Leveraging the TOE Framework: Examining the Potential of Mobile Health (mHealth) to Mitigate Health Inequalities
Abstract
1. Introduction
1.1. mHealth Potential to Reduce Health Disparities
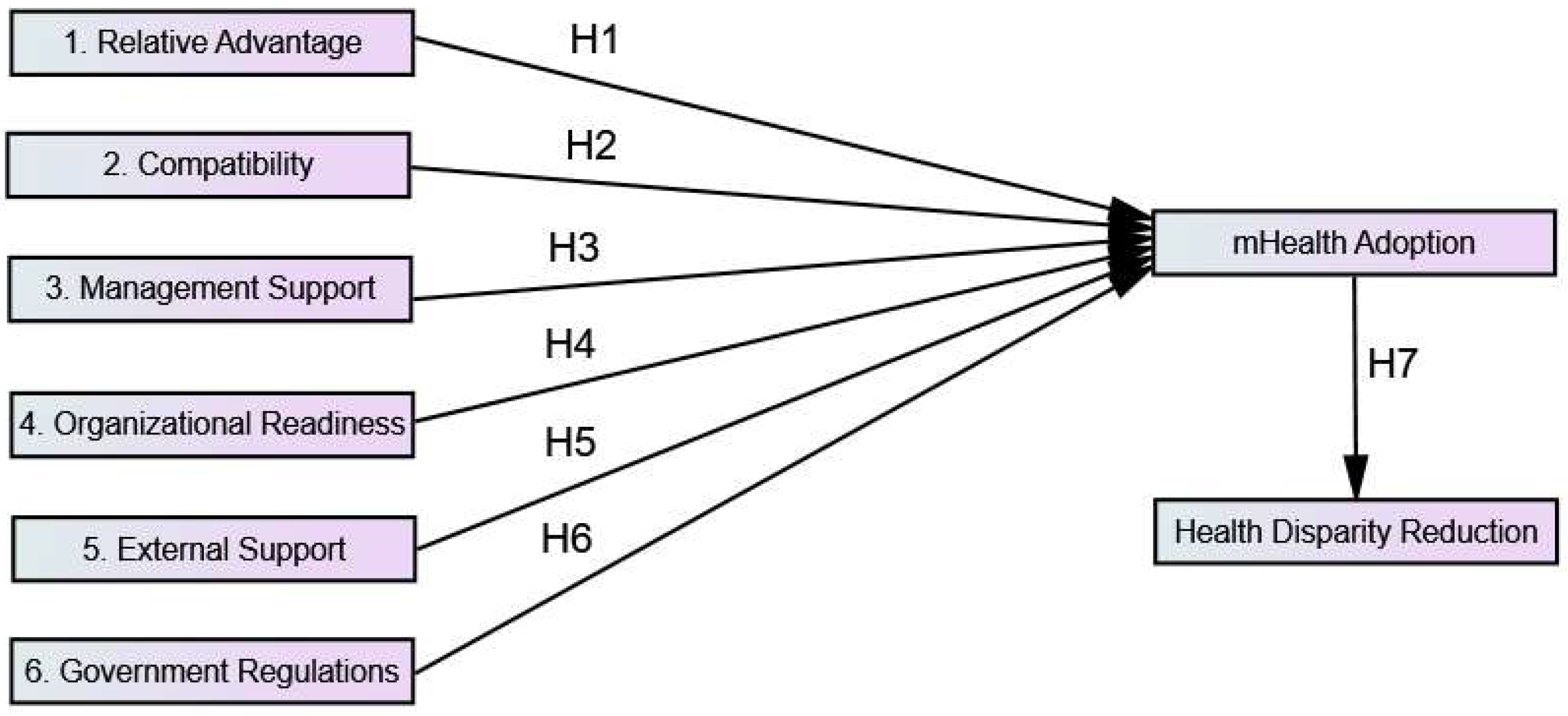
1.2. Theoretical Framework and Hypotheses Development
1.2.1. Technological Factors (TF)
1.2.2. Organizational Factors (OF)
1.2.3. Environmental Factors (EF)
1.3. Study Objectives
2. Materials and Methods
2.1. Participants and Procedure
2.2. Research Instrument
2.3. Data Collection and Analysis Procedure
2.4. Ethical Approval
3. Results
3.1. Demographic Information
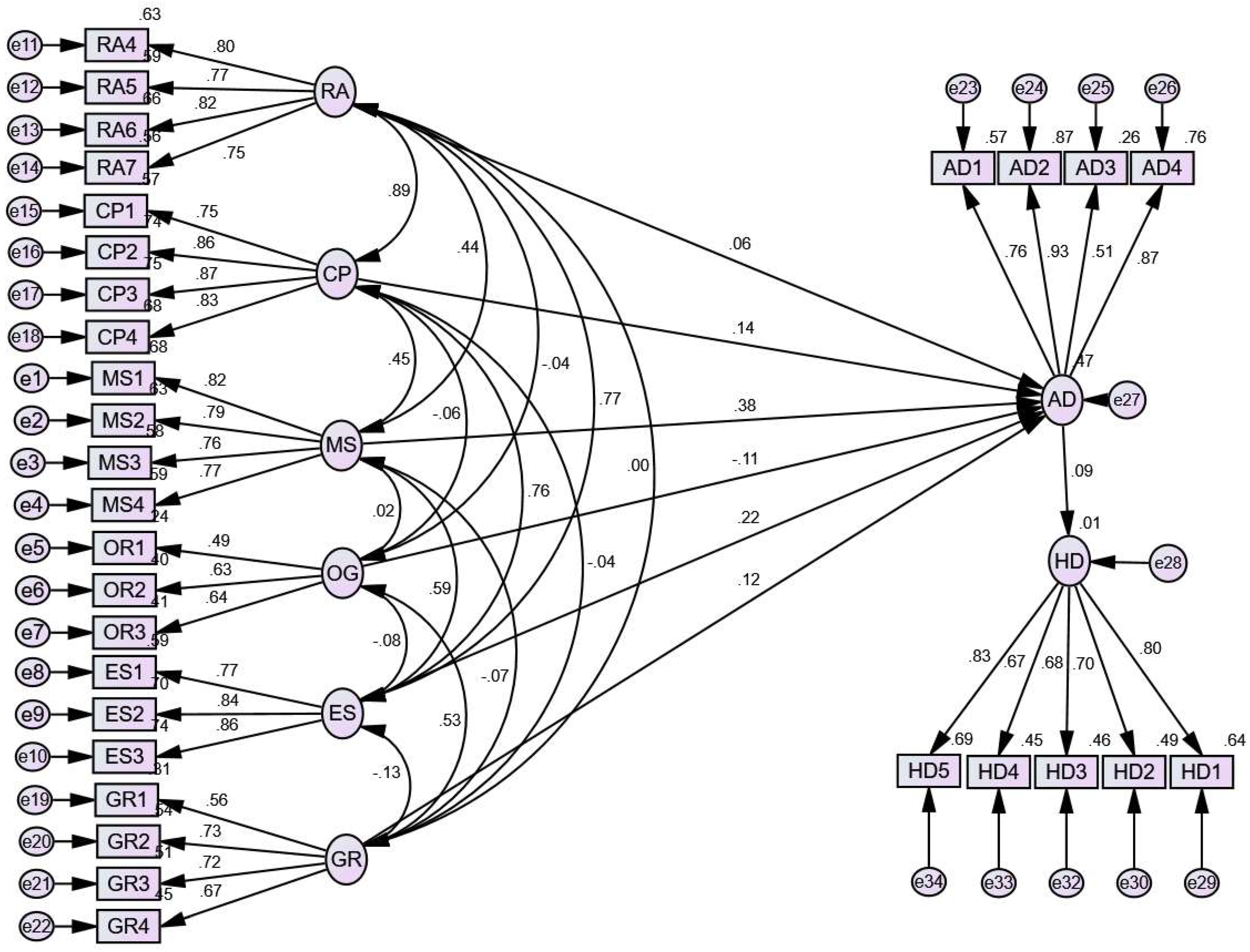
3.2. Structural Equation Model
3.2.1. Standardized Estimation of Regression Weights
3.2.2. Standardized Estimation of Correlation among Latent Variables
3.2.3. Estimation of Covariances among Latent Variables
3.2.4. Model Fit Indices
3.2.5. Standardized Estimation of Regression Weights and Validation of the Hypotheses
4. Discussion
4.1. Study Limitations
4.2. Note to Readers
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Construct | Items | Measure | Sources |
|---|---|---|---|
| Relative Advantage (RA) | RA4 | I think using mobile for healthcare improves patients’ service and lowers the costs on healthcare provision. | [27,39] |
| RA5 | I think using mobile for healthcare brings in new service opportunities. | ||
| RA6 | I think mHealth supports medical emergency response. | ||
| RA7 | I think mHealth helps improve users’ experience by offering better services. | ||
| Compatibility (CP) | CP1 | I think using mobile for healthcare is consistent with our practices. | [75] |
| CP2 | I think using mobile for healthcare fits our organizational culture. | ||
| CP3 | I think that, overall, it is easy to incorporate mHealth into our organization. | ||
| CP4 | I think mHealth apps are compatible with most of today’s hand-held devices. | ||
| Management Support (MS) | MS1 | I think the adoption of mHealth for healthcare delivery is encouraged by our senior management. | [41] |
| MS2 | I think our senior management is willing to support mHealth adoption campaigns. | ||
| MS3 | I think healthcare delivery through mHealth is a strategic endeavor that our top management places a high priority on. | ||
| MS4 | I think our top management is enthusiastic about using mobile phone technologies in the healthcare industry. | ||
| Organizational Readiness (OG) | OG1 | I think the hospital has the required resources to adopt mHealth solutions. | [56,57] |
| OG2 | The hospital/health department has organized workshops or trainings on ICT/computer proficiency in order to effectively adopt mHealth solutions for patient care. | ||
| OG3 | I think the hospital aims to encourage mHealth solutions in the future. | ||
| External Support (ES) | ES1 | I think external funding agencies (such as Asian Development Bank, WHO, etc.) encourage adopting new health ICT (e.g., mHealth for quality patient care). | [76] |
| ES2 | I think health department can offer necessary training for using mHealth in healthcare | ||
| ES3 | I think the health department can offer efficient technical assistance for the use of mHealth in healthcare | ||
| Government Regulation (GR) | GR1 | I think government regulations encourage adopting new information technology (e.g., mHealth for quality patient care). | [75] |
| GR2 | I think the government can provide the technical support, training, and funding to increase the usage of mHealth services | ||
| GR3 | I think the government can support safeguarding security and privacy concerns while using the mHealth | ||
| GR4 | I think government can be adaptable towards the regulations for advances in mHealth technologies | ||
| mHealth Adoption (AD) | AD1 | I think adopting an mHealth service will be a pleasant experience. | [17] |
| AD2 | I think mHealth can provide an opportunity to respond to patients more quickly. | ||
| AD3 | I spend a lot of time using mHealth applications. | ||
| AD4 | I think adopting mHealth can enable faster access to patient data. | ||
| Health Disparity Reduction (HD) | HD1 | I think mHealth is an effective solution for reducing health disparities. | [25] |
| HD2 | I am satisfied with the impact of mHealth adoption on improving healthcare access for marginalized communities. | ||
| HD3 | mHealth initiatives can address the specific health needs of underserved populations. | ||
| HD4 | I think mHealth has the potential to reduce health disparities in the healthcare setting/areas where I practice medicine. | ||
| HD5 | I recommend mHealth solutions to colleagues for addressing health disparities. |
References
- World Health Organization (WHO). Available online: https://www.who.int/ (accessed on 23 August 2023).
- National Institutes of Health (NIH)|Turning Discovery into Health. Available online: https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health (accessed on 30 November 2023).
- Barton, A.J. The Regulation of Mobile Health Applications.: Full Text Finder Results. BMC Med. 2012, 46, 2–5. [Google Scholar]
- Ben-Zeev, D.; Kaiser, S.M.; Brenner, C.J.; Begale, M.; Duffecy, J.; Mohr, D.C. Development and Usability Testing of FOCUS: A Smartphone System for Self-Management of Schizophrenia. Psychiatr. Rehabil. J. 2013, 36, 289–296. [Google Scholar] [CrossRef]
- Collins, F. How to Fulfill the True Promise of “MHealth”: Mobile Devices Have the Potential to Become Powerful Medical Tools. Sci. Am. 2012, 307, 16. [Google Scholar] [CrossRef]
- Silva, B.M.C.; Rodrigues, J.J.P.C.; de la Torre Díez, I.; López-Coronado, M.; Saleem, K. Mobile-Health: A Review of Current State in 2015. J. Biomed. Inform. 2015, 56, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H. 28 City Doctors Killed in Targeted Attacks over Four Years. DAWN.COM. Available online: https://www.dawn.com/news/1128612 (accessed on 7 January 2023).
- Burney, A.; Abbas, Z.; Mahmood, N.; Arifeen, Q. Prospects for Mobile Health in Pakistan and Other Developing Countries. Adv. Internet Things 2013, 3, 27–32. [Google Scholar] [CrossRef]
- Siddiqui, M.; ul Islam, M.Y.; Mufti, B.A.I.; Khan, N.; Farooq, M.S.; Muhammad, M.G.; Osama, M.; Kherani, D.; Kazi, A.N.; Kazi, A.M. Assessing Acceptability of Hypertensive/Diabetic Patients towards Mobile Health Based Behavioral Interventions in Pakistan: A Pilot Study. Int. J. Med. Inform. 2015, 84, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Kurji, Z.; Premani, Z.S.; Mithani, Y. Analysis of the Health Care System of Pakistan: Lessons Learnt and Way forward. J. Ayub Med. Coll. Abbottabad 2016, 28, 601–604. [Google Scholar] [PubMed]
- Hashmi, N.R.; Khan, S.A. Interventional Study to Improve Diabetic Guidelines Adherence Using Mobile Health (m-Health) Technology in Lahore, Pakistan. BMJ Open 2018, 8, e020094. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, A.; Memon, S.F.; Zehra, A.; Barry, S.; Jawed, H.; Akhtar, M.; Kirmani, W.; Malik, F.; Khawaja, A.W.; Barry, H.; et al. Knowledge and Attitude Regarding Telemedicine among Doctors in Karachi. Cureus 2020, 12, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Borsari, L.; Stancanelli, G.; Guarenti, L.; Grandi, T.; Leotta, S.; Barcellini, L.; Borella, P.; Benski, A.C. An Innovative Mobile Health System to Improve and Standardize Antenatal Care among Underserved Communities: A Feasibility Study in an Italian Hosting Center for Asylum Seekers. J. Immigr. Minor. Health 2018, 20, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Paduano, S.; Incerti, F.; Borsari, L.; Benski, A.C.; Ernest, A.; Mwampagatwa, I.; Lilungulu, A.; Masoi, T.; Bargellini, A.; Stornelli, F.; et al. Use of a MHealth System to Improve Antenatal Care in Low and Lower-Middle Income Countries: Report on Patients and Healthcare Workers’ Acceptability in Tanzania. Int. J. Environ. Res. Public Health 2022, 19, 15342. [Google Scholar] [CrossRef]
- Benski, A.C.; Schmidt, N.C.; Viviano, M.; Stancanelli, G.; Soaroby, A.; Reich, M.R. Improving the Quality of Antenatal Care Using Mobile Health in Madagascar: Five-Year Cross-Sectional Study. JMIR MHealth UHealth 2020, 8, e18543. [Google Scholar] [CrossRef]
- Aamir, J.; Ali, S.M.; Kamel Boulos, M.N.; Anjum, N.; Ishaq, M. Enablers and Inhibitors: A Review of the Situation Regarding MHealth Adoption in Low- and Middle-Income Countries. Health Policy Technol. 2018, 7, 88–97. [Google Scholar] [CrossRef]
- Azam, M.; Naeem, S.B.; Kamel Boulos, M.N.; Faiola, A. Modelling the Predictors of Mobile Health (MHealth) Adoption among Healthcare Professionals in Low-Resource Environments. Int. J. Environ. Res. Public Health 2023, 20, 7112. [Google Scholar] [CrossRef]
- Caleb, J.; Colón-Rodríguez; DrPH, M. Shedding Light on Healthcare Algorithmic and Artificial Intelligence Bias; American Board of Family Medicine: Lexington, KY, USA, 2023. [Google Scholar] [CrossRef]
- Nelson, A. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. J. Natl. Med. Assoc. 2002, 94, 666. [Google Scholar] [CrossRef]
- Virnig, B.A.; Lurie, N.; Huang, Z.; Musgrave, D.; Marshall McBean, A.; Dowd, B. Racial Variation in Quality of Care among Medicare + Choice Enrollees. Health Aff. 2017, 21, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.; Callaghan, W.M.; Barfield, W. Racial/Ethnic Disparities in Pregnancy-Related Deaths—United States, 2007–2016. Morb. Mortal. Wkly. Rep. 2019, 68, 762. [Google Scholar] [CrossRef] [PubMed]
- Lee, J. The Impact of Health Information Technology on Disparity of Process of Care. Int. J. Equity Health 2015, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Al Dahdah, M.; Desgrées Du LoÛ, A.; Méadel, C. Mobile Health and Maternal Care: A Winning Combination for Healthcare in the Developing World? Health Policy Technol. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Brodie, M.; Flournoy, R.E.; Altman, D.E.; Blendon, R.J.; Benson, J.M.; Rosenbaum, M.D. Health Information, the Internet, and the Digital Divide. Health Aff. 2018, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hailu, B.; Tabor, D.C.; Gold, R.; Sayre, M.H.; Sim, I.; Jean-Francois, B.; Casnoff, C.A.; Cullen, T.; Thomas, V.A.; et al. Role of Health Information Technology in Addressing Health Disparities: Patient, Clinician, and System Perspectives. Med. Care 2019, 57 (Suppl. S6 2), S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Tornatzky, L.; Fleischer, M.; Chakrabarti, A. Processes of Technological Innovation; Lexington Books: Lanham, MD, USA, 1990. [Google Scholar]
- Lippert, S.K. Technological, Organizational, and Environmental Antecedents to Web Services Adoption. Commun. IIMA 2006, 6, 14. [Google Scholar] [CrossRef]
- Alam, S.S. Adoption of Internet in Malaysian SMEs. J. Small Bus. Enterp. Dev. 2009, 16, 240–255. [Google Scholar] [CrossRef]
- Cao, Q.; Jones, D.R.; Sheng, H. Contained Nomadic Information Environments: Technology, Organization, and Environment Influences on Adoption of Hospital RFID Patient Tracking. Inf. Manag. 2014, 51, 225–239. [Google Scholar] [CrossRef]
- Chau, P.Y.K.; Tam, K.Y. Factors Affecting the Adoption of Open Systems: An Exploratory Study. MIS Q. Manag. Inf. Syst. 1997, 21, 1–24. [Google Scholar] [CrossRef]
- Kuan, K.K.Y.; Chau, P.Y.K. A Perception-Based Model for EDI Adoption in Small Businesses Using a Technology-Organization-Environment Framework. Inf. Manag. 2001, 38, 507–521. [Google Scholar] [CrossRef]
- Liang, Y.; Qi, G.; Wei, K.; Chen, J. Exploring the Determinant and Influence Mechanism of E-Government Cloud Adoption in Government Agencies in China. Gov. Inf. Q. 2017, 34, 481–495. [Google Scholar] [CrossRef]
- Lin, H.F. Understanding the Determinants of Electronic Supply Chain Management System Adoption: Using the Technology-Organization-Environment Framework. Technol. Forecast. Soc. Change 2014, 86, 80–92. [Google Scholar] [CrossRef]
- Ramdani, B.; Chevers, D.; Williams, D.A. SMEs’ Adoption of Enterprise Applications: A Technology-Organisation-Environment Model. J. Small Bus. Enterp. Dev. 2013, 20, 735–753. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, Y.S.; Yang, Y.F. Understanding the Determinants of RFID Adoption in the Manufacturing Industry. Technol. Forecast. Soc. Change 2010, 77, 803–815. [Google Scholar] [CrossRef]
- Zhu, K.; Dong, S.; Xu, S.X.; Kraemer, K.L. Innovation Diffusion in Global Contexts: Determinants of Post-Adoption Digital Transformation of European Companies. Eur. J. Inf. Syst. 2006, 15, 601–616. [Google Scholar] [CrossRef]
- Maroufkhani, P.; Tseng, M.L.; Iranmanesh, M.; Ismail, W.K.W.; Khalid, H. Big Data Analytics Adoption: Determinants and Performances among Small to Medium-Sized Enterprises. Int. J. Inf. Manag. 2020, 54, 102190. [Google Scholar] [CrossRef]
- Ngongo, B.P.; Ochola, P.; Ndegwa, J.; Katuse, P. The Technological, Organizational and Environmental Determinants of Adoption of Mobile Health Applications (m-Health) by Hospitals in Kenya. PLoS ONE 2019, 14, e0225167. [Google Scholar] [CrossRef] [PubMed]
- Ogundele, O.; Cilliers, L.; Isabirye, N. A Model to Provide Health Services to Hypertensive Patients through the Use of Mobile Health Technology. In Proceedings of the African Conference of Information and Communication Technology, Cape Town, South Africa, 9–10 July 2018. [Google Scholar]
- Nabil, A.M.; Kandil, A.; Ragheb, M.A.; Ragab, A.A.; Farouk, M. Examining the Effect of TOE Model on Cloud Computing Adoption in Egypt. Bus. Manag. Rev. 2018, 9, 113–123. [Google Scholar]
- Ngongo, B.P.; Ochola, P.; Ndegwa, J.; Katuse, P. The Moderating Role of Top Executives’ Sex, Level of Education and Knowledge on Adoption of Mobile Health Applications by Hospitals in Kenya. J. Healthc. Leadersh. 2019, 11, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, K.K.; Dwivedi, Y.K.; Williams, M.D. Innovation Adoption Attributes: A Review and Synthesis of Research Findings. Eur. J. Innov. Manag. 2014, 17, 327–348. [Google Scholar] [CrossRef]
- Alalwan, A.A.; Dwivedi, Y.K.; Rana, N.P. Factors Influencing Adoption of Mobile Banking by Jordanian Bank Customers: Extending UTAUT2 with Trust. Int. J. Inf. Manag. 2017, 37, 99–110. [Google Scholar] [CrossRef]
- Olanrewaju, A.S.T.; Hossain, M.A.; Whiteside, N.; Mercieca, P. Social Media and Entrepreneurship Research: A Literature Review. Int. J. Inf. Manag. 2020, 50, 90–110. [Google Scholar] [CrossRef]
- Jacob, C.; Sanchez-vazquez, A.; Ivory, C. Social, Organizational, and Technological Factors Impacting Clinicians’ Adoption of Mobile Health Tools: Systematic Literature Review. JMIR MHealth UHealth 2020, 8, e15935. [Google Scholar] [CrossRef]
- Asiaei, A.; Nor, N.Z. A Multifaceted Framework for Adoption of Cloud Computing in Malaysian SMEs. J. Sci. Technol. Policy Manag. 2019, 10, 708–750. [Google Scholar] [CrossRef]
- Sun, S.; Cegielski, C.G.; Jia, L.; Hall, D.J. Understanding the Factors Affecting the Organizational Adoption of Big Data. J. Comput. Inf. Syst. 2018, 58, 193–203. [Google Scholar] [CrossRef]
- Alajlani, M.; Clarke, M. Effect of Culture on Acceptance of Telemedicine in Middle Eastern Countries: Case Study of Jordan and Syria. Telemed. e-Health 2013, 19, 305–311. [Google Scholar] [CrossRef]
- Abd Ghani, M.K.; Jaber, M.M. Willingness to Adopt Telemedicine in Major Iraqi Hospitals: A Pilot Study. Int. J. Telemed. Appl. 2015, 2015, 136591. [Google Scholar] [CrossRef]
- De Souza, C.H.A.; Morbeck, R.A.; Steinman, M.; Hors, C.P.; Bracco, M.M.; Kozasa, E.H.; Leaõ, E.R. Barriers and Benefits in Telemedicine Arising between a High-Technology Hospital Service Provider and Remote Public Healthcare Units: A Qualitative Study in Brazil. Telemed. e-Health 2017, 23, 527–532. [Google Scholar] [CrossRef]
- Brewster, L.; Mountain, G.; Wessels, B.; Kelly, C.; Hawley, M. Factors Affecting Front Line Staff Acceptance of Telehealth Technologies: A Mixed-Method Systematic Review. J. Adv. Nurs. 2014, 70, 21–33. [Google Scholar] [CrossRef]
- Gagnon, M.P.; Ngangue, P.; Payne-Gagnon, J.; Desmartis, M. M-Health Adoption by Healthcare Professionals: A Systematic Review. J. Am. Med. Inform. Assoc. 2016, 23, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.L.S.; Izquierdo, M.; Querol, X.; Lieberman, R.N.; Saikia, B.K.; Silva, L.F.O. Nanoparticles from Construction Wastes: A Problem to Health and the Environment. J. Clean. Prod. 2019, 219, 236–243. [Google Scholar] [CrossRef]
- Anasi, S.N.; Ukangwa, C.C.; Fagbe, A. University Libraries-Bridging Digital Gaps and Accelerating the Achievement of Sustainable Development Goals through Information and Communication Technologies. World J. Sci. Technol. Sustain. Dev. 2018, 15, 13–25. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, H.; Ren, J. Understanding the Determinants of Big Data Analytics (BDA) Adoption in Logistics and Supply Chain Management: An Empirical Investigation. Int. J. Logist. Manag. 2018, 29, 676–703. [Google Scholar] [CrossRef]
- Verma, S.; Bhattacharyya, S.S. Perceived Strategic Value-Based Adoption of Big Data Analytics in Emerging Economy: A Qualitative Approach for Indian Firms. J. Enterp. Inf. Manag. 2017, 30, 354–382. [Google Scholar] [CrossRef]
- Hoti, E. The Technological, Organizational and Environmental Framework of IS Innovation Adaption in Small and Medium Enterprises. Int. J. Bus. Manag. 2015, 3, 1–14. [Google Scholar] [CrossRef]
- Ramdani, B.; Duan, B.; Berrou, I. Exploring the Determinants of Mobile Health Adoption by Hospitals in China: Empirical Study. JMIR Med. Inform. 2020, 8, e14795. [Google Scholar] [CrossRef]
- Bilal, W.; Qamar, K.; Siddiqui, A.; Kumar, P.; Essar, M.Y. Digital Health and Telemedicine in Pakistan: Improving Maternal Healthcare. Ann. Med. Surg. 2022, 81, 104425. [Google Scholar] [CrossRef]
- Sayibu, M.; Chu, J.; Akintunde, T.Y.; Rufai, O.H.; Amosun, T.S.; George-Ufot, G. Environmental Conditions, Mobile Digital Culture, Mobile Usability, Knowledge of App in COVID-19 Risk Mitigation: A Structural Equation Model Analysis. Smart Health 2022, 25, 100286. [Google Scholar] [CrossRef]
- Lee, Y.; Hsieh, Y.; Hsu, C.H. Adding Innovation Diffusion Theory to the Technology Acceptance Model: Supporting Employees’ Intentions to Use e-Learning Systems. J. Educ. Technol. Soc. 2011, 14, 124–137. [Google Scholar]
- Rogers, E.M. Diffuison of Innovations, 4th ed.; The Free Press: New York, NY, USA, 1995; Available online: https://search.worldcat.org/title/31604567 (accessed on 20 March 2024).
- Hwang, J.; Christensen, C.M. Disruptive Innovation in Health Care Delivery: A Framework for Business-Model Innovation. Health Aff. 2017, 27, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Herzlinger, R. Why Innovation in Health Care is so Hard. Harv. Bus. Rev. 2006, 84, 58. [Google Scholar] [PubMed]
- Economist Impact: The Inclusive Internet Index, Supported by Meta. The Economist Newspaper Limited. Available online: https://impact.economist.com/projects/inclusive-internet-index/2022/country/Pakistan (accessed on 30 December 2023).
- Global Health Council. Available online: https://globalhealth.org/?s=Pakistan+ (accessed on 30 December 2023).
- Shaikh, B.T.; Ali, N. Universal Health Coverage in Pakistan: Is the Health System Geared up to Take on the Challenge? Glob. Health 2023, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- WHO. Primary Health Care Strengthening and Health Financing Reforms—A Priority for the Federal and Provincial Governments in Pakistan. Available online: https://www.who.int/news-room/feature-stories/detail/pakistan (accessed on 28 November 2023).
- Naeem, S.B.; Bhatti, R. Barriers in Seeking Health Information from Primary Healthcare Facilities in Pakistan. Inf. Dev. 2016, 32, 1014–1026. [Google Scholar] [CrossRef]
- The World Bank. World Bank Supports Primary Healthcare and Universal Coverage to Strengthen Human Capital in Pakistan. Available online: https://www.worldbank.org/en/news/press-release/2022/06/06/-world-bank-supports-primary-healthcare-and-universal-coverage-to-strengthen-human-capital-in-pakistan (accessed on 28 November 2023).
- Bin Naeem, S.; Kamel Boulos, M.N. COVID-19 Misinformation Online and Health Literacy: A Brief Overview. Int. J. Environ. Res. Public Health 2021, 18, 8091. [Google Scholar] [CrossRef] [PubMed]
- Faiola, A.; Kamel Boulos, M.N.; Bin Naeem, S.; ur-Rehman, A. Integrating Social and Family Support as a Measure of Health Outcomes: Validity Implications from the Integrated Model of Health Literacy. Int. J. Environ. Res. Public Health 2023, 20, 729. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Dunning, D. Unskilled and Unaware of It: How Difficulties in Recognizing One’s Own Incompetence Lead to Inflated Self-Assessments. J. Pers. Soc. Psychol. 1999, 77, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Canady, B.E.; Larzo, M. Overconfidence in Managing Health Concerns: The Dunning–Kruger Effect and Health Literacy. J. Clin. Psychol. Med. Settings 2023, 30, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Maroufkhani, P.; Iranmanesh, M.; Ghobakhloo, M. Determinants of Big Data Analytics Adoption in Small and Medium-Sized Enterprises (SMEs). Ind. Manag. Data Syst. 2022, 123, 278–301. [Google Scholar] [CrossRef]
- Haleem, A. Big Data Usage Intentionusing Toe Framework: Sri Lankan Context. J. Contemp. Issues Bus. Gov. 2021, 27, 454–471. [Google Scholar]
| Doctors | Nurses | χ2 Value | p-Value | Phi/ Cramer’s V | |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 117 (78%) | 33 (22%) | 47.327 | 0.000 | 0.388 |
| Female | 65 (36.6%) | 99 (60.4%) | |||
| Total | 182 (100%) | 132 (100%) | |||
| Age | |||||
| <35 years | 174 (95.6%) | 94 (71.2%) | 42.560 | 0.000 | 0.368 |
| 36–50 years | 2 (1.1%) | 31 (23.5%) | |||
| >50 years | 6 (3.3%) | 7 (5.3%) | |||
| Total | 182 (100%) | 132 (100%) | |||
| Experience | |||||
| <5 years | 105 (57.7%) | 88 (66.7%) | 34.114 | 0.000 | 0.330 |
| 5–10 years | 63 (34.6%) | 38 (28.8%) | |||
| 11–15 Semester | 8 (4.4%) | 5 (3.8%) | |||
| >15 years | 6 (3.3%) | 1 (0.8%) | |||
| Total | 182 (100%) | 132 (100%) | |||
| Setting | |||||
| Primary Healthcare | 84 (46.1%) | 27 (20.5%) | 108.695 | 0.000 | 0.588 |
| Medical/Surgical | 56 (30.8%) | 15 (11.4%) | |||
| Intensive Care | 11 (6%) | 44 (33.3%) | |||
| Emergency Unit | 1 (0.5%) | 35 (26.5%) | |||
| Operating Unit | 30 (16.5%) | 11 (8.3%) | |||
| Total | 182 (100%) | 132 (100%) | |||
| Factor | Factor | Estimate | S.E | C.R | p-Value | Result | ||
|---|---|---|---|---|---|---|---|---|
| Relative Advantage | –> | mHealth Adoption | 0.045 | 0.141 | 0.320 | 0.749 | Rejected | |
| Compatibility | –> | mHealth Adoption | 0.126 | 0.123 | 1.018 | 0.309 | Rejected | |
| Management support | –> | mHealth Adoption | 0.357 | 0.067 | 5.318 | *** | Accepted | |
| Organization readiness | –> | mHealth Adoption | 0.047 | 0.059 | 0.800 | 0.424 | Rejected | |
| External support | –> | mHealth Adoption | 0.166 | 0.082 | 2.024 | *** | Accepted | |
| Government regulations | –> | mHealth Adoption | 0.057 | 0.043 | 1.323 | 0.186 | Rejected | |
| mHealth adoption | –> | Health disparity reduction | 0.109 | 0.076 | 1.426 | 0.154 | Rejected |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Naeem, S.; Azam, M.; Kamel Boulos, M.N.; Bhatti, R. Leveraging the TOE Framework: Examining the Potential of Mobile Health (mHealth) to Mitigate Health Inequalities. Information 2024, 15, 176. https://doi.org/10.3390/info15040176
Bin Naeem S, Azam M, Kamel Boulos MN, Bhatti R. Leveraging the TOE Framework: Examining the Potential of Mobile Health (mHealth) to Mitigate Health Inequalities. Information. 2024; 15(4):176. https://doi.org/10.3390/info15040176
Chicago/Turabian StyleBin Naeem, Salman, Mehreen Azam, Maged N. Kamel Boulos, and Rubina Bhatti. 2024. "Leveraging the TOE Framework: Examining the Potential of Mobile Health (mHealth) to Mitigate Health Inequalities" Information 15, no. 4: 176. https://doi.org/10.3390/info15040176
APA StyleBin Naeem, S., Azam, M., Kamel Boulos, M. N., & Bhatti, R. (2024). Leveraging the TOE Framework: Examining the Potential of Mobile Health (mHealth) to Mitigate Health Inequalities. Information, 15(4), 176. https://doi.org/10.3390/info15040176









