Intelligent Hybrid Modeling for Heart Disease Prediction
Abstract
1. Introduction
2. Related Work
3. Methods
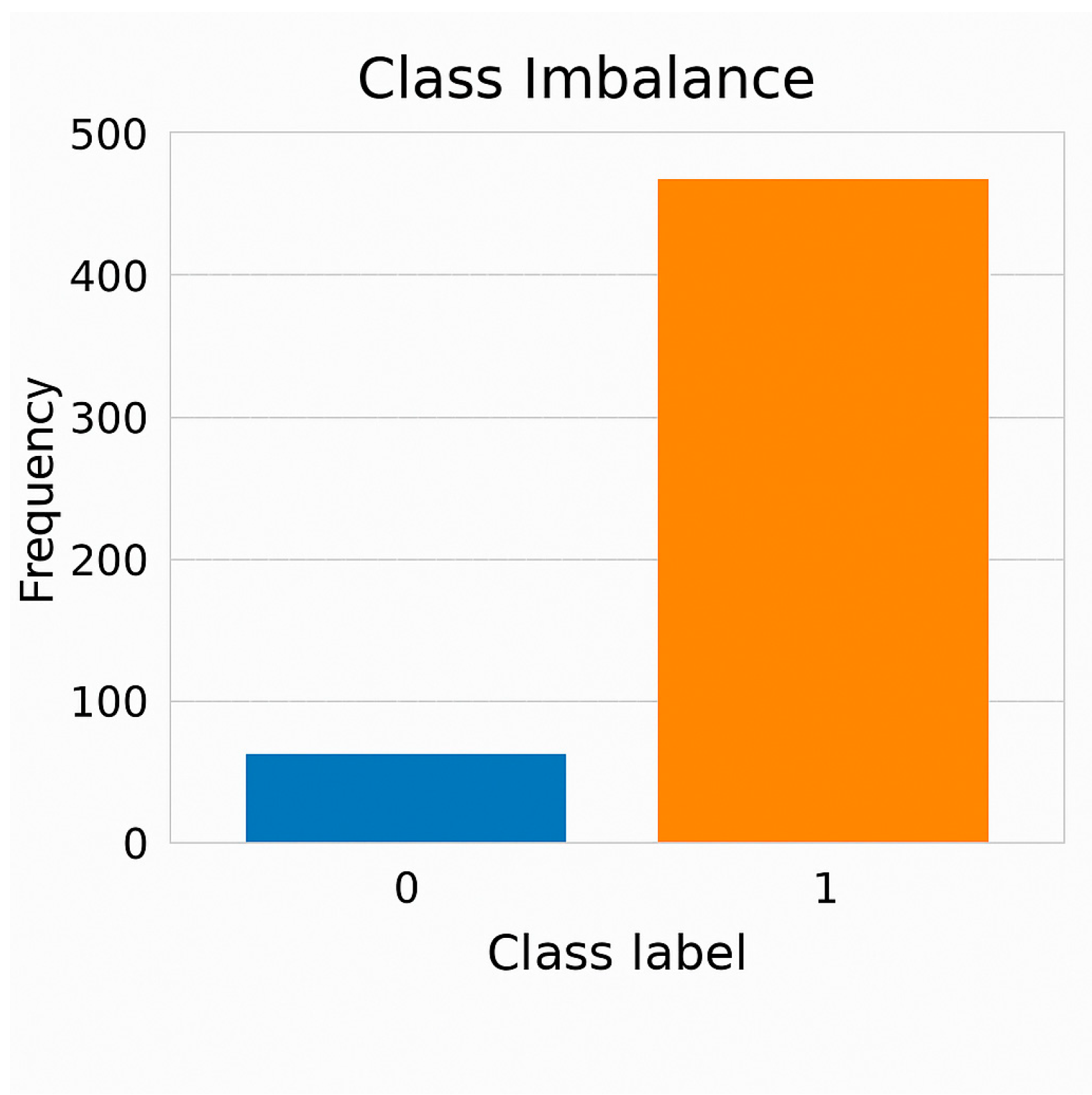
3.1. Dataset Description
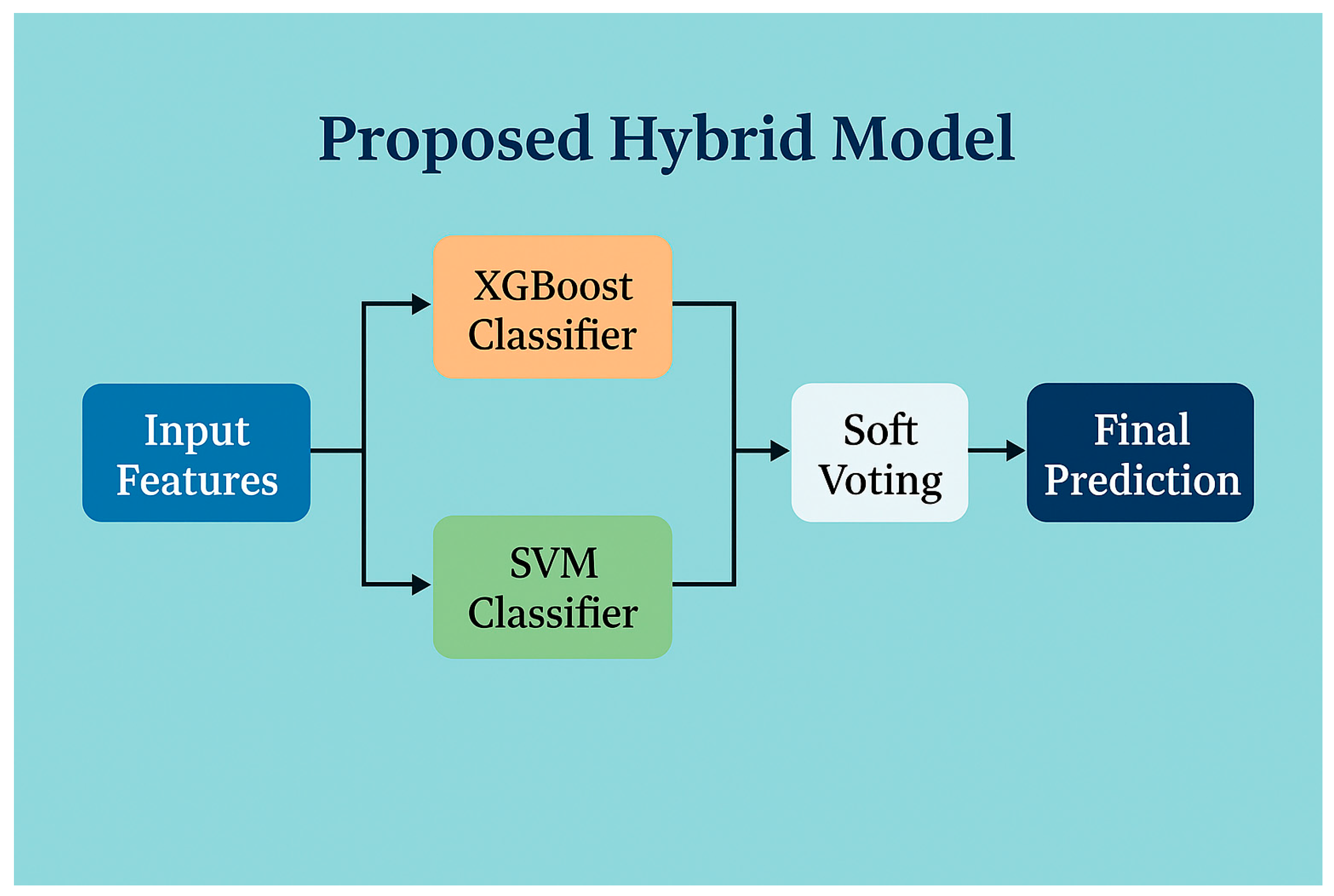
3.2. Model Development
3.3. Mathematical Formulation
3.3.1. Support Vector Machine (SVM)
- -
- w is the weight vector
- -
- x is the input feature vector
- -
- b is the bias term
3.3.2. XGBoost Classifier
- -
- l is the loss function (e.g., logistic loss)
- -
- Ω is the regularization term for controlling model complexity
3.3.3. Soft Voting Ensemble
- -
- P_XGBoost(c) and P_SVM(c) are the predicted probabilities for class c
- -
- α ∈ [0, 1] is the weighting parameter (e.g., 0.5 for equal weight)
3.4. Proposed Hybrid Model
- -
- Hyperparameter tuning for both XGBoost and SVM using cross-validation.
- -
- Feature scaling prior to training to ensure compatibility with SVM.
- -
- Stratified splitting of data to maintain class distribution during training and testing.
4. Results and Discussion
4.1. Implementation Details
4.2. Evaluation Metrics
- Recall: The proportion of actual positive cases that were correctly identified by the model. Recall indicates the model’s ability to detect relevant cases.
- F1-Score: The harmonic mean of precision and recall, offering a single metric that balances both. It is especially useful when the class distribution is uneven.
- Accuracy: The overall correctness of the model, defined as the ratio of all correct predictions (true positives and true negatives) to the total number of cases:
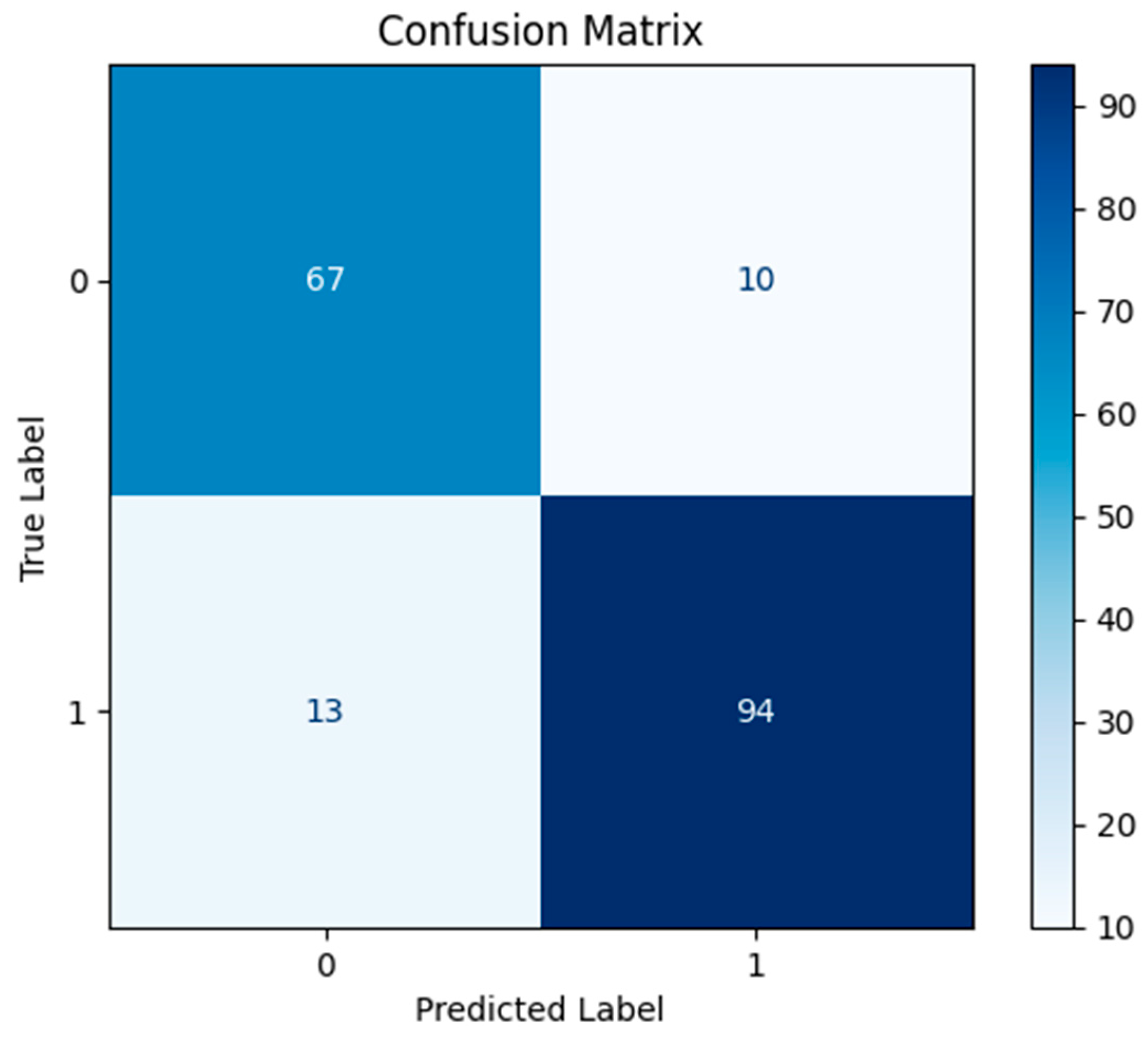
4.3. Results
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). WHO. 2020. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 March 2025).
- Towards Data Science. Understanding Random Forest. 2020. Available online: https://towardsdatascience.com/understanding-random-forest-58381e0602d2 (accessed on 1 March 2025).
- WebFocus InfoCenter. Explanation of the Decision Tree Model. 2020. Available online: https://webfocusinfocenter.informationbuilders.com/wfappent/TLs/TL_rstat/source/DecisionTree47.htm (accessed on 1 March 2025).
- Martin-Isla, C.; Campello, V.M.; Izquierdo, C.; Raisi-Estabragh, Z.; Baeßler, B.; Petersen, S.E.; Lekadir, K. Image-Based Cardiac Diagnosis With Machine Learning: A Review. Front. Cardiovasc. Med. 2020, 7, 1. [Google Scholar] [CrossRef]
- Costa, W.L.; Figueredo, L.S.; Alves, E.T.A. Application of an Artificial Neural Network for Heart Disease Diagnosis. In Proceedings of the Brazilian Congress on Biomedical Engineering, Rio de Janeiro, Brazil, 21–25 October 2018; pp. 753–758. [Google Scholar]
- Kumar, A.; Dwivedi, R. Performance Evaluation of Different Machine Learning Techniques for Prediction of Heart Disease. Neural Comput. Appl. 2018, 29, 685–693. [Google Scholar]
- Salau, A.; Admassu, T.; Chhabra, G.; Kaushik, K.; Braide, S. Heart Disease Detection Model Using Support Vector Machine with Feature Selection. In Proceedings of the InCACCT 2024, Gharuan, India, 2–3 May 2024. [Google Scholar] [CrossRef]
- Cao, K.; Liu, C.; Yang, S.; Zhang, Y.; Li, L.; Jung, H.; Zhang, S. Prediction of Cardiovascular Disease Based on Multiple Feature Selection and Improved PSO-XGBoost Model. Sci. Rep. 2025, 15, 12406. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahdi, I.S.; Darwish, S.M.; Madbouly, M.M. Heart Disease Prediction Model Using Feature Selection and Ensemble Deep Learning with Optimized Weight. Comput. Model. Eng. Sci. 2025, 143, 875–909. [Google Scholar] [CrossRef]
- Khan, M.A.; Mazhar, T.; Yaqoob, M.M.; Khan, M.B.; Saudagar, A.K.J.; Ghadi, Y.Y.; Khattak, U.F.; Shahid, M. Optimal Feature Selection for Heart Disease Prediction Using Modified Artificial Bee Colony (M-ABC) and K-Nearest Neighbors (KNN). Sci. Rep. 2024, 14, 26241. [Google Scholar] [CrossRef] [PubMed]
- Boukhatem, C.; Youssef, H.Y.; Nassif, A.B. Heart Disease Prediction Using Machine Learning. In Proceedings of the 2022 Advances in Science and Engineering Technology International Conferences (ASET), Dubai, United Arab Emirates, 21–24 February 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Udoy, I.A.; Hassan, O. AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Symmetry 2025, 17, 469. [Google Scholar] [CrossRef]
- Akter, B.; Shakil, R.; Rajbongshi, A.; Sara, U.; Barman, M.R. Utilization of Five Distinct Datasets to Diagnose and Predict Heart Disease: A Machine Learning Approach. In Proceedings of the ICCCNT 2022, Kharagpur, India, 3–5 October 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Patidar, S.; Kumar, D.; Rukwal, D. Comparative Analysis of Machine Learning Algorithms for Heart Disease Prediction; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Wang, W.; Chakraborty, G.; Chakraborty, B. Predicting the Risk of Chronic Heart Disease Using Machine Learning Algorithms. Appl. Sci. 2021, 11, 202. [Google Scholar] [CrossRef]
- Tan, K.; Teoh, E.; Yu, Q.; Goh, K.C. A Hybrid Evolutionary Algorithm for Data Mining Attribute Selection in Heart Disease Prediction. Expert Syst. Appl. 2009, 36, 8616–8630. [Google Scholar] [CrossRef]
- Elvas, L.B.; Nunes, M.; Ferreira, J.C.; Dias, M.S.; Rosário, L.B. AI-Driven Decision Support for Early Detection of Cardiac Events: Unveiling Patterns and Predicting Myocardial Ischemia. J. Pers. Med. 2023, 13, 1421. [Google Scholar] [CrossRef]
- Garza-Frias, E.; Kaviani, P.; Karout, L.; Fahimi, R.; Hosseini, S.; Putha, P.; Tadepalli, M.; Kiran, S.; Arora, C.; Robert, D.; et al. Early Detection of Heart Failure with Autonomous AI-Based Model Using Chest Radiographs: A Multicenter Study. Diagnostics 2024, 14, 1635. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, A.; Pal, S. Applications of Machine Learning Techniques to Predict Diagnostic Breast Cancer. SN Comp. Sci. 2020, 1, 270. [Google Scholar]
- Parthiban, G.; Srivatsa, S.K. Applying machine learning methods in diagnosing heart disease for diabetic patients. Int. J. Appl. Inf. Syst. 2012, 3, 25–30. [Google Scholar]
- Alwakid, G.; Haq, F.U.; Tariq, N.; Humayun, M.; Shaheen, M.; Alsadun, M. Optimized Machine Learning Framework for Cardiovascular Disease Diagnosis: A Novel Ethical Perspective. BMC Cardiovasc. Disord. 2025, 25, 123. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Faa, G.; Johri, A.M.; Mantella, L.E.; Fernandes, J.F.E.; Teji, J.S.; et al. Artificial Intelligence for Cardiovascular Disease Risk Assessment in Personalised Framework: A Scoping Review. eClinicalMedicine 2024, 73, 102660. [Google Scholar] [CrossRef]
- Saranya, G.; Pravin, A. Grid Search Based Optimum Feature Selection by Tuning Hyperparameters for Heart Disease Diagnosis in Machine Learning. Open Biomed. Eng. J. 2023, 17, e187412072304061. [Google Scholar] [CrossRef]
- Bilal, H.; Tian, Y.; Ali, A.; Muhammad, Y.; Yahya, A.; Abu Izneid, B.; Ullah, I. An Intelligent Approach for Early and Accurate Prediction of Cardiac Disease Using Hybrid Artificial Intelligence Techniques. Bioengineering 2024, 11, 1290. [Google Scholar] [CrossRef]
- Ogunpola, A.; Saeed, F.; Basurra, S.; Albarrak, A.M.; Qasem, S.N. Machine Learning-Based Predictive Models for Detection of Cardiovascular Diseases. Diagnostics 2024, 14, 144. [Google Scholar] [CrossRef]
- Wang, D. Semantic Representation and Inference for NLP. arXiv 2021, arXiv:2106.08117. [Google Scholar] [CrossRef]
- Kero, A.; Demissie, D. Leveraging Ontology-Driven Machine Learning for Public Policy Analysis: A Systematic Review. Int. J. Inform. Dev. 2024, 13, 485–503. [Google Scholar]
- Ge, X.; Wang, Y.C.; Wang, B.; Kuo, C.-C.J. Knowledge Graph Embedding: An Overview. arXiv 2023, arXiv:arXiv:2309.12501. [Google Scholar] [CrossRef]
- Zhang, J.; Shin, B.; Choi, J.D.; Ho, J.C. SMAT: An Attention-Based Deep Learning Solution for Schema Matching. Adv. Databases Inf. Syst. (ADBIS) 2021, 12843, 260–274. [Google Scholar]
- Shraga, R.; Gal, A.; Roitman, H. AdNeV: Cross-Domain Schema Matching Using Deep Similarity Matrix. Proc. VLDB Endow. 2020, 13, 1401–1415. [Google Scholar] [CrossRef]
- Duan, B.; Chen, S.; Guo, Y.; Xie, G.-S.; Ding, W.; Wang, Y. Visual–Semantic Graph Matching Net for Zero-Shot Learning. IEEE Trans. Neural Netw. Learn. Syst. 2024, 36, 10171–10185. [Google Scholar] [CrossRef]
- Hoseinzade, E.; Wang, K. Graph Neural Network Approach to Semantic Type Detection in Tables. In Proceedings of the Pacific-Asia Conference on Knowledge Discovery and Data Mining (PAKDD), Taipei, Taiwan, 7–10 May 2024. [Google Scholar]
- Xue, X.; Huang, Y.; Zhang, Z. Deep Reinforcement Learning-Based Ontology Meta-Matching Technique. IEICE Trans. Inf. Syst. 2023, 106, 635–643. [Google Scholar] [CrossRef]
- Sun, X.; Yin, Y.; Yang, Q.; Huo, T. Artificial intelligence in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. J. Med. Res. 2023, 28, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhenya, Q.; Zhang, Z. A hybrid cost-sensitive ensemble for heart disease prediction. BMC Med. Inform. Decis. Mak. 2021, 21, 73. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| Programming Language | Python 3.10 |
| Machine Learning Libraries | Scikit-learn 1.3.0, XGBoost 1.7.6, TensorFlow 2.14.0 (Keras API) |
| Hardware | Intel Core i7, 32 GB RAM, NVIDIA RTX 3060 GPU (12 GB VRAM) |
| Operating System | Windows 11 with CUDA 11.8 |
| Optimizer (NN) | Adam |
| Initial Learning Rate | 1 × 10−4 |
| Loss Function | Binary Cross-Entropy |
| Batch Size | 32 |
| Epochs | 50 (with early stopping) |
| Model Ensemble | Soft Voting (XGBoost + SVM) |
| Data Split Ratio | 80:20 (Train:Test, Stratified) |
| Scaling Method | StandardScaler |
| Study/Method | Dataset | Accuracy | Precision | Recall | F1-Score | Ref. |
|---|---|---|---|---|---|---|
| Hybrid AI Model | Multi-center clinical dataset | 87.5% | 0.86 | 0.87 | 0.865 | [24] |
| Ensemble Deep Learning | UCI and clinical datasets | 88.1% | 0.87 | 0.88 | 0.875 | [9] |
| M-ABC + KNN Feature Selection | UCI Heart dataset | 86.7% | 0.85 | 0.86 | 0.855 | [10] |
| PSO-XGBoost | Clinical dataset | 88.5% | 0.88 | 0.88 | 0.880 | [8] |
| Proposed Hybrid Model (XGBoost + SVM) | Kaggle Heart Failure dataset | 89.3% | 0.90 | 0.91 | 0.905 | Our proposed model |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, M.; Dardouri, S. Intelligent Hybrid Modeling for Heart Disease Prediction. Information 2025, 16, 869. https://doi.org/10.3390/info16100869
Almutairi M, Dardouri S. Intelligent Hybrid Modeling for Heart Disease Prediction. Information. 2025; 16(10):869. https://doi.org/10.3390/info16100869
Chicago/Turabian StyleAlmutairi, Mona, and Samia Dardouri. 2025. "Intelligent Hybrid Modeling for Heart Disease Prediction" Information 16, no. 10: 869. https://doi.org/10.3390/info16100869
APA StyleAlmutairi, M., & Dardouri, S. (2025). Intelligent Hybrid Modeling for Heart Disease Prediction. Information, 16(10), 869. https://doi.org/10.3390/info16100869





