Survey on Knowledge Representation Models in Healthcare
Abstract
1. Introduction
2. Background of Knowledge Concepts and Types
2.1. Data, Information, Knowledge and Wisdom
2.2. Knowledge Types
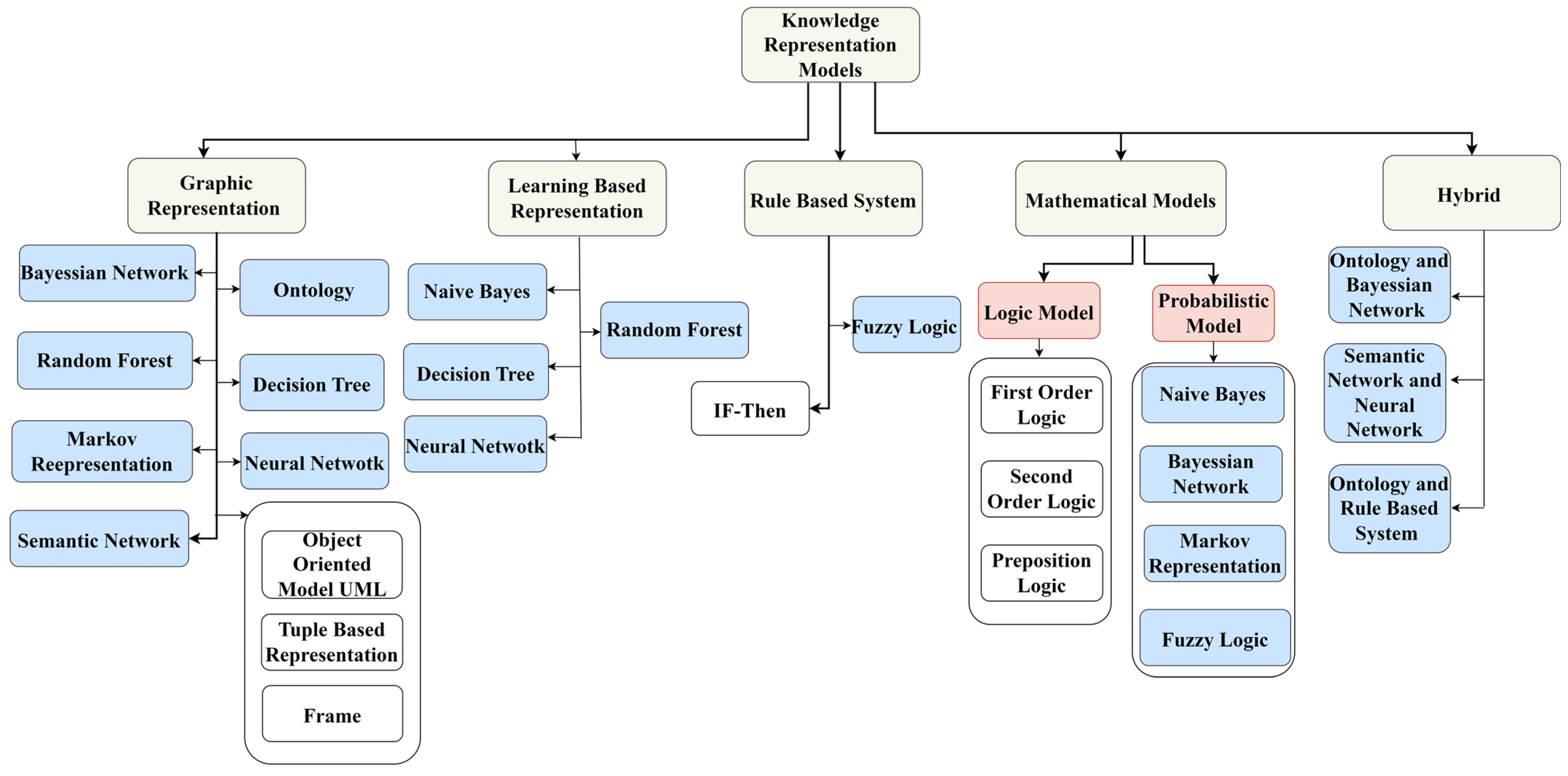
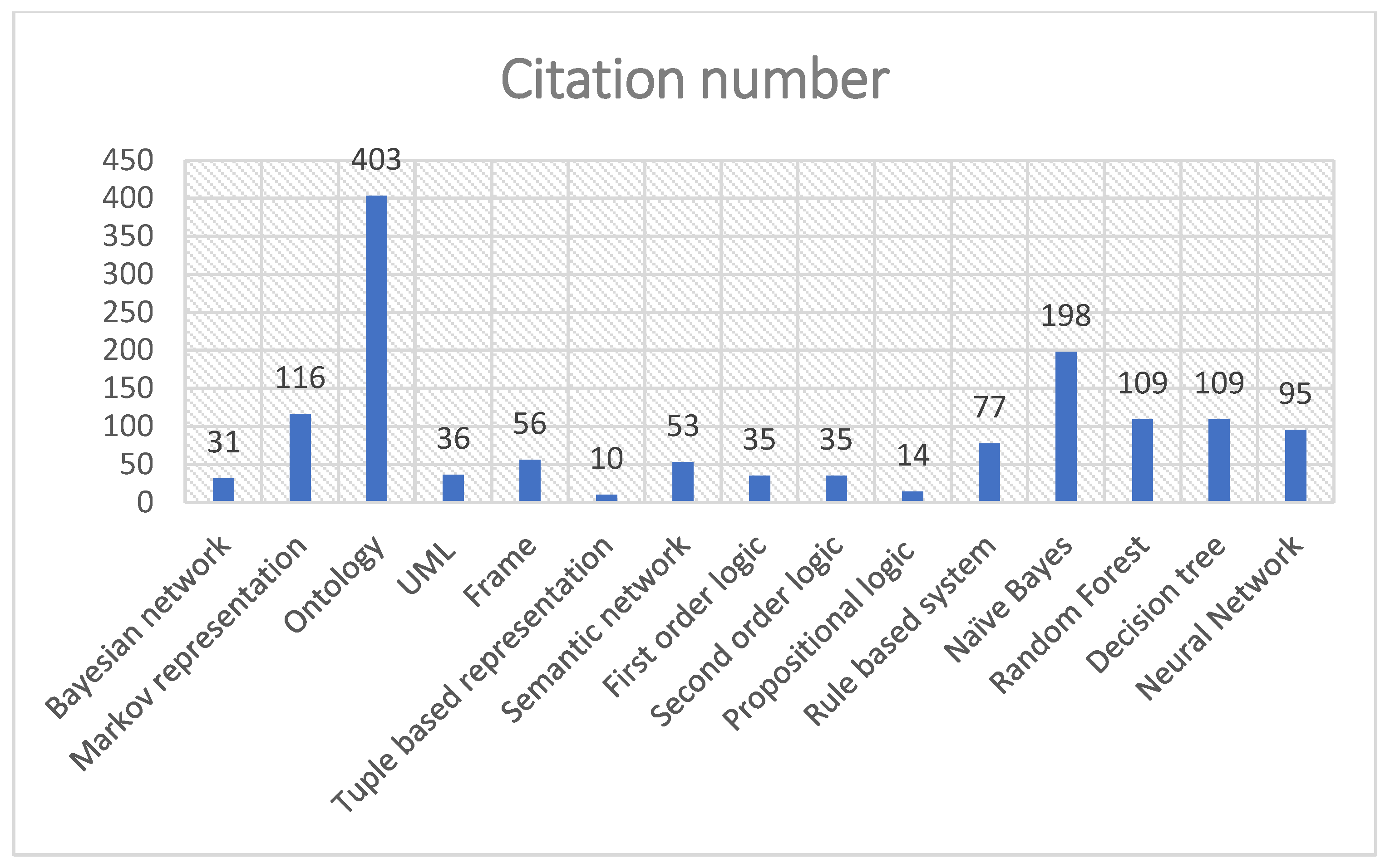
3. Knowledge Representation Models (KRMs)
3.1. Graphical Representation Models
3.2. Learning-Based Models
3.3. Rule-Based Models
3.4. Mathematical Models
3.5. Hybrid Models
4. Importance of Knowledge Representation Models in the Medical Domain
5. Requirements in the Medical Domain
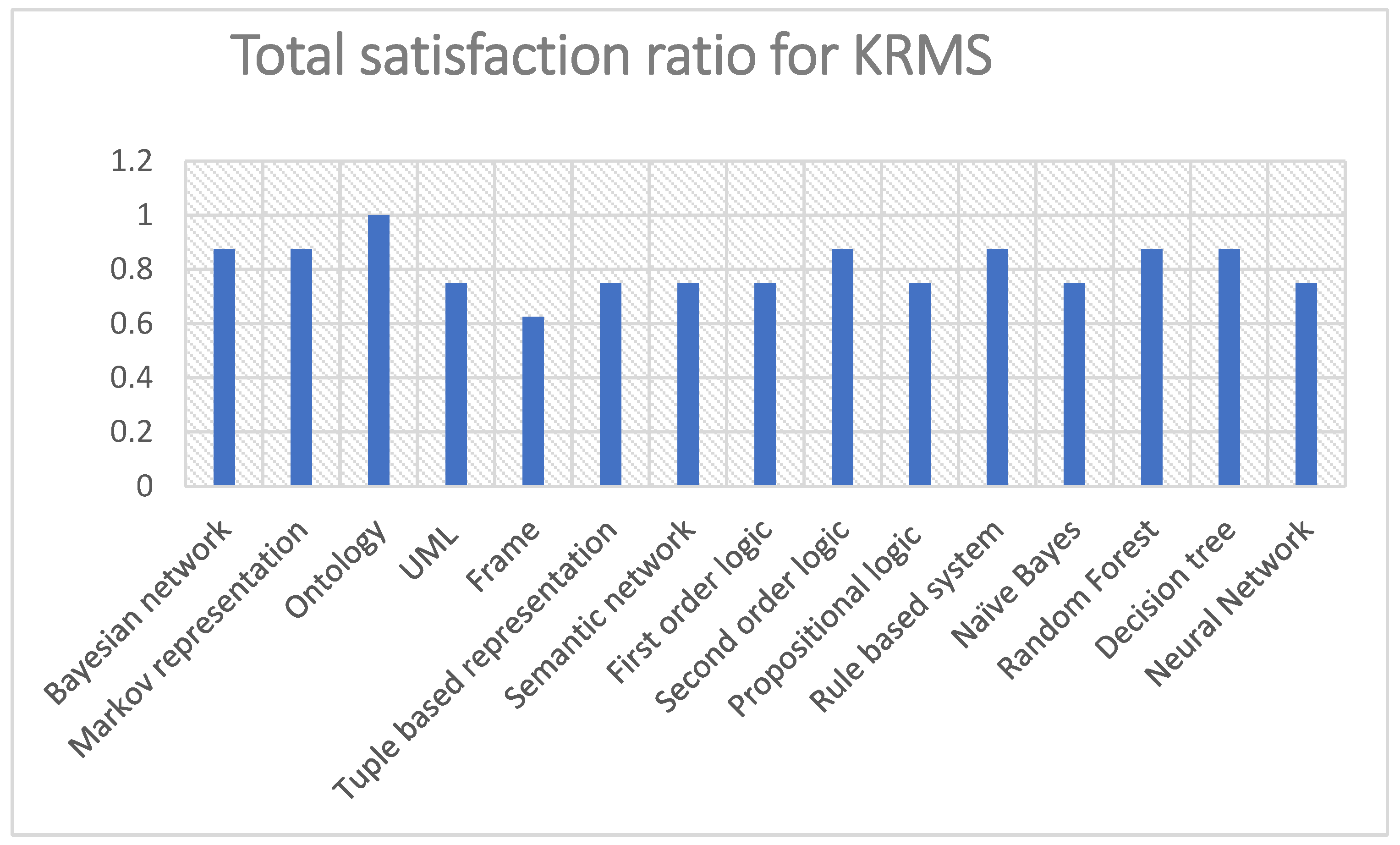
6. Results and Discussion
7. Conclusions and Future Work
Funding
Conflicts of Interest
References
- Stafford, S.P. Data, information, knowledge, and wisdom. Knowl. Manag. Organ. Intell. Learn. Complex. 2009, 3, 179. [Google Scholar]
- Kamala, S.P.R.; Justus, S. A Study on Knowledge Representation Models. Eur. J. Mol. Clin. Med. 2020. [Google Scholar]
- Praveenkumar, T.; Sabhrish, B.; Saimurugan, M.; Ramachandran, K. Pattern recognition based on-line vibration monitoring system for fault diagnosis of automobile gearbox. Measurement 2018, 114, 233–242. [Google Scholar] [CrossRef]
- Abu-Salih, B. Domain-specific knowledge graphs: A survey. J. Netw. Comput. Appl. 2021, 185, 103076. [Google Scholar] [CrossRef]
- Lenz, R.; Miksch, S.; MPeleg Reichert, M.; Riano, D.; Teije, A. Process Support and Knowledge Representation in Health Care; Springer: Tallin, Estonia, 2013. [Google Scholar]
- Wang, F.; Preininger, A. AI in health: State of the art, challenges, and future directions. Yearb. Med. Inform. 2019, 28, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Vellido, A. The importance of interpretability and visualization in machine learning for applications in medicine and health care. Neural Comput. Appl. 2019, 32, 18069–18083. [Google Scholar] [CrossRef]
- Shi, L.; Li, S.; Yang, X.; Qi, J.; Pan, G.; Zhou, B. Semantic Health Knowledge Graph: Semantic Integration of Heterogeneous Medical Knowledge and Services. BioMed Res. Int. 2017, 2017, 2858423. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Wu, X.; Luo, Z.; Boland, M.R.; Theodoratos, D.; Johnson, S.B. EliXR: An approach to eligibility criteria extraction and representation. J. Am. Med. Inform. Assoc. 2011, 18, i116–i124. [Google Scholar] [CrossRef]
- Blasch, E.; Kadar, I.; Salerno, J.; Kokar, M.M.; Das, S.; Powell, G.M.; Corkill, D.D.; Ruspini, E.H. Issues and challenges of knowledge representation and reasoning methods in situation assessment (Level 2 Fusion). Signal Process. Sens. Fusion Target Recognit. XV 2006, 6235, 355–368. [Google Scholar]
- Cooper, P. Data, information, knowledge and wisdom. Anaesth. Intensive Care Med. 2017, 18, 55–56. [Google Scholar] [CrossRef]
- Lee, J.; Lapira, E.; Bagheri, B.; Kao, H.-A. Recent advances and trends in predictive manufacturing systems in big data environment. Manuf. Lett. 2013, 1, 38–41. [Google Scholar] [CrossRef]
- Bright, C.; Kay, A.; Feeney, A. The effects of domain and type of knowledge on category-based inductive reasoning. In Proceedings of the Annual Meeting of the Cognitive Science Society, Portland, Oregon, USA, 11–14 August 2010; Volume 32. [Google Scholar]
- Lieto, A.; Minieri, A.; Piana, A.; Radicioni, D.P. A knowledge-based system for prototypical reasoning. Connect. Sci. 2015, 27, 137–152. [Google Scholar] [CrossRef]
- Sowa, J.F. Common Logic. A Framework for a Family of Logic-Based Languages. 2008. Available online: http://www.jfsowa.com/talks/clprop.htm (accessed on 16 June 2024).
- Holsapple, C.W. Knowledge and its attributes. In Handbook on Knowledge Management 1: Knowledge Matters; Springer: Berlin/Heidelberg, Germany, 2004; pp. 165–188. [Google Scholar]
- Nobécourt, J.; Brigitte, B. Md: A Modelling Language to Build a Formal Ontology in Either Description Logics or Conceptual Graphs. In International Conference on Knowledge Engineering and Knowledge Management; Springer: Berlin/Heidelberg, Germany, 2000; Volume 1937. [Google Scholar]
- Edward, E.O.; Nlerum, P.A. Knowledge representation in artificial intelligence and expert systems using inference rule. Int. J. Sci. Eng. Res. 2020, 11, 1886–1900. [Google Scholar]
- Chandrasegaran, S.K.; Ramani, K.; Sriram, R.D.; Horváth, I.; Bernard, A.; Harik, R.F.; Gao, W. The evolution, challenges, and future of knowledge representation in product design systems. Comput.-Aided Des. 2012, 45, 204–228. [Google Scholar] [CrossRef]
- Pinker, S. A theory of graph comprehension. In Artificial Intelligence and the Future of Testing. Psychology Press; Psychology Press: London, UK, 2014; pp. 73–126. [Google Scholar]
- Wang, F.; Li, H.; Dong, C.; Ding, L. Knowledge representation using non-parametric Bayesian networks for tunneling risk analysis. Reliab. Eng. Syst. Saf. 2019, 191, 106529. [Google Scholar] [CrossRef]
- Frank, A.U. Tiers of ontology and consistency constraints in geographical information systems. Int. J. Geogr. Inf. Sci. 2001, 15, 667–678. [Google Scholar] [CrossRef]
- Azad, M.; Chikalov, I.; Moshkov, M. Representation of Knowledge by Decision Trees for Decision Tables with Multiple Decisions. Procedia Comput. Sci. 2020, 176, 653–659. [Google Scholar] [CrossRef]
- Myles, A.J.; Feudale, R.N.; Liu, Y.; Woody, N.A.; Brown, S.D. An introduction to decision tree modeling. J. Chemom. A J. Chemom. Soc. 2004, 18, 275–285. [Google Scholar] [CrossRef]
- Levine, D.S.; Aparicio, M., IV (Eds.) Neural Networks for Knowledge Representation and Inference; Psychology Press: London, UK, 2013. [Google Scholar]
- Hotz, L.; Felfernig, A.; Stumptner, M.; Ryabokon, A.; Bagley, C.; Wolter, K. Configuration Knowledge Representation and Reasoning; Morgan Kaufmann: San Francisco, CA, USA, 2014. [Google Scholar]
- Minock, M. Knowledge Representation using Schema Tuple Queries; KRDB: Umea, Sweden, 2003. [Google Scholar]
- Kenett, Y.N.; Faust, M. A Semantic Network Cartography of the Creative Mind. Trends Cogn. Sci. 2019, 23, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Sipper, M.; Moore, J.H. Conservation machine learning: A case study of random forests. Sci. Rep. 2021, 11, 3629. [Google Scholar] [CrossRef] [PubMed]
- Prajwala, T.R. A comparative study on decision tree and random forest using R tool. Int. J. Adv. Res. Comput. Commun. Eng. 2015, 4, 196–199. [Google Scholar]
- Jadhav, S.D.; Channe, H.P. Comparative study of K-NN, naive Bayes and decision tree classification techniques. Int. J. Sci. Res. 2016, 5, 1842–1845. [Google Scholar]
- Prieto, J.; Corchado, J.M. A Review of k-NN Algorithm Based on Classical and Quantum Machine Learning. In Distributed Computing and Artificial Intelligence, Special Sessions, 17th International Conference; Springer Nature: L’Aquila, Italy, 2020; Volume 1242, p. 189. [Google Scholar]
- Schuster-Böckler, B.; Bateman, A. An Introduction to Hidden Markov Models. Curr. Protoc. Bioinform. 2007, 18, A.3A.1–A.3A.9. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.M.; Rushdi, M.A. Mathematics and examples of the modern syllogistic method of propositional logic. Math. Appl. Inf. Syst. Bentham Sci. Publ. Emir. Sharjah United Arab. Emir. 2018, 6, 123–167. [Google Scholar]
- Bartels, P.H.; Hiessl, H. Expert systems in histopathology. II. Knowledge representation and rule-based systems. Anal. Quant. Cytol. Histol. 1989, 11, 147–153. [Google Scholar] [PubMed]
- Lifschitz, V.; Morgenstern, L.; Plaisted, D. Knowledge representation and classical logic. Found. Artif. Intell. 2008, 3, 3–88. [Google Scholar]
- Frick, M.; Grohe, M. The complexity of first-order and monadic second-order logic revisited. Ann. Pure Appl. Log. 2004, 130, 3–31. [Google Scholar] [CrossRef]
- Fan, C.-Y.; Chang, P.-C.; Lin, J.-J.; Hsieh, J. A hybrid model combining case-based reasoning and fuzzy decision tree for medical data classification. Appl. Soft Comput. 2011, 11, 632–644. [Google Scholar] [CrossRef]
- Setiawan, F.A.; Budiardjo, E.K.; Basaruddin, T.; Aminah, S. A Systematic Literature Review on Combining Ontology with Bayesian Network to Support Logical and Probabilistic Reasoning. In Proceedings of the 2017 International Conference on Software and e-Business, Hong Kong, China, 28–30 December 2017. [Google Scholar]
- Yan, Z.; Zhang, H.; Jia, Y.; Breuel, T.; Yu, Y. Combining the best of convolutional layers and recurrent layers: A hybrid network for semantic segmentation. arXiv 2016, arXiv:1603.04871. [Google Scholar]
- Vu, D.-H.; Vu, T.-S.; Luong, T.-D. An efficient and practical approach for privacy-preserving Naive Bayes classification. J. Inf. Secur. Appl. 2022, 68, 103215. [Google Scholar] [CrossRef]
- Nikam, S.S. A comparative study of classification techniques in data mining algorithms. Orient. J. Comput. Sci. Technol. 2015, 8, 13–19. [Google Scholar]
- Fletcher, S.; Islam, M.Z. Decision tree classification with differential privacy: A survey. ACM Comput. Surv. 2019, 52, 1–33. [Google Scholar] [CrossRef]
- Kotsiantis, S.B.; Zaharakis, I.; Pintelas, P. Supervised machine learning: A review of classification techniques. Emerg. Artif. Intell. Appl. Comput. Eng. 2007, 160, 3–24. [Google Scholar]
- Uusitalo, L. Advantages and challenges of Bayesian networks in environmental modelling. Ecol. Model. 2007, 203, 312–318. [Google Scholar] [CrossRef]
- Schlüter, F. A survey on independence-based Markov networks learning. Artif. Intell. Rev. 2012, 42, 1069–1093. [Google Scholar] [CrossRef][Green Version]
- Weng, C.; Gennari, J.H.; Fridsma, D.B. User-centered semantic harmonization: A case study. J. Biomed. Inform. 2007, 40, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Kulakowski, K.; Nalepa, G.J. Using UML state diagrams for visual modeling of business rules. In Proceedings of the 2008 International Multiconference on Computer Science and Information Technology, Wisla, Poland, 20–22 October 2008. [Google Scholar]
- Borge-Holthoefer, J.; Arenas, A. Semantic Networks: Structure and Dynamics. Entropy 2010, 12, 1264–1302. [Google Scholar] [CrossRef]
- Konopka, B.M. Biomedical ontologies—A review. Biocybern. Biomed. Eng. 2015, 35, 75–86. [Google Scholar] [CrossRef]
- Callahan, T.J.; Tripodi, I.J.; Pielke-Lombardo, H.; Hunter, L.E. Knowledge-Based Biomedical Data Science. Annu. Rev. Biomed. Data Sci. 2020, 3, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Yang, W.; Le Grange, J.M.; Wang, P.; Huang, W.; Ye, Z. Smart healthcare: Making medical care more intelligent. Glob. Health J. 2019, 3, 62–65. [Google Scholar] [CrossRef]
- Anwar, S.S.; Ahmad, U.; Khan, M.M.; Haider, M.F.; Akhtar, J. Artificial Intelligence in Healthcare: An Overview. Int. J. Eng. Res. Adv. Technol. 2020, 6, 38–45. [Google Scholar]
- Narula, S.; Shameer, K.; Omar, A.M.S.; Dudley, J.T.; Sengupta, P.P. Machine-Learning Algorithms to Automate Morphological and Functional Assessments in 2D Echocardiography. J. Am. Coll. Cardiol. 2016, 68, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Tylman, W.; Waszyrowski, T.; Napieralski, A.; Kamiński, M.; Trafidło, T.; Kulesza, Z.; Kotas, R.; Marciniak, P.; Tomala, R.; Wenerski, M. Real-time prediction of acute cardiovascular events using hardware-implemented Bayesian networks. Comput. Biol. Med. 2016, 69, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, D.; Torra, A.M.; Soriano-Payá, A.; Marín-Alonso, O.; Palencia, E.T. Aid decision algorithms to estimate the risk in congenital heart surgery. Comput. Methods Programs Biomed. 2016, 126, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.D.; Noh, J.; Lee, H.; Kim, D.K.; Lim, C.S.; Kim, Y.H.; Lee, J.P.; Kim, G.; Kim, Y.S. A Machine Learning Approach Using Survival Statistics to Predict Graft Survival in Kidney Transplant Recipients: A Multicenter Cohort Study. Sci. Rep. 2017, 7, 8904. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.; Larson, D.W.; Habermann, E.B.; Naessens, J.M.; Alabbad, J.Y.; Liu, H. Detection of clinically important colorectal surgical site infection using Bayesian network. J. Surg. Res. 2016, 209, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Lezcano-Valverde, J.M.; Salazar, F.; León, L.; Toledano, E.; Jover, J.A.; Fernandez-Gutierrez, B.; Soudah, E.; González-Álvaro, I.; Abasolo, L.; Rodriguez-Rodriguez, L. Development and validation of a multivariate predictive model for rheumatoid arthritis mortality using a machine learning approach. Sci. Rep. 2017, 7, 10189. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Zardar, A.A.; Bhatti, Z. Artificial Intelligence based Smart Doctor using Decision Tree Algorithm. arXiv 2018, arXiv:1808.01884. [Google Scholar]
- Wang, C.; Zhang, J.J.; Tang, B.B.; Fu, S.Y. Comparison of equivalent current systems for the substorm event of 8 March 2008 derived from the global PPMLR-MHD model and the KRM algorithm. J. Geophys. Res. 2011, 116, A07207. [Google Scholar] [CrossRef]
- Bleakley, K.; Yamanishi, Y. Supervised prediction of drug–target interactions using bipartite local models. Bioinformatics 2009, 25, 2397–2403. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, P.; Yan, J.; Wang, Y.; Li, S.; Jiang, J.; Sun, Z.; Tang, B.; Chang, T.-H.; Wang, S.; et al. Real-world data medical knowledge graph: Construction and applications. Artif. Intell. Med. 2020, 103, 101817. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jiang, J.; Wang, Y.; Guan, Y.; Guo, X. Learning an expandable EMR-based medical knowledge network to enhance clinical diagnosis. Artif. Intell. Med. 2020, 107, 101927. [Google Scholar] [CrossRef] [PubMed]
- Monroy, N.; Altuve, M. Analysis of the observation sequence duration of hidden Markov models for QRS complex detection in single-lead ECG recordings. In Proceedings of the 2018 Computing in Cardiology Conference (CinC), Maastricht, The Netherlands, 23–26 September 2018; Volume 45. [Google Scholar]
- Sandag, G.A.; Tedry, N.E.; Lolong, S. Classification of Lower Back Pain Using K-Nearest Neighbor Algorithm. In Proceedings of the 2018 6th International Conference on Cyber and IT Service Management (CITSM), Parapat, Indonesia, 7–9 August 2018. [Google Scholar]
- Lin, H.; Long, E.; Ding, X.; Diao, H.; Chen, Z.; Liu, R.; Huang, J.; Cai, J.; Xu, S.; Zhang, X.; et al. Prediction of myopia development among Chinese school-aged children using refraction data from electronic medical records: A retrospective, multicentre machine learning study. PLOS Med. 2018, 15, e1002674. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Majumder, D.D. A mathematical theory of shape and neuro-fuzzy methodology-based diagnostic analysis: A comparative study on early detection and treatment planning of brain cancer. Int. J. Clin. Oncol. 2017, 18, 349–681. [Google Scholar] [CrossRef] [PubMed]
- Johansson, F.D.; Collins, J.E.; Yau, V.; Guan, H.; Kim, S.C.; Losina, E.; Sontag, D.; Stratton, J.; Trinh, H.; Greenberg, J.; et al. Predicting Response to Tocilizumab Monotherapy in Rheumatoid Arthritis: A Real-world Data Analysis Using Machine Learning. J. Rheumatol. 2021, 48, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.Q.B.; du Toit, C.; Padmanabhan, S. Artificial intelligence in healthcare—The road to precision medicine. J. Hosp. Manag. Health Policy 2021, 5, 29. [Google Scholar] [CrossRef]
- Allalou, A.; Nalla, A.; Prentice, K.J.; Liu, Y.; Zhang, M.; Dai, F.F.; Ning, X.; Osborne, L.R.; Cox, B.J.; Gunderson, E.P.; et al. A Predictive Metabolic Signature for the Transition From Gestational Diabetes Mellitus to Type 2 Diabetes. Diabetes 2016, 65, 2529–2539. [Google Scholar] [CrossRef]
- Bertaud-Gounot, V.; Duvauferrier, R.; Burgun, A. Ontology and medical diagnosis. Inform. Health Soc. Care 2012, 37, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Nyangaresi, V.O.; El-Omari, N.K.T.; Nyakina, J.N. Efficient Feature Selection and ML Algorithm for Accurate Diagnostics. J. Comput. Sci. Res. 2022, 4, 10–19. [Google Scholar] [CrossRef]
- Kasbekar, P.U.; Goel, P.; Jadhav, S.P. A Decision Tree Analysis of Diabetic Foot Amputation Risk in Indian Patients. Front. Endocrinol. 2017, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Topuz, K.; Zengul, F.D.; Dag, A.; Almehmi, A.; Yildirim, M.B. Predicting graft survival among kidney transplant recipients: A Bayesian decision support model. Decis. Support Syst. 2018, 106, 97–109. [Google Scholar] [CrossRef]
- Boldsen, J.K.; Engedal, T.S.; Pedraza, S.; Cho, T.-H.; Thomalla, G.; Nighoghossian, N.; Baron, J.-C.; Fiehler, J.; Østergaard, L.; Mouridsen, K. Better Diffusion Segmentation in Acute Ischemic Stroke Through Automatic Tree Learning Anomaly Segmentation. Front. Neurosci. 2018, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.M.; Adiwijaya; Utama, D.Q. Snake bite classification using Chain code and K nearest neighbour. J. Phys. Conf. Ser. 2019, 1192, 012015. [Google Scholar] [CrossRef]
- Dan, N.; Long, T.; Jia, X.; Lu, W.; Gu, X.; Iqbal, Z.; Jiang, S. A feasibility study for predicting optimal radiation therapy dose distributions of prostate cancer patients from patient anatomy using deep learning. Sci. Rep. 2019, 9, 1076. [Google Scholar]
- Dash, D.P.; Kolekar, M.H. Hidden Markov model based epileptic seizure detection using tunable Q wavelet transform. J. Biomed. Res. 2020, 34, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, Ž.; Pokvić, L.G.; Spahić, L.; Badnjević, A. Prediction of medical device performance using machine learning techniques: Infant incubator case study. Health Technol. 2020, 10, 151–155. [Google Scholar] [CrossRef]
- Tran, L.; Li, Y.; Nocera, L.; Shahabi, C.; Xiong, L. MultiFusionNet: Atrial Fibrillation Detection With Deep Neural Networks. AMIA Summits Transl. Sci. Proc. 2020, 2020, 654–663. [Google Scholar] [PubMed]
- Jiang, X.; Ding, H.; Shi, H.; Li, C. Novel QoS optimization paradigm for IoT systems with fuzzy logic and visual information mining integration. Neural Comput. Appl. 2020, 32, 16427–16443. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Zhou, J.; Lei, F.; Zhou, F.; Qin, J.-J.; Zhang, X.-J.; Zhu, L.; Liu, Y.-M.; Wang, H.; et al. A risk score based on baseline risk factors for predicting mortality in COVID-19 patients. Curr. Med Res. Opin. 2021, 37, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.J.; Cabral, C.; Hay, A.D.; Horwood, J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand. J. Prim. Health Care 2015, 33, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bettini, C.; Brdiczka, O.; Henricksen, K.; Indulska, J.; Nicklas, D.; Ranganathan, A.; Riboni, D. A survey of context modelling and reasoning techniques. Pervasive Mob. Comput. 2010, 6, 161–180. [Google Scholar] [CrossRef]
- Kassam, A.; Kassam, N. Artificial intelligence in healthcare: A Canadian context. Health Manag. Forum 2019, 33, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Barua, S.; Begum, S. Artificial Intelligence, Machine Learning and Reasoning in Health Informatics—Case Studies. In Signal Processing Techniques for Computational Health Informatics; Springer: Berlin/Heidelberg, Germany, 2021; pp. 261–291. [Google Scholar]
- Ben Charif, A.; Zomahoun, H.T.V.; Gogovor, A.; Abdoulaye Samri, M.; Massougbodji, J.; Wolfenden, L.; Ploeg, J.; Zwarenstein, M.; Milat, A.J.; Rheault, N.; et al. Tools for assessing the scalability of innovations in health: A systematic review. Health Res. Policy Syst. 2022, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Kyrimi, E.; Dube, K.; Fenton, N.; Fahmi, A.; Neves, M.R.; Marsh, W.; McLachlan, S. Bayesian networks in healthcare: What is preventing their adoption? Artif. Intell. Med. 2021, 116, 102079. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L. Overview of Bayesian Network. Sci. J. Math. Stat. 2013. [Google Scholar]
- Mengshoel, O.J. Understanding the scalability of Bayesian network inference using clique tree growth curves. Artif. Intell. 2010, 174, 984–1006. [Google Scholar] [CrossRef]
- Jain, D. Knowledge engineering with markov logic networks: A review. Evol. Knowl. Theory Appl. 2011, 16, 50–75. [Google Scholar]
- Faghih-Roohi, S.; Xie, M.; Ng, K.M. Accident risk assessment in marine transportation via Markov modelling and Markov Chain Monte Carlo simulation. Ocean Eng. 2014, 91, 363–370. [Google Scholar] [CrossRef]
- Amith, M.F.; He, Z.; Bian, J.; Lossio-Ventura, J.A.; Tao, C. Assessing the practice of biomedical ontology evaluation: Gaps and opportunities. J. Biomed. Inform. 2018, 80, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lee, J.; Lee, C.H.; Lin, J.; Serres, O.; Chung, C.W. An efficient and scalable management of ontology. In Proceedings of the Advances in Databases: Concepts, Systems and Applications: 12th International Conference on Database Systems for Advanced Applications, DASFAA 2007, Bangkok, Thailand, 9–12 April 2007; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Ma, X.; Ma, C.; Wang, C. A new structure for representing and tracking version information in a deep time knowledge graph. Comput. Geosci. 2020, 145, 104620. [Google Scholar] [CrossRef]
- Weinstein, M.C.; O’Brien, B.; Hornberger, J.; Jackson, J.; Johannesson, M.; McCabe, C.; Luce, B.R. Principles of Good Practice for Decision Analytic Modeling in Health-Care Evaluation: Report of the ISPOR Task Force on Good Research Practices—Modeling Studies. Value Health 2003, 6, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yao, L.; Chen, K.; Wang, S.; Haghighi, P.D.; Sullivan, C. A Graph-Based Hierarchical Attention Model for Movement Intention Detection from EEG Signals. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Enos, J.R. Merging system architecture and social network analysis to better understand emergent networks of systems. In Proceedings of the International Annual Conference of the American Society for Engineering Management, Charlotte, NC, USA, 26–29 October 2016; American Society for Engineering Management (ASEM): Huntsville, AL, USA, 2016. [Google Scholar]
- Monte-Serrat, D.M.; Cattani, C. Interpretability in neural networks towards universal consistency. Int. J. Cogn. Comput. Eng. 2021, 2, 30–39. [Google Scholar] [CrossRef]
- Navigli, R.; Bevilacqua, M.; Conia, S.; Montagnini, D.; Cecconi, F. Ten Years of BabelNet: A Survey. IJCAI 2021, 4559–4567. [Google Scholar]
- Park, S.H. Semantic network analysis of presidential debates in 2007 election in Korea. Korean J. Commun. Inf. 2009, 45, 220–254. [Google Scholar]
- Song, S.; Lin, Y.; Guo, B.; Di, Q.; Lv, R. Scalable Distributed Semantic Network for knowledge management in cyber physical system. J. Parallel Distrib. Comput. 2018, 118, 22–33. [Google Scholar] [CrossRef]
- Lakemeyer, G.; Levesque, H.J. A first-order logic of limited belief based on possible worlds. In Proceedings of the International Conference on Principles of Knowledge Representation and Reasoning, Rhodes, Greece, 12–18 September 2020; Volume 17. [Google Scholar]
- Laurent, S. Reasoning with propositional logic: From sat solvers to knowledge compilation. In A Guided Tour of Artificial Intelligence Research: Volume II: AI Algorithms; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–152. [Google Scholar]
- García-García, J.; Enríquez, J.; Domínguez-Mayo, F. Characterizing and evaluating the quality of software process modeling language: Comparison of ten representative model-based languages. Comput. Stand. Interfaces 2018, 63, 52–66. [Google Scholar] [CrossRef]
- Kalcheva, N.; Todorova, M.; Marinova, G. Naive Bayes Classifier, Decision Tree and AdaBoost Ensemble Algorithm–Advantages and Disadvantages. In Proceedings of the 6th ERAZ Conference Proceedings (Part of ERAZ Conference Collection), Online, 21 May 2020. [Google Scholar]
- Ahmad, M.A.; Eckert, C.; Teredesai, A. Interpretable machine learning in healthcare. In Proceedings of the 2018 ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics, New York, NY, USA, 15 August 2018. [Google Scholar]
- Jackins, V.; Vimal, S.; Kaliappan, M.; Lee, M.Y. AI-based smart prediction of clinical disease using random forest classifier and Naive Bayes. J. Supercomput. 2021, 77, 5198–5219. [Google Scholar] [CrossRef]
- Alghunaim, S.; Al-Baity, H.H. On the Scalability of Machine-Learning Algorithms for Breast Cancer Prediction in Big Data Context. IEEE Access 2019, 7, 91535–91546. [Google Scholar] [CrossRef]
- Renganathan, V. Overview of artificial neural network models in the biomedical domain. Bratisl. Med J. 2019, 120, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Ajami, H.; Mcheick, H. Ontology-Based Model to Support Ubiquitous Healthcare Systems for COPD Patients. Electronics 2018, 7, 371. [Google Scholar] [CrossRef]
- Eshghishargh, A.; Milton, S.; Egan, G.F.; Lonie, A.; Kolbe, S.; Killeen, N.E.; Lohrey, J.M. An ontology-based semantic question complexity model and its applications in neuroinformatics. Front. Neurosci. 2015, 9. [Google Scholar]
- Khattak, A.M.; Batool, R.; Pervez, Z.; Khan, A.M.; Lee, S. Ontology Evolution and Challenges. J. Inf. Sci. Eng. 2013, 29, 851–871. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Shahpori, R.; Doig, C. Systematized Nomenclature of Medicine–Clinical Terms direction and its implications on critical care. J. Crit. Care 2010, 25, 364.e1–364.e9. [Google Scholar] [CrossRef] [PubMed]
- Hastings, J.; Owen, G.; Dekker, A.; Ennis, M.; Kale, N.; Muthukrishnan, V.; Turner, S.; Swainston, N.; Mendes, P.; Steinbeck, C. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2015, 44, D1214–D1219. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Jiang, G.; Oniki, T.A.; Freimuth, R.R.; Zhu, Q.; Sharma, D.; Pathak, J.; Huff, S.M.; Chute, C.G. A semantic-web oriented representation of the clinical element model for secondary use of electronic health records data. J. Am. Med Inform. Assoc. 2012, 20, 554–562. [Google Scholar] [CrossRef]






| Knowledge Types | Description | Examples |
|---|---|---|
| Explicit knowledge | A form of knowledge that can be expressed in various formats such as plain text, documents, spreadsheets, databases, images, etc., and can exist in structured or unstructured forms [10,11]. | Scientific formulas; recipes for a chef; books. For example: The capital of France is Paris. |
| Implicit knowledge | Knowledge gained through incidental activities, or without awareness that learning is occurring [12]. | How to walk, run, ride a bicycle or swim. |
| Tacit knowledge | This type of knowledge is subjective, based on personal experience, and often difficult to express. It resides in the human brain in an inexpressible form and is of an intellectual nature. This original form of knowledge is primarily obtained through learning and experiences [13]. | Doctor’s diagnosis; musician’s improvisation. |
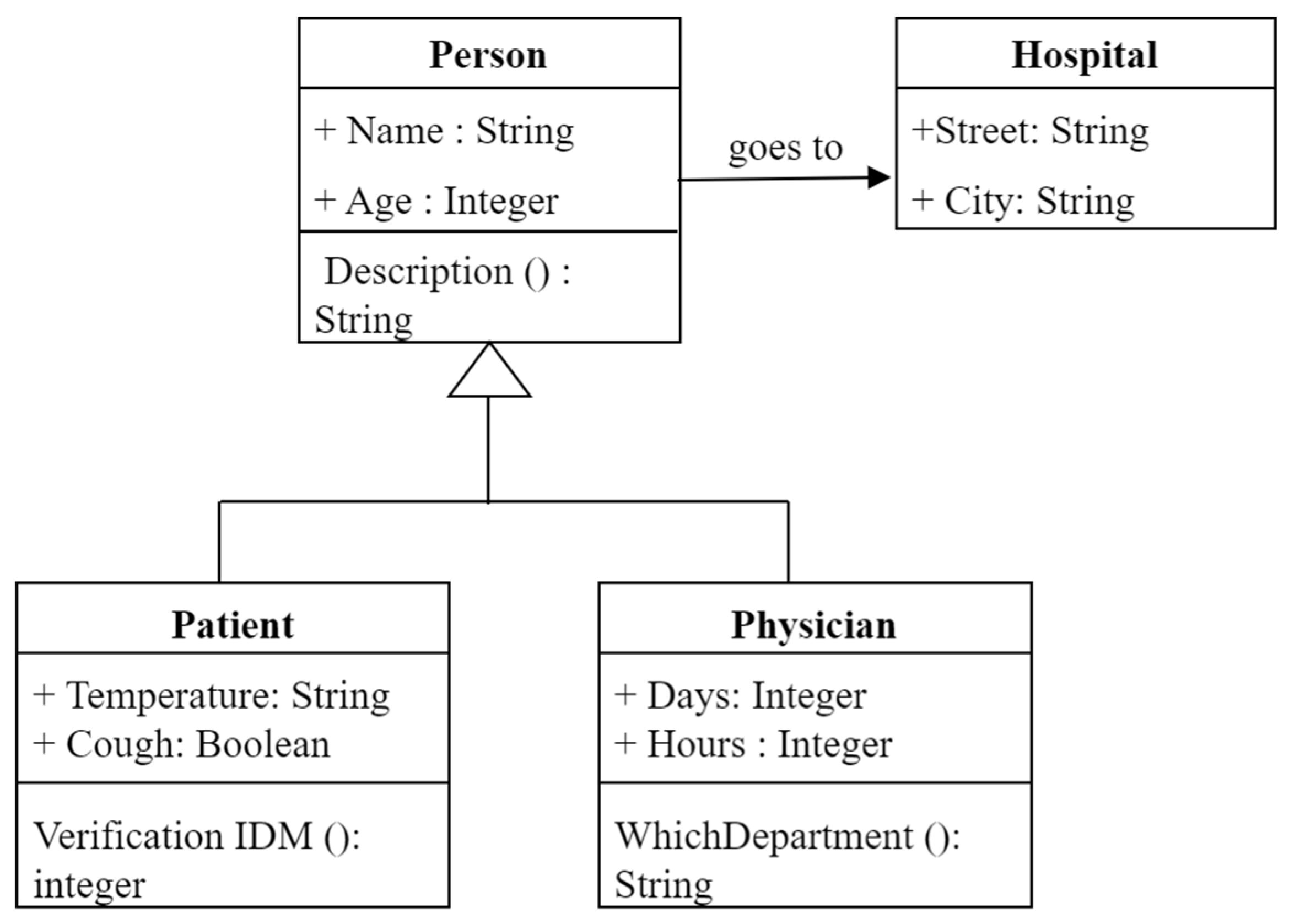
| Name | Age | Sex | Description | Temperature | Cough |
|---|---|---|---|---|---|
| Sami | 32 | Male | Patient | 38 °C | Yes |
| Model | Advantages | Disadvantages |
|---|---|---|
| Naïve Bayes [39,40] | Simple; manages noisy data and missing values. | Inability to handle continuous features. |
| Decision tree [41] | Intuitive interpretation; manages missing values. | Difficulty in designing large trees; low ability to manage noisy data. |
| Random forest [42] | Ability to manage noisy data; suitable for large and heterogeneous data. | Requires more memory; difficult implementation and complicated in some cases; low ability to manage missing values. |
| Bayesian network [43] | Handles missing or incomplete data; graphical structure facilitates interoperability. | Memory requirements increase with the number of variables. Large and complex networks may face scalability issues. |
| Markov representation [44] | Ability to represent large data; robustness in handling noisy data. | Future transitions depend only on the current state, not on the entire history. This memoryless property can be limiting, especially when considering long-term effects or complex dependencies. |
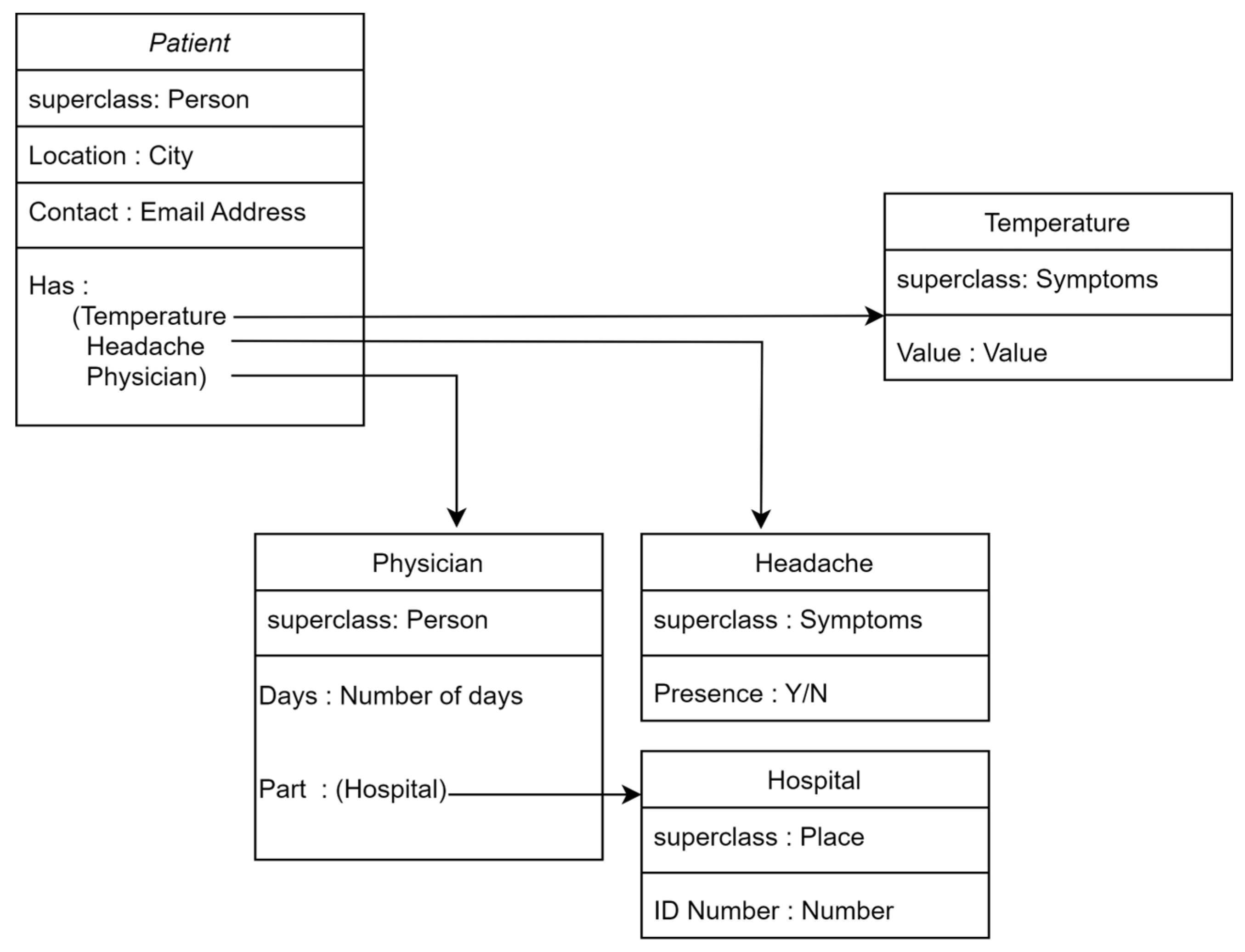
| UML [45,46] | Graphical representation for software design. | Becomes complex for large systems. |
| Frame [47] | Hierarchical organization of knowledge; easy to construct. | Difficulty in representing complex relationships. |
| Semantic network [48] | Effective when representing relationships between entities. | Faces challenges with large graphs. |
| Ontology [49] | Structured representation of data; allows navigation of complex processes. Enables integration of domain knowledge for high-level reasoning. | Creating an ontology model can be time-consuming. |
| First-order logic [50] | Expressive; handles complex relationships. | Faces challenges in representing certain types of data. |
| Second-order logic [50] | Adds expressiveness over first-order logic. | Increased complexity in reasoning. |
| Propositional logic [50] | Simple and easy to understand. | Limited expressiveness for complex relationships. |
| Ref. | KRMs | Health Domain |
|---|---|---|
| [64] | Mathematical representation models | Disease diagnosis. |
| [65] | Random forest | Treatment for rheumatoid arthritis. |
| [66] | Random forest | Detection of inflammatory bowel disease. |
| [67] | Decision tree | Patient and disease diagnosis. |
| [68] | Ontology | Data representation in medical domain. |
| [69] | K-nearest neighbours | Diagnosis and data in X-ray image. |
| [70] | Neural network | Finding signs of diabetic retinopathy. |
| [71,72] | Naïve Bayes | Data representation in congenital heart surgery. |
| [73] | Decision tree | Evaluation of the risk of amputation in individuals with diabetic foot. |
| [74] | Random forest | Antibody data representation in kidney transplantation. |
| [75] | Naïve Bayes | Data representation from ultrasound images. |
| [76] | Bayesian network | Used in treatment process. |
| [77] | Decision tree | Specific details in the diagnosis process. |
| [78] | K-nearest neighbours | Forecasting the risk of retinopathy. |
| [79] | Random forest | Monitoring for school children. |
| [80] | Decision tree | Detection of spread patterns in recent ischemic stroke. |
| [81] | K-nearest neighbours | Detection of thyroid disease. |
| [82] | Neural network | Used in cancer treatment using advanced therapy techniques. |
| [83] | Markov model | Disease diagnosis using ECG. |
| [84] | Decision tree | Patient monitoring. |
| [85] | Neural network | Identification of coronary calcium. |
| [86] | Fuzzy logic | Medical record representation for diagnosis prediction. |
| [87] | Random forest | Diagnosing CKD disease. |
| KRMs | Heterogeneity | Interpretability | Reasoning | Scalability |
|---|---|---|---|---|
| Bayesian network | Satisfy [93] | Satisfy [94] | Satisfy [93] | Partial satisfaction [94] |
| Markov representation | Satisfy [75] | Partial satisfaction [95] | Satisfy [95] | Satisfy [96] |
| Ontology | Satisfy [97] | Satisfy [97] | Satisfy [97] | Satisfy [98] |
| UML | Satisfy [99] | Satisfy [99] | Partial satisfaction [100] | Partial satisfaction [99] |
| Frame | Satisfy [101] | Satisfy [99] | Not satisfy [100] | Partial satisfaction [101] |
| Tuple-based representation | Satisfy [102] | Satisfy [102] | Partial satisfaction [102] | Partial satisfaction [102] |
| Semantic network | Satisfy [103] | Satisfy [103] | Partial satisfaction [104] | Partial satisfaction [104] |
| First-order logic | Partial satisfaction [105] | Satisfy [105] | Satisfy [105] | Partial satisfaction [105] |
| Second-order logic | Satisfy [106] | Satisfy [107] | Satisfy [106] | Partial satisfaction [106] |
| Propositional logic | Partial satisfaction [106] | Satisfy [106] | Partial satisfaction [107] | Not satisfy [107] |
| Rule-based system | Satisfy [108] | Satisfy [108] | Satisfy [109] | Partial satisfaction [108] |
| Naïve Bayes | Partial satisfaction [110] | Partial satisfaction [110] | Satisfy [110] | Satisfy [110] |
| Random forest | Satisfy [111] | Partial satisfaction [112] | Satisfy [112] | Satisfy [112] |
| Decision tree | Satisfy [112] | Satisfy [111] | Satisfy [112] | Partial satisfaction [112] |
| Neural network | Satisfy [113] | Not satisfy [114] | Satisfy [114] | Satisfy [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msheik, B.; Adda, M.; Mcheick, H.; Dbouk, M. Survey on Knowledge Representation Models in Healthcare. Information 2024, 15, 435. https://doi.org/10.3390/info15080435
Msheik B, Adda M, Mcheick H, Dbouk M. Survey on Knowledge Representation Models in Healthcare. Information. 2024; 15(8):435. https://doi.org/10.3390/info15080435
Chicago/Turabian StyleMsheik, Batoul, Mehdi Adda, Hamid Mcheick, and Mohamed Dbouk. 2024. "Survey on Knowledge Representation Models in Healthcare" Information 15, no. 8: 435. https://doi.org/10.3390/info15080435
APA StyleMsheik, B., Adda, M., Mcheick, H., & Dbouk, M. (2024). Survey on Knowledge Representation Models in Healthcare. Information, 15(8), 435. https://doi.org/10.3390/info15080435









