: An Asymptomatic Japanese Cohort Study. Int. Angiol. 2022, 41, 9–23. [Google Scholar] [CrossRef]
- Lin, Y.; Huang, J.; Chen, Y.; Chen, Q.; Li, Z.; Cao, Q. Intelligent Segmentation of Intima–Media and Plaque Recognition in Carotid Artery Ultrasound Images. Ultrasound Med. Biol. 2022, 48, 469–479. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Giannopoulos, A.A.; Saba, L.; Nicolaides, A.; Suri, J.S. Hybrid Deep Learning Segmentation Models for Atherosclerotic Plaque in Internal Carotid Artery B-Mode Ultrasound. Comput. Biol. Med. 2021, 136, 104721. [Google Scholar] [CrossRef]
- Jain, P.K.; Sharma, N.; Saba, L.; Paraskevas, K.I.; Kalra, M.K.; Johri, A.; Laird, J.R.; Nicolaides, A.N.; Suri, J.S. Unseen Artificial Intelligence—Deep Learning Paradigm for Segmentation of Low Atherosclerotic Plaque in Carotid Ultrasound: A Multicenter Cardiovascular Study. Diagnostics 2021, 11, 2257. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Huang, Y.; Zhu, W.; Wang, Y.; Chen, M.; Yu, J. Automatic Multi-Plaque Tracking and Segmentation in Ultrasonic Videos. Med. Image Anal. 2021, 74, 102201. [Google Scholar] [CrossRef]
- Zhou, R.; Azarpazhooh, M.R.; Spence, J.D.; Hashemi, S.; Ma, W.; Cheng, X.; Gan, H.; Ding, M.; Fenster, A. Deep Learning-Based Carotid Plaque Segmentation from B-Mode Ultrasound Images. Ultrasound Med. Biol. 2021, 47, 2723–2733. [Google Scholar] [CrossRef]
- Zhou, R.; Guo, F.; Azarpazhooh, M.R.; Hashemi, S.; Cheng, X.; Spence, J.D.; Ding, M.; Fenster, A. Deep Learning-Based Measurement of Total Plaque Area in B-Mode Ultrasound Images. IEEE J. Biomed. Health Inform. 2021, 25, 2967–2977. [Google Scholar] [CrossRef]
- Biswas, M.; Saba, L.; Chakrabartty, S.; Khanna, N.N.; Song, H.; Suri, H.S.; Sfikakis, P.P.; Mavrogeni, S.; Viskovic, K.; Laird, J.R.; et al. Two-Stage Artificial Intelligence Model for Jointly Measurement of Atherosclerotic Wall Thickness and Plaque Burden in Carotid Ultrasound: A Screening Tool for Cardiovascular/Stroke Risk Assessment. Comput. Biol. Med. 2020, 123, 103847. [Google Scholar] [CrossRef] [PubMed]
- Meshram, N.H.; Mitchell, C.C.; Wilbrand, S.; Dempsey, R.J.; Varghese, T. Deep Learning for Carotid Plaque Segmentation Using a Dilated U-Net Architecture. Ultrason Imaging 2020, 42, 221–230. [Google Scholar] [CrossRef] [PubMed]
- del Mar Vila, M.; Remeseiro, B.; Grau, M.; Elosua, R.; Betriu, À.; Fernandez-Giraldez, E.; Igual, L. Semantic Segmentation with DenseNets for Carotid Artery Ultrasound Plaque Segmentation and CIMT Estimation. Artif. Intell. Med. 2020, 103, 101784. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Guo, F.; Azarpazhooh, M.R.; Spence, J.D.; Ukwatta, E.; Ding, M.; Fenster, A. A Voxel-Based Fully Convolution Network and Continuous Max-Flow for Carotid Vessel-Wall-Volume Segmentation From 3D Ultrasound Images. IEEE Trans. Med. Imaging 2020, 39, 2844–2855. [Google Scholar] [CrossRef]
- Zhou, R.; Fenster, A.; Xia, Y.; Spence, J.D.; Ding, M. Deep Learning-based Carotid Media-adventitia and Lumen-intima Boundary Segmentation from Three-dimensional Ultrasound Images. Med. Phys. 2019, 46, 3180–3193. [Google Scholar] [CrossRef]
- Saba, L.; Biswas, M.; Suri, H.S.; Viskovic, K.; Laird, J.R.; Cuadrado-Godia, E.; Nicolaides, A.; Khanna, N.N.; Viswanathan, V.; Suri, J.S. Ultrasound-Based Carotid Stenosis Measurement and Risk Stratification in Diabetic Cohort: A Deep Learning Paradigm. Cardiovasc. Diagn. Ther. 2019, 9, 439–461. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, Y.; Hu, X.; Mi, J.; Zhang, P.; Sun, G.; Mukhopadhyay, S.C.; Li, Y.; Liu, Z. A Semi-Automatic Cardiovascular Annotation and Quantification Toolbox Utilizing Prior Knowledge-Guided Feature Learning. Biomed. Signal Process. Control 2025, 102, 107201. [Google Scholar] [CrossRef]
- Prajapati, N.K.; Patel, A.; Mewada, H. Automated Diagnosis of Atherosclerosis Using Multi-Layer Ensemble Models and Bio-Inspired Optimization in Intravascular Ultrasound Imaging. Med. Biol. Eng. Comput. 2025, 63, 213–227. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.-G.; Jeong, G.-J.; Lee, G.; Min, H.; Cho, H.; Min, D.; Lee, S.-W.; Cho, J.H.; Cho, S.; et al. Deep Learning Model for Intravascular Ultrasound Image Segmentation with Temporal Consistency. Int. J. Cardiovasc. Imaging 2024, 40, 2283–2292. [Google Scholar] [CrossRef]
- Li, X.; Song, P.; Lv, T.; Jiao, Y.; Guo, Y.; Zhang, Y.; Wang, N.; Yang, J.; Cui, Y. An Automatic Pipeline for Segmentation and Quantification of Intravascular Ultrasound Images. Biomed. Signal Process. Control 2024, 94, 106208. [Google Scholar] [CrossRef]
- Liu, X.; Feng, T.; Liu, W.; Song, L.; Yuan, Y.; Hau, W.K.; Del Ser, J.; Gao, Z. Scale Mutualized Perception for Vessel Border Detection in Intravascular Ultrasound Images. IEEE/ACM Trans. Comput. Biol. Bioinform. 2024, 21, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.-J.; Lee, G.; Lee, J.-G.; Kang, S.-J. Deep Learning-Based Lumen and Vessel Segmentation of Intravascular Ultrasound Images in Coronary Artery Disease. Korean Circ. J. 2024, 54, 30. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bajaj, R.; Li, Y.; Ye, X.; Lin, J.; Pugliese, F.; Ramasamy, A.; Gu, Y.; Wang, Y.; Torii, R.; et al. POST-IVUS: A Perceptual Organisation-Aware Selective Transformer Framework for Intravascular Ultrasound Segmentation. Med. Image Anal. 2023, 89, 102922. [Google Scholar] [CrossRef]
- Kyriakidis, S.; Rigas, G.; Kigka, V.; Zaridis, D.; Karanasiou, G.; Tsompou, P.; Karanasiou, G.; Lakkas, L.; Nikopoulos, S.; Naka, K.K.; et al. An All-in-One Tool for 2D Atherosclerotic Disease Assessment and 3D Coronary Artery Reconstruction. J. Cardiovasc. Dev. Dis. 2023, 10, 130. [Google Scholar] [CrossRef]
- Meng, L.; Jiang, M.; Zhang, C.; Zhang, J. Deep Learning Segmentation, Classification, and Risk Prediction of Complex Vascular Lesions on Intravascular Ultrasound Images. Biomed. Signal Process. Control 2023, 82, 104584. [Google Scholar] [CrossRef]
- Prajapati, N.K.; Patel, A.V. Optimal Deep Learning Based Atherosclerotic Plaque Classification on Intravascular Ultrasound Images. Int. J. Intell. Eng. Syst. 2023, 16, 691–708. [Google Scholar] [CrossRef]
- Zhu, F.; Gao, Z.; Zhao, C.; Zhu, H.; Nan, J.; Tian, Y.; Dong, Y.; Jiang, J.; Feng, X.; Dai, N.; et al. A Deep Learning-Based Method to Extract Lumen and Media-Adventitia in Intravascular Ultrasound Images. Ultrason Imaging 2022, 44, 191–203. [Google Scholar] [CrossRef]
- Blanco, P.J.; Ziemer, P.G.P.; Bulant, C.A.; Ueki, Y.; Bass, R.; Räber, L.; Lemos, P.A.; García-García, H.M. Fully Automated Lumen and Vessel Contour Segmentation in Intravascular Ultrasound Datasets. Med. Image Anal. 2022, 75, 102262. [Google Scholar] [CrossRef]
- Du, H.; Ling, L.; Yu, W.; Wu, P.; Yang, Y.; Chu, M.; Yang, J.; Yang, W.; Tu, S. Convolutional Networks for the Segmentation of Intravascular Ultrasound Images: Evaluation on a Multicenter Dataset. Comput. Methods Programs Biomed. 2022, 215, 106599. [Google Scholar] [CrossRef]
- Bajaj, R.; Huang, X.; Kilic, Y.; Ramasamy, A.; Jain, A.; Ozkor, M.; Tufaro, V.; Safi, H.; Erdogan, E.; Serruys, P.W.; et al. Advanced Deep Learning Methodology for Accurate, Real-Time Segmentation of High-Resolution Intravascular Ultrasound Images. Int. J. Cardiol. 2021, 339, 185–191. [Google Scholar] [CrossRef]
- Li, Y.-C.; Shen, T.-Y.; Chen, C.-C.; Chang, W.-T.; Lee, P.-Y.; Huang, C.-C.J. Automatic Detection of Atherosclerotic Plaque and Calcification From Intravascular Ultrasound Images by Using Deep Convolutional Neural Networks. IEEE Trans. Ultrason Ferroelectr. Freq. Control 2021, 68, 1762–1772. [Google Scholar] [CrossRef] [PubMed]
- Oktay, O.; Schlemper, J.; Folgoc, L.L.; Lee, M.; Heinrich, M.; Misawa, K.; Mori, K.; McDonagh, S.; Hammerla, N.Y.; Kainz, B.; et al. Attention U-Net: Learning Where to Look for the Pancreas. arXiv 2018, arXiv:1804.03999. [Google Scholar]
- Litjens, G.; Ciompi, F.; Wolterink, J.M.; de Vos, B.D.; Leiner, T.; Teuwen, J.; Išgum, I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc. Imaging 2019, 12, 1549–1565. [Google Scholar] [CrossRef] [PubMed]
- Biccirè, F.G.; Mannhart, D.; Kakizaki, R.; Windecker, S.; Räber, L.; Siontis, G.C.M. Automatic Assessment of Atherosclerotic Plaque Features by Intracoronary Imaging: A Scoping Review. Front. Cardiovasc. Med. 2024, 11, 1332925. [Google Scholar] [CrossRef]
- Balocco, S.; Gatta, C.; Ciompi, F.; Wahle, A.; Radeva, P.; Carlier, S.; Unal, G.; Sanidas, E.; Mauri, J.; Carillo, X.; et al. Standardized Evaluation Methodology and Reference Database for Evaluating IVUS Image Segmentation. Comput. Med. Imaging Graph. 2014, 38, 70–90. [Google Scholar] [CrossRef]

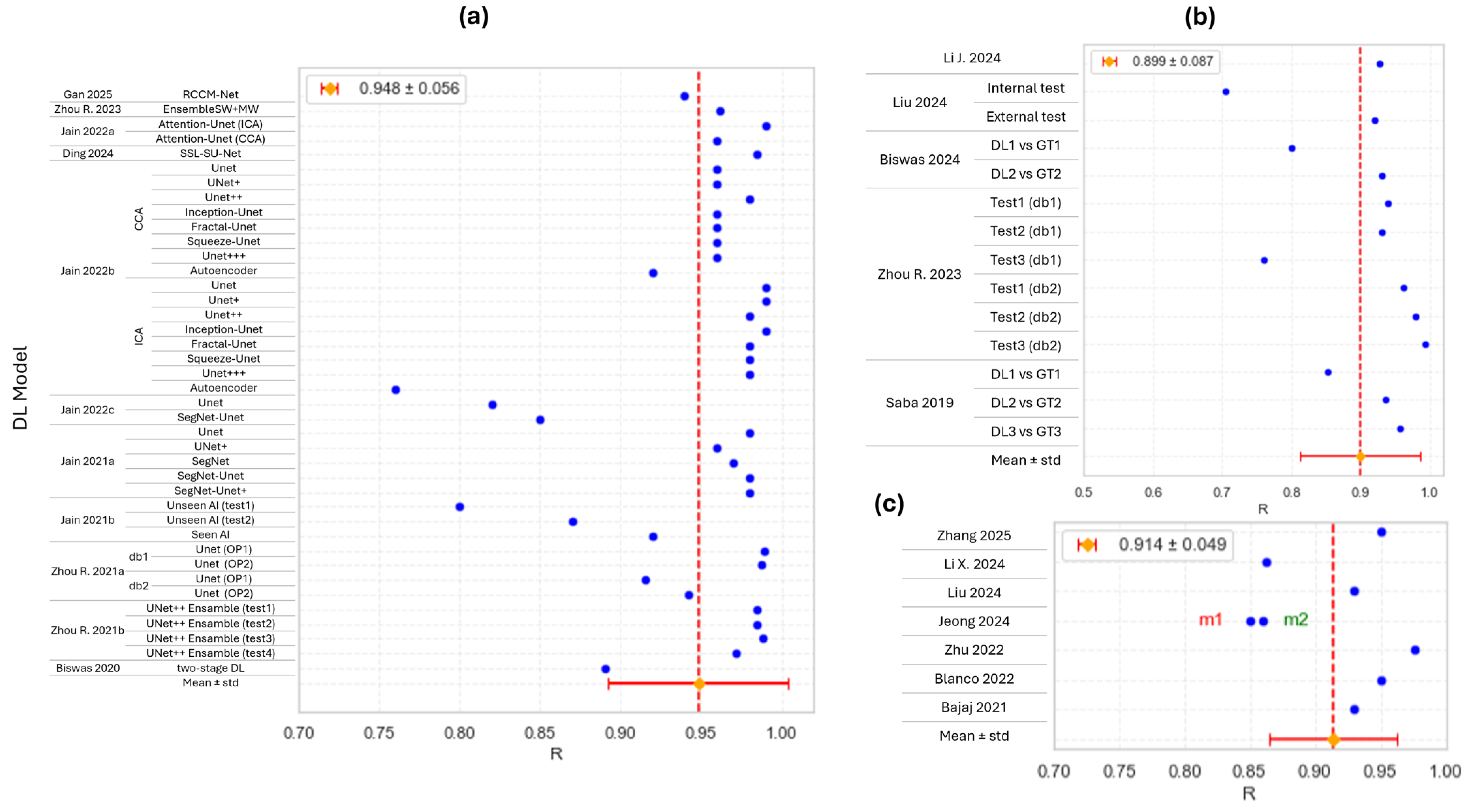

| Ref. | N Subjects/N Images | US Imaging | Carotid Site | Aim of the Study | Proposed Task | Main Results |
|---|---|---|---|---|---|---|
| [32] | 844/1270 | CUS | CCA, ICA, ECA | Plaque segmentation and classification with multi-task learning framework using RCCM-Net | Segmentation, Classification | DSC: 84.92 ± 0.40; R TPA: 0.939; Acc: 91.1/92.3 hyper-/hypo-echoic plaques |
| [33] | na/450 | CUS | na | Plaque segmentation using UNet integrating a self-attention mechanism | Segmentation | DSC: 80.8 ± 15.8 |
| [34] | 83/7036 | CUS | CCA | SSI assessment and presence/absence plaque classification via portable free-hand 3D-US system | Classification, Quantification | R SSI%: 0.76; Acc plaque yes/no 92/80 frame-/video-based |
| [35] | 844/1270 | CUS | na | Plaque segmentation/classification (hypo-/hyper-/mixed-echoic) using an end-to-end multi-task learning network | Segmentation, Classification | Acc: 90.7/92.1 hyper-/hypo-echoic plaques |
| [36] | 88/11,048 | CUS | CCA | Quantification of maximum PB on US-video | Quantification | R PB: 0.61 |
| [37] | 413/4652 | CUS | ICA | Automated plaque stability prediction and plaque width assessment | Segmentation, Quantification, Classification | Sen/Spe vulnerable/stable plaque: 72.5/48.5; R thickness: 0.32 |
| [38] | 276/276 | CUS | CCA, ICA, ECA | Plaque segmentation and effect of image standardization | Segmentation | DSC: 84.4 ± 8.1 |
| [39] | 491/512 | CUS | CCA | Segmentation of IMT and quantification of SSI | Quantification | R SSI%: 0.928/0.704 internal/external test |
| [40] | 134/659 | CUS | na | Plaque segmentation using a U-Net integrating attention mechanism | Segmentation | DSC: 82.54 ± 0.73 |
| [41] | 204/407 | CUS | CCA | Lumen segmentation and SSI measurement using DL with attention mechanisms | Classification, Quantification | R SSI%: 0.92/0.8 DL1/DL2; AUC stenosis risk: 0.88/0.98/1 and 0.93/0.97/1 low/moderate/high risk for DL1 and DL2, respectively |
| [42] | 144/506, 497/636 | CUS | na | Plaque segmentation using an image registration-based self-supervised learning method | Segmentation, Quantification | DSC: 80.25 ± 9.57 (n = 10), 85.40 ± 6.67 (n = 33), 86.72 ± 5.72 (n = 50), 89.18 ± 4.56 (n = 100); R TPA: 0.985 |
| [43] | >200/na | CUS | na | Plaque segmentation | Segmentation | DSC: 93.81 |
| [44] | 157/5662, 8/4889 | CUS | na | Real-time plaque segmentation using a Spatial–Temporal Feature Filter and multiscale features | Segmentation | DSC: 85.98 (DB1), 89.44 (DB2) |
| [45] | na/84 (baseline) + 84 (FU) volumes | 3D US | na | Evaluation of 3D-US image segmentation workflow and VWV/WVT quantification | Quantification | R VWV: 0.69/0.77 patient-/time-based; R VWT: 0.69/0.73 patient-/time-based |
| [46] | 144/510 | CUS | na | Combination of CNN models to improve accuracy and segmentation performance | Segmentation, Quantification | DSC: 88.88 ± 4.36, R TPA: 0.967 |
| [47] | 117/117 | CUS | na | Optimization of plaque segmentation using texture information from US images | Segmentation | Acc mean: 80.33 |
| [48] | na/568 | Doppler US | na | Plaque segmentation and vulnerable/stable classification with DL system | Segmentation, Classification | Acc and AUC vulnerable/stable: 92.94/89.41 and 0.915/0.853 Inception_v3/ResNet50 |
| [49] | 245/na | CUS + CEUS | na | Classification of fibrous cap integrity of plaque | Segmentation, Classification | Acc and AUC vulnerable/stable: 92.35 and 0.935 |
| [50] | 144/510, 497/638 | CUS | na | Plaque segmentation and TPA quantification in limited labeled training dataset using self-supervised learning | Segmentation, Quantification | DSC/R TPA: 80.61 ± 9.75/0.852 DB1, 84.91 ± 6.75/0.936 DB2, 85.69 ± 6.71/0.957 DB3, on external Zhongnan test |
| [51] | 2379/8448 | CUS | CCA, Bulb | Framework for quantification of IMT and classification based on presence/absence plaque | Classification | Acc plaque yes/no: 97/81 CCA/bulb |
| [52] | 99/970, 190/379, 50/300 | CUS | ICA, CCA | Plaque segmentation and stroke risk assessment with attention-based DL model | Segmentation, Quantification | DSC: 89.90 ± 3.69 ICA, 86.50 ± 5.94 CCA; R TPA: 0.99 ICA, 0.96 CCA |
| [53] | 99/970, 190/379, 50/300 | CUS | ICA, CCA | Plaque segmentation and measurement of TPA by hybrid DL architectures | Segmentation, Quantification | DSC min–max: 78.88–88.37 (CCA); 75.14–90.02 (ICA) |
| [54] | na/4384, na/431 | CUS | na | Encoder–decoder architecture for automated plaque segmentation | Segmentation | DSC: 83.65 |
| [55] | 90/115 | CUS | ICA, CCA | Improvement of plaque segmentation and estimation of TPA using transfer learning | Segmentation, Quantification | DSC: 82.1 ± 5.3 |
| [56] | 108/67 | CUS + Doppler US | CCA | Identification of plaque components using a patch-based DL method | Segmentation | JSC: 67.34/25.17/26.54 fibrous/lipid/calcified plaque |
| [57] | 190/379 | CUS | CCA | High-risk plaque segmentation and TPA quantification with a hybrid DL method | Segmentation, Quantification, Classification | DSC: 88.23 ± 7.75, R TPA: 0.82 (UNet), 0.85 (SegNet-UNet); AUC stenosis risk: 0.94 (UNet), 0.93 (SegNet-UNet) |
| [58] | na/2096 | CUS | na | Intima–media segmentation and plaque thickness assessment based on 2D images | Quantification | R2 thickness: 0.982 |
| [59] | 99/970 | CUS | left/right ICA | Comparison among solo DL and hybrid DL models for plaque segmentation and quantification | Segmentation, Quantification | DSC/R TPA: 88.98 ± 1.04/0.974 (cross entropy-loss), 86.98 ± 0.74/0.978 (DSC-loss) |
| [60] | 165/630, 50/300 | CUS | CCA | Investigation of “unseen AI” paradigm for plaque segmentation across ethnic groups | Segmentation, Quantification | DSC/R TPA: 78.38 ± 10.11/0.8 (UnseenAI-1), 82.49 ± 8.44/0.87 (UnseenAI-2), 86.89 ± 6.43/0.92 (SeenAI/Mixed) |
| [61] | 295/25,289 | CUS | na | Multi-plaque tracking and segmentation in US-video | Segmentation | DSC: 78 ±15 (MSTUnet), 69 ± 13 (Dual Attention U-Net), 83 ± 12 (Test1), 80 ± 2 (Test2) |
| [62] | 144/510, 497/638 | CUS | CCA, ICA, ECA | Plaque segmentation and TPA measurement | Segmentation, Quantification | R TPA: test1 0.989/0.987 (OP1/OP2), Zhongnan 0.915/0.942 (OP1/OP2) |
| [63] | 144/510, 497/638 | CUS | CCA, ICA, ECA | Automated plaque segmentation and TPA measurement | Segmentation, Quantification | DSC: 83.3 ± 10.0 (DB1), 85.3 ± 8.3 (DB2), 85.0 ± 7.8 (DB3); R TPA: 0.972 (Zhongnan) |
| [64] | 204/250 | CUS | CCA | Joint detection and measurement of VWT and PB using a two-stage model | Segmentation, Quantification | R TPA: 0.89 two-stage DL |
| [65] | 101/862 | CUS | ICA, CCA, bifurcation | Plaque segmentation in severely stenotic cases | Segmentation | DSC: 55 ± 19 dilated U-Net, 84 ± 5 semi-dilated U-Net |
| [66] | 2379/8484, 27/4751 | CUS | CCA, Bulb | Plaque detection and IMT measurement using single-step semantic segmentation | Classification | Acc plaque presence: 96/78 CCA/bulb |
| [67] | na/1007, 21/21 | 3D US | CCA, bifurcation | Segmentation of MA/LI borders from 3DUS for VWV measurements | Quantification | R VWV: 0.945 external SPARC dataset |
| [68] | 38/144 | 3D US | CCA | Segmentation of MA/LI borders from 3DUS for VWV measurements | Quantification | R VWV: 0.96 |
| [69] | 204/407 | CUS | CCA | SSI measurement and risk stratification in diabetic patients | Classification, Quantification | R SSI%: 0.93/0.94/0.93 DL1/DL2/DL3; AUC stenosis risk: 0.9/0.94/0.86 DL1/DL2/DL3 |
| Ref. | N Subjects/N Images | US Imaging | Aim of the Study | Proposed Task | Main Results |
|---|---|---|---|---|---|
| [70] | 11/5625, 5/791 | IVUS | Plaque detection and classification using a toolbox for semi-automatic annotation and PB quantification | Quantification | R PB: 0.951 |
| [71] | 10/2175 | IVUS | Plaque detection and classification using a DL hybrid technique | Segmentation, Classification | DSC: 96.16 ± 1.3 (Dice-loss), 98.88 ± 1.0 (Focal-loss), 98.97 ± 2.3 (Tversky-loss); Acc plaque/calcification: 97.33/96.94 |
| [72] | 1240/191,407 | IVUS | PB quantification based on EEM/lumen segmentation | Quantification | ICC volume: 0.94 |
| [73] | 292/35,930 | IVUS | Postprocessing pipeline for automated calculation of clinical parameters of vessel and plaque | Quantification | R PB: 0.862 |
| [74] | 153/68,549 | IVUS | Vessel segmentation and PB quantification reducing scale-dependent interference | Quantification | R PB: 0.93 |
| [75] | 1063/na | IVUS | Quantification of vessel and plaque parameters from lumen/EEM segmentation | Quantification | R PB: 0.86/0.85 OP1/OP2 |
| [76] | 70/23,774, 77/435 | NIRS—IVUS | EEM/lumen segmentation using POST-IVUS framework and TPA quantification | Quantification | Modified William index: 1.248 |
| [77] | na/4197 | IVUS | 3D reconstruction and calcified/noncalcified plaques characterization tool | Segmentation, Classification | Characterization Acc: 91.43 |
| [78] | 100/5089 | IVUS | Classification of vascular lesions including plaques (fibrous/lipid/calcified) | Segmentation, Classification | DSC and Acc: 86.29 ± 1.03/84.31 ± 0.99/84.48 ± 1.18 and 95.08/95.47/94.33 for fibrous/lipid/calcific |
| [79] | 10/2175 | IVUS | Classification of plaques and calcification | Classification | Acc plaque/calcification: 96.45/97.94 |
| [80] | 18/1746 | IVUS | Lumen/MA segmentation using feature pyramid network and PB quantification | Segmentation, Quantification | R PB: 0.976 |
| [81] | 63/13,435 | IVUS | Segmentation of lumen/vessel and PB estimation using ML approach | Quantification | R PB: 0.95 |
| [82] | na/175 pullbacks | IVUS | Comparison among different CNNs for lumen/EEL segmentation and TPA quantification | Quantification | R TPA: 0.98 |
| [83] | 65/824,750 | IVUS | Segmentation of lumen in real-time high-resolution IVUS images and TPA estimation | Quantification | R PB: 0.93 |
| [84] | 18/713 | IVUS | Segmentation of MA/lumen/calcific plaque | Segmentation | DSC: 59 ± 39 (0–10% plaque), 74 ± 22 (0–10%), 84 ± 9 (>30%), 67 ± 15 (mean) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rosa, L.; L’Abbate, S.; Mota da Silva, E.; Andretta, M.; Bianchini, E.; Gemignani, V.; Kusmic, C.; Faita, F. Deep Learning Segmentation Techniques for Atherosclerotic Plaque on Ultrasound Imaging: A Systematic Review. Information 2025, 16, 491. https://doi.org/10.3390/info16060491
De Rosa L, L’Abbate S, Mota da Silva E, Andretta M, Bianchini E, Gemignani V, Kusmic C, Faita F. Deep Learning Segmentation Techniques for Atherosclerotic Plaque on Ultrasound Imaging: A Systematic Review. Information. 2025; 16(6):491. https://doi.org/10.3390/info16060491
Chicago/Turabian StyleDe Rosa, Laura, Serena L’Abbate, Eduarda Mota da Silva, Mauro Andretta, Elisabetta Bianchini, Vincenzo Gemignani, Claudia Kusmic, and Francesco Faita. 2025. "Deep Learning Segmentation Techniques for Atherosclerotic Plaque on Ultrasound Imaging: A Systematic Review" Information 16, no. 6: 491. https://doi.org/10.3390/info16060491
APA StyleDe Rosa, L., L’Abbate, S., Mota da Silva, E., Andretta, M., Bianchini, E., Gemignani, V., Kusmic, C., & Faita, F. (2025). Deep Learning Segmentation Techniques for Atherosclerotic Plaque on Ultrasound Imaging: A Systematic Review. Information, 16(6), 491. https://doi.org/10.3390/info16060491










