A Lightweight Neural Network for Cell Segmentation Based on Attention Enhancement
Abstract
1. Introduction
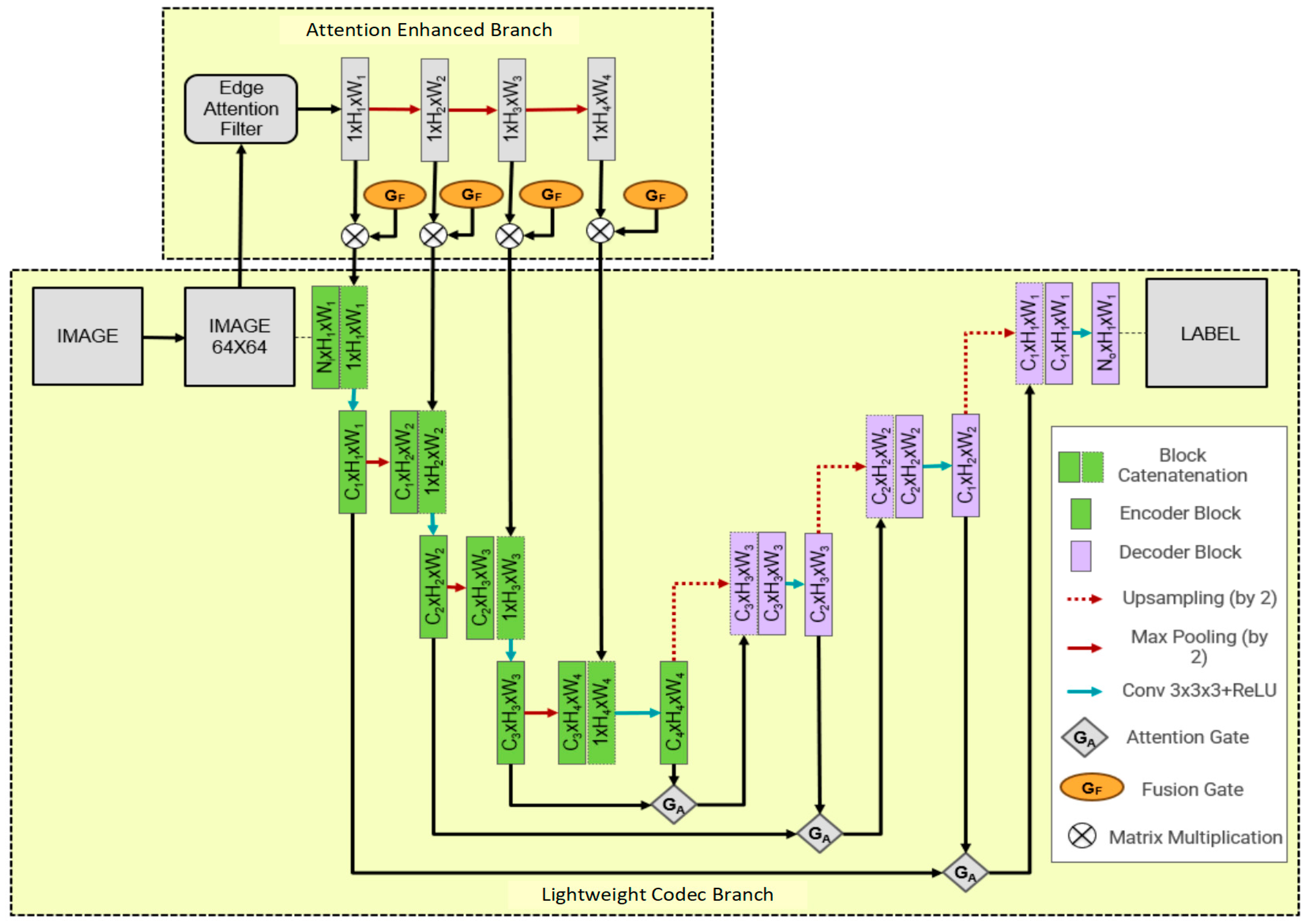
2. AttE-Unet Structure Introduction
2.1. Lightweight Feature Encoding and Decoding Branch
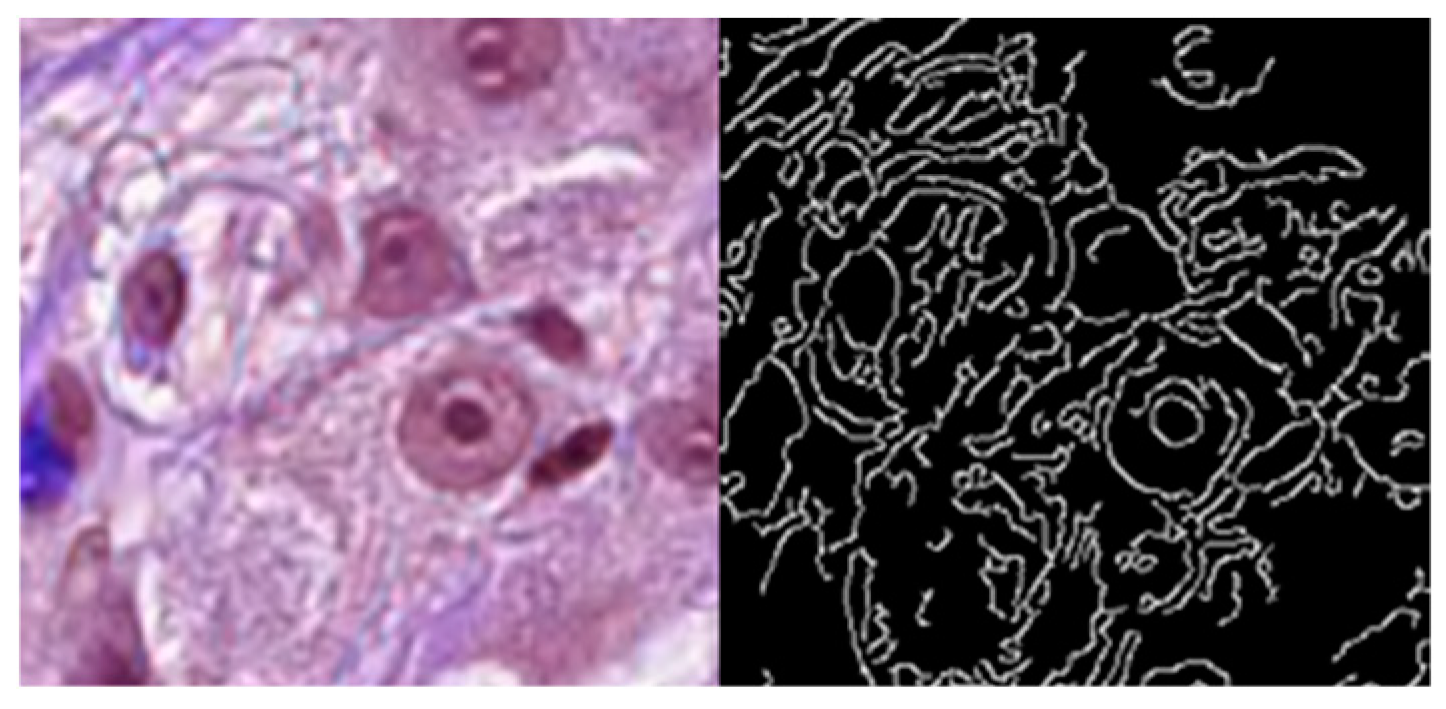
2.2. Attention Enhancement Branch
| Algorithm 1. Feature Map Fusion Process of the Edge Attention Enhancement Branch |
| Input: Original Canny edge feature maps , where k = 1, 2, 3, … Output: Fused feature maps , where k = 1, 2, 3, … |
| 1. # Max pooling operation across height and width for each level k 2. for k = 1, 2, 3, … 3. for h = 0 to H − 1 4. for w = 0 to W − 1 5. x[i, j, k + 1] = max(x[i, j, k]) # Max pooling 6. end for 7. end for 8. end for 9. 10. # Fusion of edge features with gated fusion unit (GF) 11. for k = 1, 2, 3, … 12. y[k] = [k] * x[k] # Weighted fusion through GF unit 13. end for |
| Definitions: : Edge feature maps after average pooling at level k. : Fused feature maps after modulation by fusion gates (GF). x[i, j, k]: Pixel value at coordinate (i, j) in feature map at level k. : Pixel value at coordinate (i, j) in fused feature map at level k. : Fusion weight for level k, provided by the GF unit. |
3. Results and Discussion
3.1. Experimental Setup
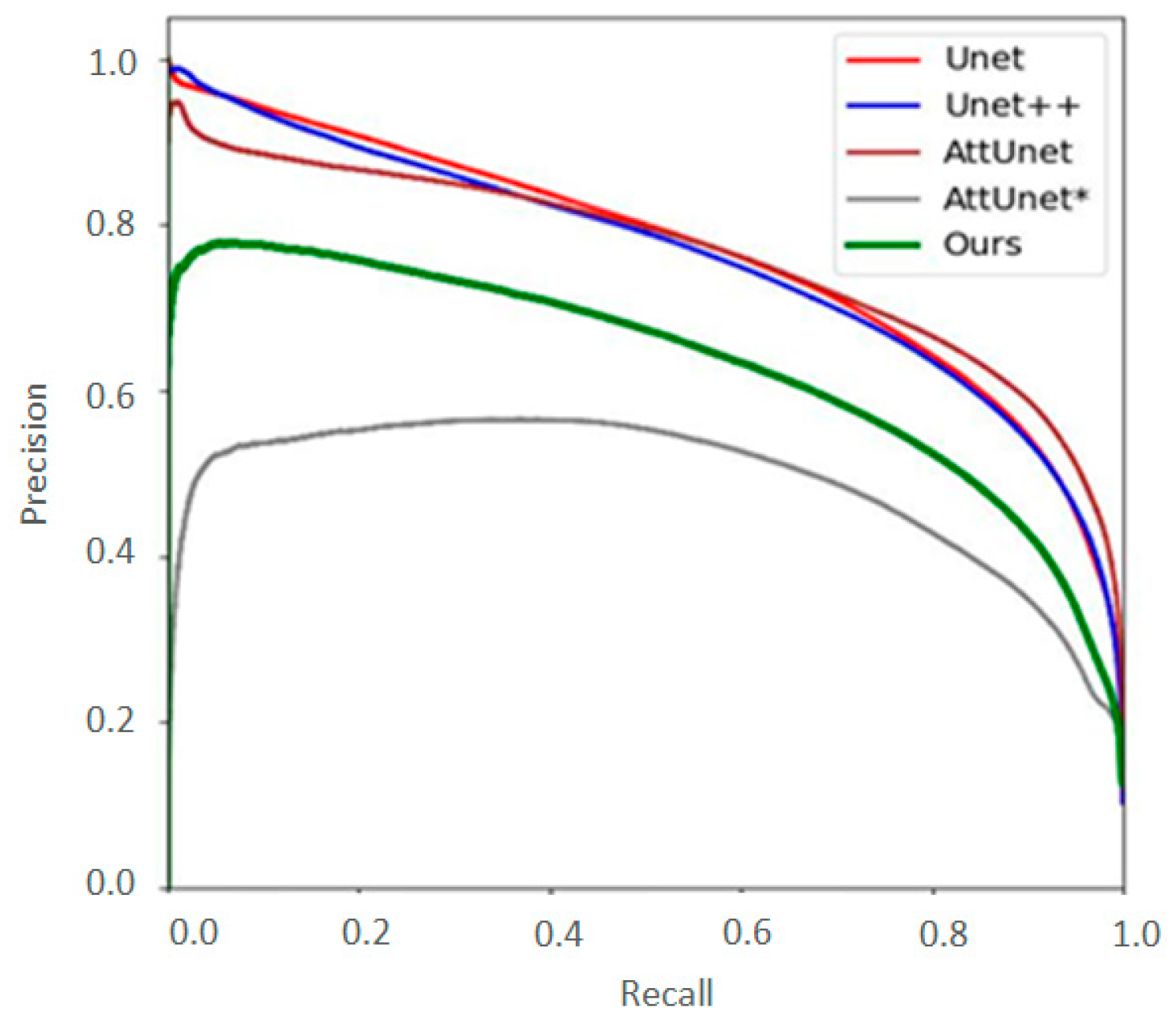
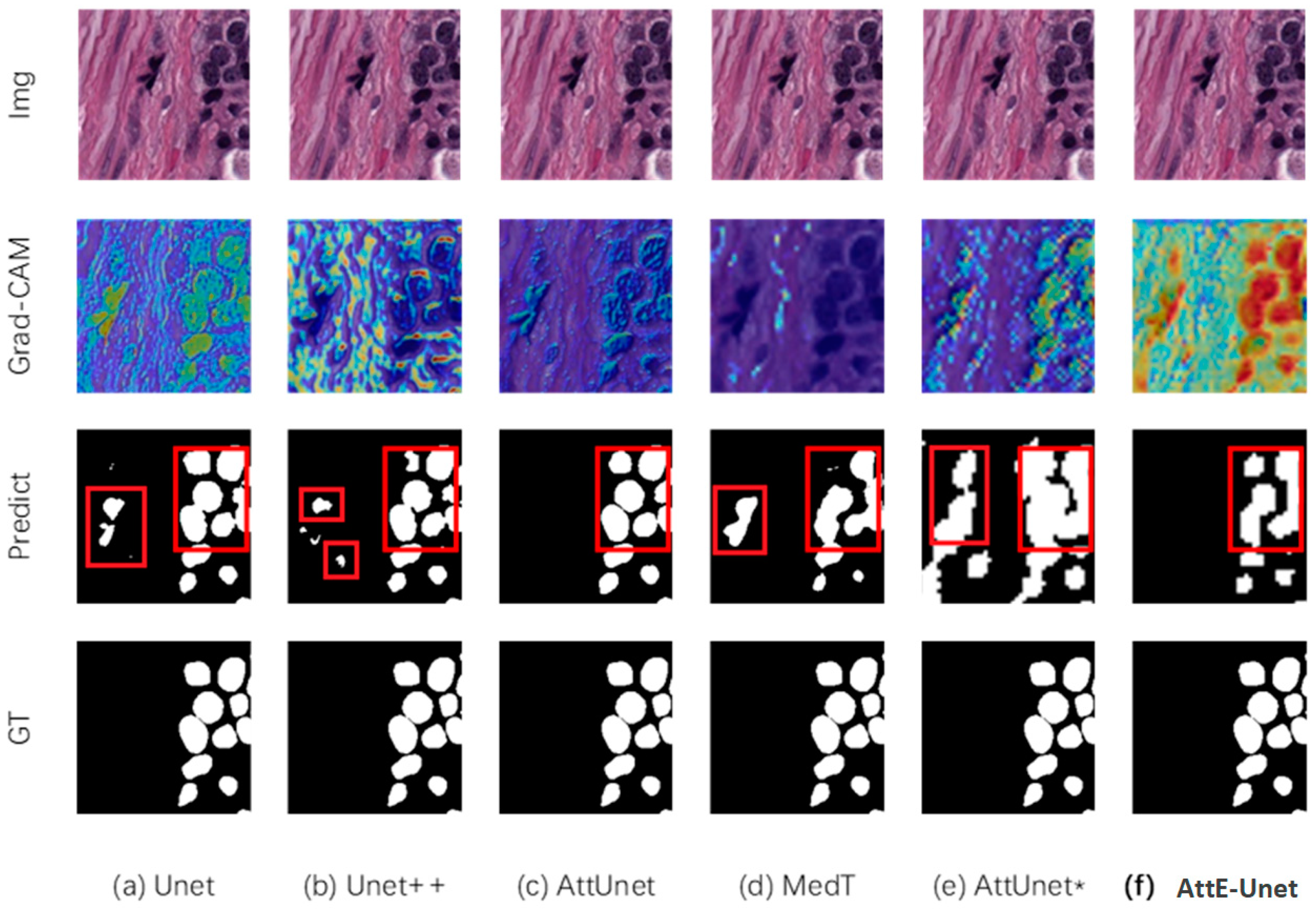
3.2. Algorithm Evaluation
3.3. Hardware Implementation and Evaluation
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shen, D.; Wu, G.; Suk, H.-I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017, 19, 221–248. [Google Scholar] [PubMed]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; van der Laak, J.A.W.M.; van Ginneken, B.; Sanchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed]
- Hesamian, M.H.; Jia, W.; He, X.; Kennedy, P. Deep learning techniques for medical image segmentation: Achievements and challenges. J. Digit. Imaging 2019, 32, 582–596. [Google Scholar] [PubMed]
- Vicar, T.; Balvan, J.; Jaros, J.; Jug, F.; Kolar, R.; Masarik, M.; Gumulec, J. Cell segmentation methods for label-free contrast microscopy: Review and comprehensive comparison. BMC Bioinform. 2019, 20, 360. [Google Scholar] [CrossRef]
- Wen, T.; Tong, B.; Liu, Y.; Pan, T.; Du, Y.; Chen, Y.; Zhang, S. Review of research on the instance segmentation of cell images. Comput. Methods Programs Biomed. 2022, 227, 107211. [Google Scholar]
- Huang, Q.; Zhang, W.; Chen, Y.; Chen, J.; Yang, Z. Review of cervical cell segmentation. Multimed. Tools Appl. 2024, 1–40. [Google Scholar] [CrossRef]
- Long, J.; Shelhamer, E.; Darrell, T. Fully Convolutional Networks for Semantic Segmentation. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Boston, MA, USA, 7–12 June 2015; pp. 3431–3440. [Google Scholar]
- Xu, Y.; Quan, R.; Xu, W.; Huang, Y.; Chen, X.; Liu, F. Advances in Medical Image Segmentation: A Comprehensive Review of Traditional, Deep Learning and Hybrid Approaches. Bioengineering 2024, 11, 1034. [Google Scholar] [CrossRef]
- Ding, L.; Goshtasby, A. On the Canny edge detector. Pattern Recognit. 2001, 34, 721–725. [Google Scholar]
- Iglovikov, V.; Shvets, A. Ternausnet: U-net with vgg11 encoder pre-trained on imagenet for image segmentation. arXiv 2018, arXiv:1801.05746. [Google Scholar]
- Suzuki, K. Overview of deep learning in medical imaging. Radiol. Phys. Technol. 2017, 10, 257–273. [Google Scholar]
- Wang, J.; Zhu, H.; Wang, S.-H.; Zhang, Y.-D. A review of deep learning on medical image analysis. Mob. Netw. Appl. 2021, 26, 351–380. [Google Scholar] [CrossRef]
- Chen, L.-C.; Papandreou, G.; Kokkinos, I.; Murphy, K.; Yuille, A.L. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected crfs. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 40, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Badrinarayanan, V.; Kendall, A.; Cipolla, R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017, 39, 2481–2495. [Google Scholar] [CrossRef] [PubMed]
- Milletari, F.; Navab, N.; Ahmadi, S.-A. V-net: Fully convolutional neural networks for volumetric medical image segmentation. In Proceedings of the 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 25–28 October 2016; pp. 565–571. [Google Scholar]
- Wang, R.S.; Lei, T.; Cui, R.X.; Zhang, B.T.; Meng, H.Y.; Nandi, A.K. Medical image segmentation using deep learning: A survey. IET Image Process. 2022, 16, 1243–1267. [Google Scholar] [CrossRef]
- Liu, X.B.; Song, L.P.; Liu, S.; Zhang, Y.D. A Review of Deep-Learning-Based Medical Image Segmentation Methods. Sustainability 2021, 13, 1224. [Google Scholar] [CrossRef]
- Siddique, N.; Paheding, S.; Elkin, C.P.; Devabhaktuni, V. U-Net and Its Variants for Medical Image Segmentation: A Review of Theory and Applications. IEEE Access 2021, 9, 82031–82057. [Google Scholar] [CrossRef]
- Xu, Q.; Ma, Z.; Na, H.; Duan, W. DCSAU-Net: A deeper and more compact split-attention U-Net for medical image segmentation. Comput. Biol. Med. 2023, 154, 106626. [Google Scholar] [CrossRef]
- Ghaznavi, A.; Rychtáriková, R.; Saberioon, M.; Štys, D. Cell segmentation from telecentric bright-field transmitted light microscopy images using a Residual Attention U-Net: A case study on HeLa line. Comput. Biol. Med. 2022, 147, 105805. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Li, Z.; Ren, Z. Residual-attention unet++: A nested residual-attention u-net for medical image segmentation. Appl. Sci. 2022, 12, 7149. [Google Scholar] [CrossRef]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the 18th International Conference on Medical Image Computing and Computer-Assisted Intervention (MICCAI), Munich, Germany, 5–9 October 2015; pp. 234–241. [Google Scholar]
- Zhou, Z.W.; Siddiquee, M.M.R.; Tajbakhsh, N.; Liang, J.M. UNet plus plus: A Nested U-Net Architecture for Medical Image Segmentation. In Proceedings of the 4th International Workshop on Deep Learning in Medical Image Analysis (DLMIA)/8th International Workshop on Multimodal Learning for Clinical Decision Support (ML-CDS), Granada, Spain, 20 September 2018; pp. 3–11. [Google Scholar]
- Alom, M.Z.; Yakopcic, C.; Taha, T.M.; Asari, V.K. Nuclei Segmentation with Recurrent Residual Convolutional Neural Networks based U-Net (R2U-Net). In Proceedings of the IEEE National Aerospace and Electronics Conference (NAECON), Dayton, OH, USA, 23–26 July 2018; pp. 228–233. [Google Scholar]
- Oktay, O.; Schlemper, J.; Le Folgoc, L.; Lee, M.; Heinrich, M.; Misawa, K.; Mori, K.; McDonagh, S.; Hammerla, N.Y.; Kainz, B.; et al. Attention U-Net: Learning Where to Look for the Pancreas. arXiv 2018, arXiv:1804.03999. [Google Scholar]
- Xiao, H.; Li, L.; Liu, Q.; Zhu, X.; Zhang, Q. Transformers in medical image segmentation: A review. Biomed. Signal Process. Control 2023, 84, 104791. [Google Scholar] [CrossRef]
- Valanarasu, J.M.J.; Oza, P.; Hacihaliloglu, I.; Patel, V.M. Medical Transformer: Gated Axial-Attention for Medical Image Segmentation. In Proceedings of the International Conference on Medical Image Computing and Computer Assisted Intervention (MICCAI), Electr Network, Strasbourg, France, 27 September–1 October 2021; pp. 36–46. [Google Scholar]
- Bernal, J.; Sánchez, F.J.; Fernández-Esparrach, G.; Gil, D.; Rodríguez, C.; Vilariño, F. WM-DOVA maps for accurate polyp highlighting in colonoscopy: Validation vs. saliency maps from physicians. Comput. Med. Imaging Graph. 2015, 43, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, J.C.; Goodman, A.; Karhohs, K.W.; Cimini, B.A.; Ackerman, J.; Haghighi, M.; Heng, C.; Becker, T.; Doan, M.; McQuin, C. Nucleus segmentation across imaging experiments: The 2018 Data Science Bowl. Nat. Methods 2019, 16, 1247–1253. [Google Scholar] [CrossRef]
- Tschandl, P.; Rosendahl, C.; Kittler, H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Sci. Data 2018, 5, 180161. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Gehlot, S.; Goswami, S. Segpc-2021: Segmentation of Multiple Myeloma Plasma Cells in Microscopic Images, 2021. Available online: https://doi.org/10.21227/7np1-2q42 (accessed on 2 April 2025).
- Baid, U.; Ghodasara, S.; Mohan, S.; Bilello, M.; Calabrese, E.; Colak, E.; Farahani, K.; Kalpathy-Cramer, J.; Kitamura, F.C.; Pati, S. The rsna-asnr-miccai brats 2021 benchmark on brain tumor segmentation and radiogenomic classification. arXiv 2021, arXiv:2107.02314. [Google Scholar]
- Gamper, J.; Alemi Koohbanani, N.; Benet, K.; Khuram, A.; Rajpoot, N. Pannuke: Anopen pan-cancer histology dataset for nuclei instance segmentation andclassification. In Proceedings of the Digital Pathology: 15th European Congress, ECDP 2019, Warwick, UK, USA, 10–13 April 2019; Proceedings 15. pp. 11–19. [Google Scholar]
- Sahasrabudhe, M.; Christodoulidis, S.; Salgado, R.; Michiels, S.; Loi, S.; André, F.; Paragios, N.; Vakalopoulou, M. Self-supervised nuclei segmentation in histopathological images using attention. In Proceedings of the Medical Image Computing and Computer Assisted Intervention–MICCAI 2020: 23rd International Conference, Lima, Peru, 4–8 October 2020; Proceedings, Part V 23. pp. 393–402. [Google Scholar]
- De Vita, F.; Nocera, G.; Bruneo, D.; Tomaselli, V.; Falchetto, M. On-device training of deep learning models on edge microcontrollers. In Proceedings of the 2022 IEEE International Conferences on Internet of Things (iThings) and IEEE Green Computing & Communications (GreenCom) and IEEE Cyber, Physical & Social Computing (CPSCom) and IEEE Smart Data (SmartData) and IEEE Congress on Cybermatics (Cybermatics), Espoo, Finland, 22–25 August 2022; pp. 62–69. [Google Scholar]
- Falaschetti, L.; Bruschi, S.; Alessandrini, M.; Biagetti, G.; Crippa, P.; Turchetti, C. An U-Net Semantic Segmentation Vision System on a Low-Power Embedded Microcontroller Platform. Procedia Comput. Sci. 2023, 225, 4473–4482. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, K.; Sun, Q.; Wang, Z.; Liu, M. Edge-Guided Cell Segmentation on Small Datasets Using an Attention-Enhanced U-Net Architecture. Information 2024, 15, 198. [Google Scholar] [CrossRef]
- Cui, Z.; Yao, J.; Zeng, L.; Yang, J.; Liu, W.; Wang, X. LKCell: Efficient Cell Nuclei Instance Segmentation with Large Convolution Kernels. arXiv 2024, arXiv:2407.18054. [Google Scholar]
- Hörst, F.; Rempe, M.; Heine, L.; Seibold, C.; Keyl, J.; Baldini, G.; Ugurel, S.; Siveke, J.; Grünwald, B.; Egger, J. Cellvit: Vision transformers for precise cell segmentation and classification. Med. Image Anal. 2024, 94, 103143. [Google Scholar] [CrossRef]
| Network Layer | Number of Kernels/Stride/Size | Number of Input Channels | Size of Output Feature Map/Number of Channels |
|---|---|---|---|
| Input | - | 3 | 64 × 64/3 |
| Block1_Catenate1 | - | 1 | 64 × 64/4 |
| Block1_conv1 | 7/1/3 × 3 | 4 | 64 × 64/7 |
| Max-pool1 | -/2/2 × 2 | 7 | 32 × 32/7 |
| Block2_Catenate1 | - | 7 | 32 × 32/8 |
| Block2_conv1 | 15/1/3 × 3 | 8 | 32 × 32/15 |
| Max-pool2 | -/2/2 × 2 | 15 | 16 × 16/15 |
| Block3_Catenate1 | - | 15 | 16 × 16/16 |
| Block3_conv1 | 31/1/3 × 3 | 16 | 16 × 16/31 |
| Max-pool3 | -/2/2 × 2 | 31 | 8 × 8/31 |
| Block4_Catenate1 | - | 31 | 8 × 8/32 |
| Block4_conv1 | 63/1/3 × 3 | 32 | 8 × 8/63 |
| Max-pool4 | -/2/2 × 2 | 63 | 4 × 4/63 |
| Block5_Catenate1 | - | 63 | 4 × 4/64 |
| Block5_conv1 | 128/1/3 × 3 | 64 | 4 × 4/128 |
| Network Layer | Number of Kernels/Stride/Size | Number of Input Channels | Size of Output Feature Map/Number of Channels |
|---|---|---|---|
| Block6_up-conv1 | 64/1/2 × 2 | 128 | 8 × 8/64 |
| Block6_Catenate1 | - | - | 8 × 8/128 |
| Block6_conv1 | 64/1/3 × 3 | 128 | 8 × 8/64 |
| Block7_up-conv1 | 32/1/2 × 2 | 64 | 16 × 16/32 |
| Block7_Catenate1 | - | - | 16 × 16/64 |
| Block7_conv1 | 32/1/3 × 3 | 64 | 16 × 16/32 |
| Block8_up-conv1 | 16/1/2 × 2 | 32 | 32 × 32/16 |
| Block8_Catenate1 | - | - | 32 × 32/32 |
| Block8_conv1 | 16/1/3 × 3 | 32 | 32 × 32/16 |
| Block9_up-conv1 | 64/1/2 × 2 | 16 | 64 × 64/8 |
| Block9_Catenate1 | - | - | 64 × 64/16 |
| Block9_conv1 | 64/1/3 × 3 | 16 | 64 × 64/8 |
| Block9_conv2 | 2/1/3 × 3 | 8 | 64 × 64/2 |
| Output | - | - | 64 × 64/- |
| Network Layer | Number of Kernels/Stride/Size | Number of Input Channels | Size of Output Feature Map/Number of Channels |
|---|---|---|---|
| Input | - | 1 | 64 × 64/1 |
| Avg-pool 1 | 1/2/2 × 2 | 1 | 32 × 32/1 |
| Avg-pool 2 | 1/2/2 × 2 | 1 | 16 × 16/1 |
| Avg-pool 3 | 1/2/2 × 2 | 1 | 8 × 8/1 |
| Avg-pool 4 | 1/2/2 × 2 | 1 | 4 × 4/1 |
| Output | - | - | 4 × 4/1 |
| Models | F1 | IoU | FLOPS (M) | Parameter (M) |
|---|---|---|---|---|
| U-Net | 0.820 | 0.718 | 65,526 | 34.52 |
| UNet++ | 0.817 | 0.714 | 138,664 | 36.52 |
| Attention U-Net | 0.832 | 0.732 | 66,636 | 34.87 |
| Attention U-Net * | 0.696 | 0.588 | 69 | 0.543 |
| MedT | 0.683 | 0.578 | 487,951 | 156.4 |
| AttE-Unet | 0.763 | 0.654 | 70 | 0.548 |
| Model | Minimum Flash Occupancy (MB) | Minimum RAM Occupancy (MB) |
|---|---|---|
| U-Net | 131.69 | 64.75 |
| UNet++ | 139.70 | 232.62 |
| Attention U-Net | 133.02 | 69.29 |
| Attention U-Net * | 2.11 | 1.30 |
| AttE-Unet | 2.09 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Sun, Q.; Zhou, Y.; Wang, Z.; You, C.; Ma, K.; Liu, M. A Lightweight Neural Network for Cell Segmentation Based on Attention Enhancement. Information 2025, 16, 295. https://doi.org/10.3390/info16040295
Xia S, Sun Q, Zhou Y, Wang Z, You C, Ma K, Liu M. A Lightweight Neural Network for Cell Segmentation Based on Attention Enhancement. Information. 2025; 16(4):295. https://doi.org/10.3390/info16040295
Chicago/Turabian StyleXia, Shuang, Qian Sun, Yiheng Zhou, Zhaoyuxuan Wang, Chaoxing You, Kainan Ma, and Ming Liu. 2025. "A Lightweight Neural Network for Cell Segmentation Based on Attention Enhancement" Information 16, no. 4: 295. https://doi.org/10.3390/info16040295
APA StyleXia, S., Sun, Q., Zhou, Y., Wang, Z., You, C., Ma, K., & Liu, M. (2025). A Lightweight Neural Network for Cell Segmentation Based on Attention Enhancement. Information, 16(4), 295. https://doi.org/10.3390/info16040295








