Hemodynamic and Electrophysiological Biomarkers of Interpersonal Tuning during Interoceptive Synchronization
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure: Motor Synchronization Task and IA Manipulation
2.3. EEG Recording and Signal Processing
2.4. fNIRS Data Recording and Data Reduction
3. Results
3.1. Step 1: EEG and fNIRS Coherence Results
3.1.1. Step 1: EEG Coherence
3.1.2. Step 1: fNIRS Coherence
3.2. Step 2: Correlational Analyses between EEG and fNIRS Coherence Indices
3.3. Step 3: ANOVAs Applied to the Correlational Values
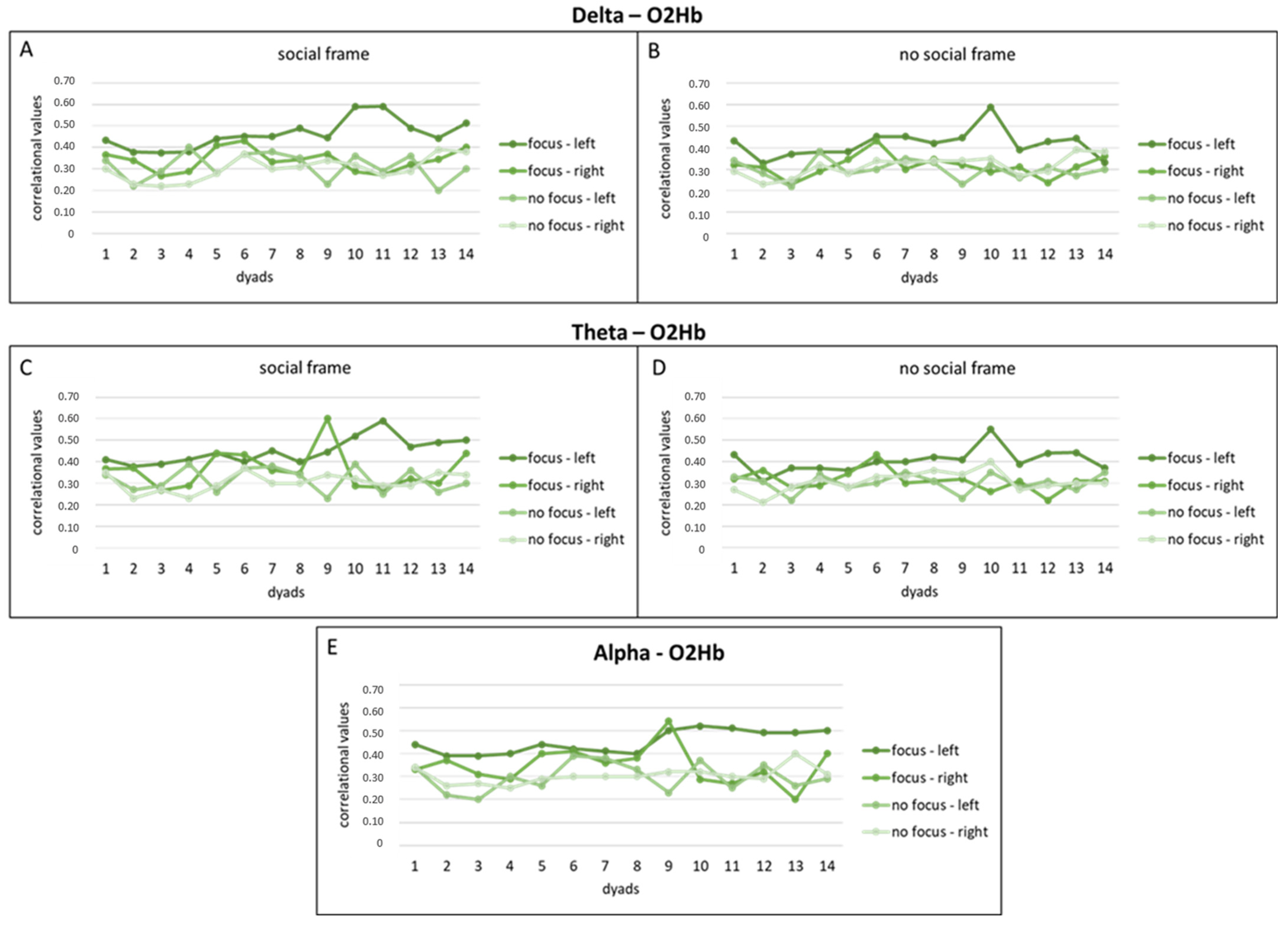
3.3.1. Delta Band and O2Hb Correlation Values
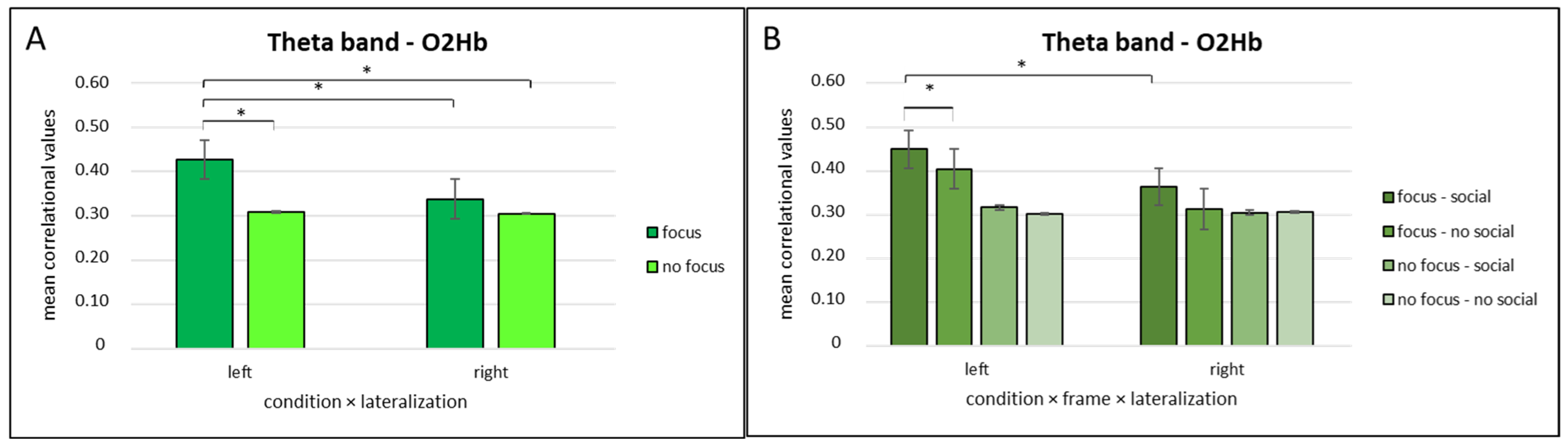
3.3.2. Theta Band and O2Hb Correlation Values
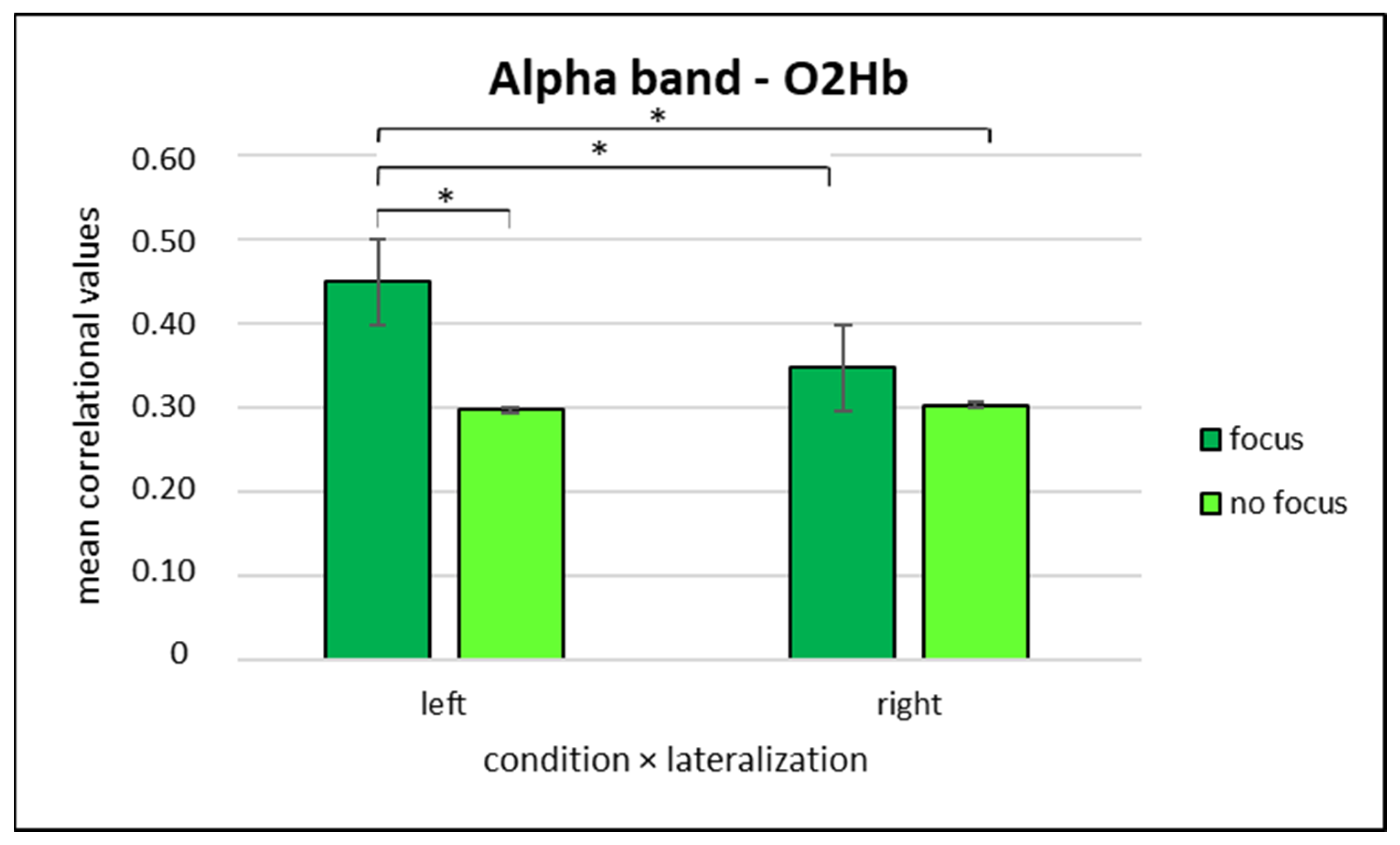
3.3.3. Alpha Band and O2Hb Correlation Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palmer, C.E.; Tsakiris, M. Going at the heart of social cognition: Is there a role for interoception in self-other distinction? Curr. Opin. Psychol. 2018, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ping, X.; Chen, W. Body influences on social cognition through interoception. Front. Psychol. 2019, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Burleson, M.H.; Quigley, K.S. Social interoception and social allostasis through touch: Legacy of the Somatovisceral Afference Model of Emotion. Soc. Neurosci. 2021, 16, 92–102. [Google Scholar] [CrossRef]
- Arnold, A.J.; Winkielman, P.; Dobkins, K. Interoception and Social Connection. Front. Psychol. 2019, 10, 2589. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Inter-Brain Hemodynamic Coherence Applied to Interoceptive Attentiveness in Hyperscanning: Why Social Framing Matters. Information 2023, 14, 58. [Google Scholar] [CrossRef]
- Schulz, S.M. Neural correlates of heart-focused interoception: A functional magnetic resonance imaging meta-analysis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160018. [Google Scholar] [CrossRef]
- Tsakiris, M.; De Preester, H. The Interoceptive Mind: From Homeostasis to Awareness; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Gvirts, H.Z.; Perlmutter, R. What Guides Us to Neurally and Behaviorally Align With Anyone Specific? A Neurobiological Model Based on fNIRS Hyperscanning Studies. Neuroscientist 2020, 26, 108–116. [Google Scholar] [CrossRef]
- Balconi, M.; Grippa, E.; Vanutelli, M.E. What hemodynamic (fNIRS), electrophysiological (EEG) and autonomic integrated measures can tell us about emotional processing. Brain Cogn. 2015, 95, 67–76. [Google Scholar] [CrossRef]
- Mu, Y.; Fan, Y.; Mao, L.; Han, S. Event-related theta and alpha oscillations mediate empathy for pain. Brain Res. 2008, 1234, 128–136. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. EEG brain oscillations are modulated by interoception in response to a synchronized motor vs. cognitive task. Front. Neuroanat. 2022, 16, 991522. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. Delta-Alpha EEG pattern reflects the interoceptive focus effect on interpersonal motor synchronization. Front. Neuroergonomics 2022, 3, 1012810. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. One’s Interoception Affects the Representation of Seeing Others’ Pain: A Randomized Controlled qEEG Study. Pain Res. Manag. 2021, 2021, 5585060. [Google Scholar] [CrossRef]
- Balconi, M.; Angioletti, L. Interoception as a social alarm amplification system. What multimethod (EEG-fNIRS) integrated measures can tell us about interoception and empathy for pain? Neuropsychol. Trends 2021, 29, 39–64. [Google Scholar] [CrossRef]
- Biallas, M.; Trajkovic, I.; Haensse, D.; Marcar, V.; Wolf, M. Reproducibility and sensitivity of detecting brain activity by simultaneous electroencephalography and near-infrared spectroscopy. Exp. Brain Res. 2012, 222, 255–264. [Google Scholar] [CrossRef]
- Angioletti, L.; Vanutelli, M.E.; Fronda, G.; Balconi, M. Exploring the Connected Brain by fNIRS: Human-to-Human Interactions Engineering. Appl. Mech. Mater. 2019, 893, 13–19. [Google Scholar] [CrossRef]
- Liu, D.; Liu, S.; Liu, X.; Zhang, C.; Li, A.; Jin, C.; Chen, Y.; Wang, H.; Zhang, X. Interactive brain activity: Review and progress on EEG-based hyperscanning in social interactions. Front. Psychol. 2018, 9, 1862. [Google Scholar] [CrossRef]
- Montague, P.R.; Berns, G.S.; Cohen, J.D.; McClure, S.M.; Pagnoni, G.; Dhamala, M.; Wiest, M.C.; Karpov, I.; King, R.D.; Apple, N.; et al. Hyperscanning: Simultaneous fMRI during linked social interactions. Neuroimage 2002, 16, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Vanutelli, M.E. Cooperation and competition with hyperscanning methods: Review and future application to emotion domain. Front. Comput. Neurosci. 2017, 11, 86. [Google Scholar] [CrossRef]
- Crivelli, D.; Balconi, M. Near-infrared spectroscopy applied to complex systems and human hyperscanning networking. Appl. Sci. 2017, 7, 922. [Google Scholar] [CrossRef]
- Czeszumski, A.; Eustergerling, S.; Lang, A.; Menrath, D.; Gerstenberger, M.; Schuberth, S.; Schreiber, F.; Rendon, Z.Z.; König, P. Hyperscanning: A Valid Method to Study Neural Inter-brain Underpinnings of Social Interaction. Front. Hum. Neurosci. 2020, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Mok, C.; Witt, E.E.; Pradhan, A.H.; Chen, J.E.; Reiss, A.L. NIRS-Based Hyperscanning Reveals Inter-brain Neural Synchronization during Cooperative Jenga Game with Face-to-Face Communication. Front. Hum. Neurosci. 2016, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Wang, P.; Liu, H.; Lin, P.; Gao, J.; Wang, R.; Iramina, K.; Zhang, Q.; Zheng, W. Neural Activity and Decoding of Action Observation Using Combined EEG and fNIRS Measurement. Front. Hum. Neurosci. 2019, 13, 357. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Jun, S.C. Multi-modal integration of EEG-fNIRS for brain-computer interfaces—Current limitations and future directions. Front. Hum. Neurosci. 2017, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and Effective Connectivity: A Review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef]
- Hasson, U.; Ghazanfar, A.A.; Galantucci, B.; Garrod, S.; Keysers, C. Brain-to-brain coupling: A mechanism for creating and sharing a social world. Trends Cogn. Sci. 2012, 16, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Fronda, G.; Balconi, M. What hyperscanning and brain connectivity for hemodynamic (fNIRS), electrophysiological (EEG) and behavioral measures can tell us about prosocial behavior? Psychol. Neurosci. 2022, 15, 147–162. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Interbrains cooperation: Hyperscanning and self-perception in joint actions. J. Clin. Exp. Neuropsychol. 2017, 39, 607–620. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V. Expanding the mirror: Vicarious activity for actions, emotions, and sensations. Curr. Opin. Neurobiol. 2009, 19, 666–671. [Google Scholar] [CrossRef]
- Richardson, M.J.; Marsh, K.L.; Isenhower, R.W.; Goodman, J.R.L.; Schmidt, R.C. Rocking together: Dynamics of intentional and unintentional interpersonal coordination. Hum. Mov. Sci. 2007, 26, 867–891. [Google Scholar] [CrossRef] [PubMed]
- Sänger, J.; Müller, V.; Lindenberger, U. Intra- and interbrain synchronization and network properties when playing guitar in duets. Front. Hum. Neurosci. 2012, 6, 312. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Pezard, L.; Nandrino, J.-L.; Vanutelli, M.E. Two is better than one: The effects of strategic cooperation on intra- and inter-brain connectivity by fNIRS. PLoS ONE 2017, 12, e0187652. [Google Scholar] [CrossRef]
- Shiraishi, M.; Shimada, S. Inter-brain synchronization during a cooperative task reflects the sense of joint agency. Neuropsychologia 2021, 154, 107770. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Interoceptive attentiveness induces significantly more PFC activation during a synchronized linguistic task compared to a motor task as revealed by functional Near-Infrared Spectroscopy. Brain Sci. 2022, 12, 301. [Google Scholar] [CrossRef] [PubMed]
- Angioletti, L.; Balconi, M. The Increasing Effect of Interoception on Brain Frontal Responsiveness During a Socially Framed Motor Synchronization Task. Front. Hum. Neurosci. 2022, 16, 1–9. [Google Scholar] [CrossRef]
- Kubota, Y.; Sato, W.; Toichi, M.; Murai, T.; Okada, T.; Hayashi, A.; Sengoku, A. Frontal midline theta rhythm is correlated with cardiac autonomic activities during the performance of an attention demanding meditation procedure. Cogn. Brain Res. 2001, 11, 281–287. [Google Scholar] [CrossRef]
- Tripathi, V.; Bhasker, L.; Kharya, C.; Bhatia, M.; Kochupillai, V. Electroencephalographic dynamics of rhythmic breath-based meditation. bioRxiv 2022. bioRxiv:2022.03.09.483685. [Google Scholar] [CrossRef]
- Lindenberger, U.; Li, S.C.; Gruber, W.; Müller, V. Brains swinging in concert: Cortical phase synchronization while playing guitar. BMC Neurosci. 2009, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Aching face and hand: The interoceptive attentiveness and social context in relation to empathy for pain. J. Integr. Neurosci. 2022, 21, 34. [Google Scholar] [CrossRef]
- Balconi, M.; Vanutelli, M.E. Empathy in negative and positive interpersonal interactions. What is the relationship between central (EEG, fNIRS) and peripheral (autonomic) neurophysiological responses? Adv. Cogn. Psychol. 2017, 13, 105–120. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Ludwig, A.; Miriani, R.M.; Langhals, N.B.; Joseph, M.D.; David, J. Using a common average reference to improve cortical neuron recordings from microelectrode arrays. J. Neurophysiol. 2008, 101, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, P.; Perdue, K.L.; Diamond, S.G. Algorithm to find high density EEG scalp coordinates and analysis of their correspondence to structural and functional regions of the brain. J. Neurosci. Methods 2014, 229, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Koessler, L.; Maillard, L.; Benhadid, A.; Vignal, J.P.; Felblinger, J.; Vespignani, H.; Braun, M. Automated cortical projection of EEG sensors: Anatomical correlation via the international 10-10 system. Neuroimage 2009, 46, 64–72. [Google Scholar] [CrossRef]
- Balconi, M.; Fronda, G.; Vanutelli, M.E. Donate or receive? Social hyperscanning application with fNIRS. Curr. Psychol. 2019, 38, 991–1002. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, M.J.; Hong, K.S. Online binary decision decoding using functional near-infrared spectroscopy for the development of brain-computer interface. Exp. Brain Res. 2014, 232, 555–564. [Google Scholar] [CrossRef]
- Naseer, N.; Hong, K.S. Classification of functional near-infrared spectroscopy signals corresponding to the right- and left-wrist motor imagery for development of a brain-computer interface. Neurosci. Lett. 2013, 553, 84–89. [Google Scholar] [CrossRef]
- Wheland, D.; Joshi, A.; McMahon, K.; Hansell, N.; Martin, N.; Wright, M.; Thompson, P.; Shattuck, D.; Leahy, R. Robust identification of partial-correlation based networks with applications to cortical thickness data. In Proceedings of the 2012 9th IEEE International Symposium on Biomedical Imaging (ISBI), Barcelona, Spain, 2–5 May 2012; pp. 1551–1554. [Google Scholar]
- Balconi, M.; Vanutelli, M.E.; Gatti, L. Functional brain connectivity when cooperation fails. Brain Cogn. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Wang, M.Y.; Luan, P.; Zhang, J.; Xiang, Y.T.; Niu, H.; Yuan, Z. Concurrent mapping of brain activation from multiple subjects during social interaction by hyperscanning: A mini-review. Quant. Imaging Med. Surg. 2018, 8, 819–837. [Google Scholar] [CrossRef]
- Harmony, T.; Fernández, T.; Silva, J.; Bernal, J.; Díaz-Comas, L.; Reyes, A.; Marosi, E.; Rodríguez, M.; Rodríguez, M. EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef]
- Knyazev, G.G. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neurosci. Biobehav. Rev. 2007, 31, 377–395. [Google Scholar] [CrossRef]
- Harmony, T. The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Aftanas, L.I.; Golocheikine, S.A. Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neurosci. Lett. 2001, 310, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Murata, T.; Hamada, T.; Omori, M.; Kosaka, H.; Kikuchi, M.; Yoshida, H.; Wada, Y. Changes in EEG and autonomic nervous activity during meditation and their association with personality traits. Int. J. Psychophysiol. 2005, 55, 199–207. [Google Scholar] [CrossRef]
- Matthews, S.C.; Paulus, M.P.; Simmons, A.N.; Nelesen, R.A.; Dimsdale, J.E. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. Neuroimage 2004, 22, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-Y.; Ma, Y.; Fan, Y.; Feng, H.; Wang, J.; Feng, S.; Lu, Q.; Hu, B.; Lin, Y.; Li, J.; et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc. Natl. Acad. Sci. USA 2009, 106, 8865–8870. [Google Scholar] [CrossRef]
- Coomans, E.; Geraedts, I.K.; Deijen, J.B.; Keeser, D.; Pogarell, O.; Engelbregt, H.J. Intersubject EEG Coherence in Healthy Dyads during Individual and Joint Mindful Breathing Exercise: An EEG-Based Experimental Hyperscanning Study. Adv. Cogn. Psychol. 2021, 17, 250–260. [Google Scholar] [CrossRef]
- Aftanas, L.I.; Lotova, N.V.; Koshkarov, V.I.; Makhnev, V.P.; Mordvintsev, Y.N.; Popov, S.A. Non-linear dynamic complexity of the human EEG during evoked emotions. Int. J. Psychophysiol. 1998, 28, 63–76. [Google Scholar] [CrossRef]
- Beauregard, M.; Courtemanche, J.; Paquette, V. Brain activity in near-death experiencers during a meditative state. Resuscitation 2009, 80, 1006–1010. [Google Scholar] [CrossRef]
- Balconi, M.; Mazza, G. Lateralisation effect in comprehension of emotional facial expression: A comparison between EEG alpha band power and behavioural inhibition (BIS) and activation (BAS) systems. Laterality Asymmetries Body Brain Cogn. 2010, 15, 361–384. [Google Scholar] [CrossRef]
- Davidson, R.J. Anterior Cerebral Asymmetry and the Nature of Emotion. Brain Cogn. 1992, 20, 125–151. [Google Scholar] [CrossRef]
- Koslov, K.; Mendes, W.B.; Pajtas, P.E.; Pizzagalli, D.A. Asymmetry in resting intracortical activity as a buffer to social threat. Psychol. Sci. 2011, 22, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Harmon-Jones, E.; Gable, P.A.; Peterson, C.K. The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biol. Psychol. 2010, 84, 451–462. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balconi, M.; Angioletti, L. Hemodynamic and Electrophysiological Biomarkers of Interpersonal Tuning during Interoceptive Synchronization. Information 2023, 14, 289. https://doi.org/10.3390/info14050289
Balconi M, Angioletti L. Hemodynamic and Electrophysiological Biomarkers of Interpersonal Tuning during Interoceptive Synchronization. Information. 2023; 14(5):289. https://doi.org/10.3390/info14050289
Chicago/Turabian StyleBalconi, Michela, and Laura Angioletti. 2023. "Hemodynamic and Electrophysiological Biomarkers of Interpersonal Tuning during Interoceptive Synchronization" Information 14, no. 5: 289. https://doi.org/10.3390/info14050289
APA StyleBalconi, M., & Angioletti, L. (2023). Hemodynamic and Electrophysiological Biomarkers of Interpersonal Tuning during Interoceptive Synchronization. Information, 14(5), 289. https://doi.org/10.3390/info14050289






