Environmental Impact on Health across Generations: Policy Meets Biology. A Review of Animal and Human Models
Abstract
1. Introduction
1.1. Susceptibility Windows for Environmental Exposures, Policy Meets Biology
1.2. Approaches to Studying Environmental Impact across Generations
2. Epigenetic Research: Building Maps for Predicting and Preventing Disease
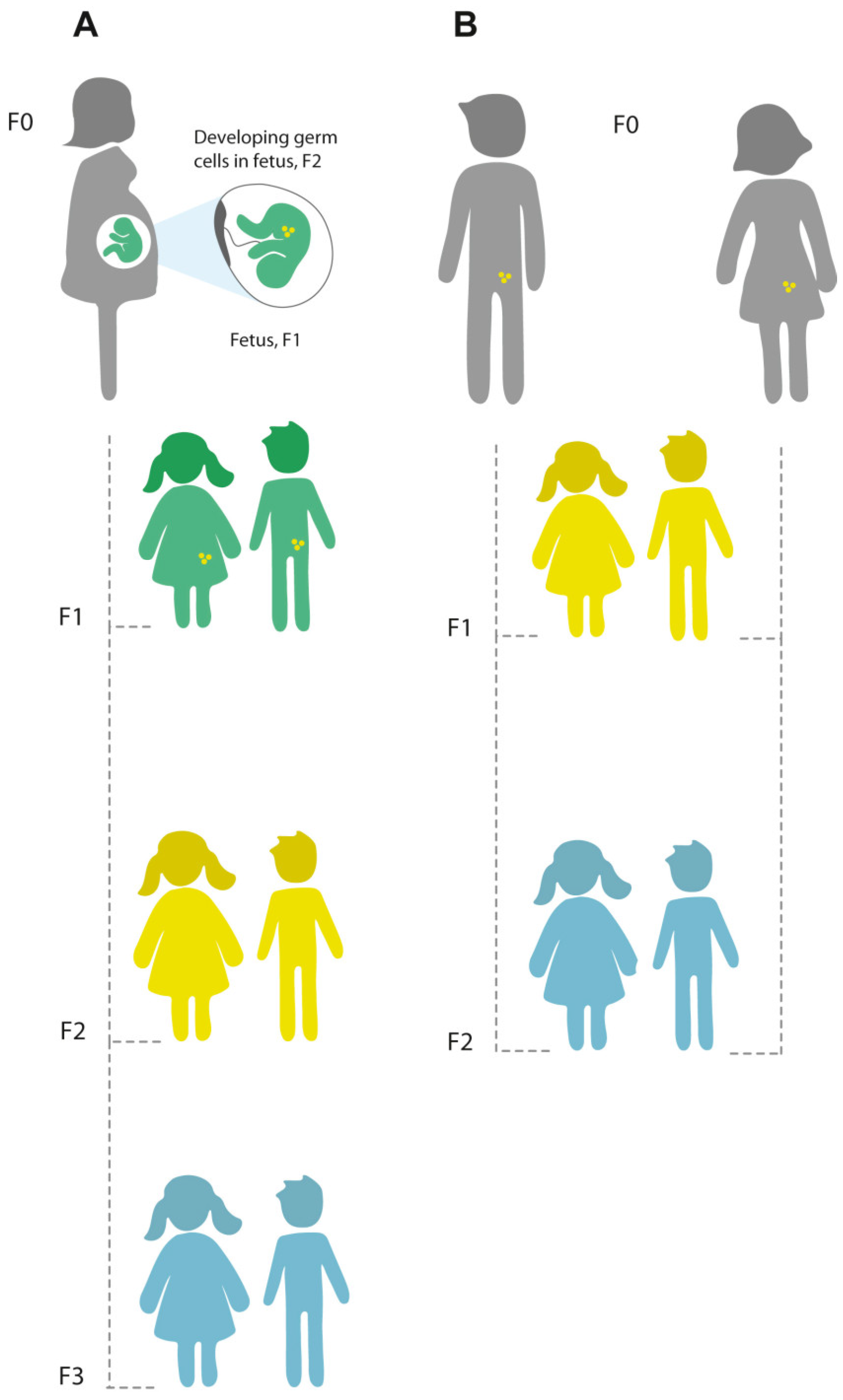
What Are the Mechanisms of Inter and Transgenerational Inheritance?
3. Studying Multi-Generational Epigenetic Effects Using Animal Models
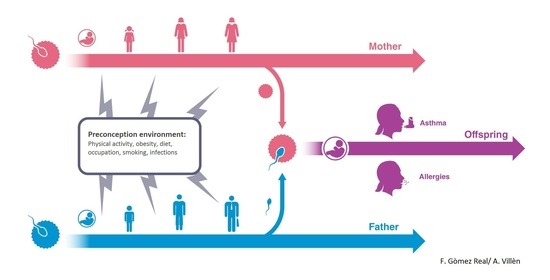
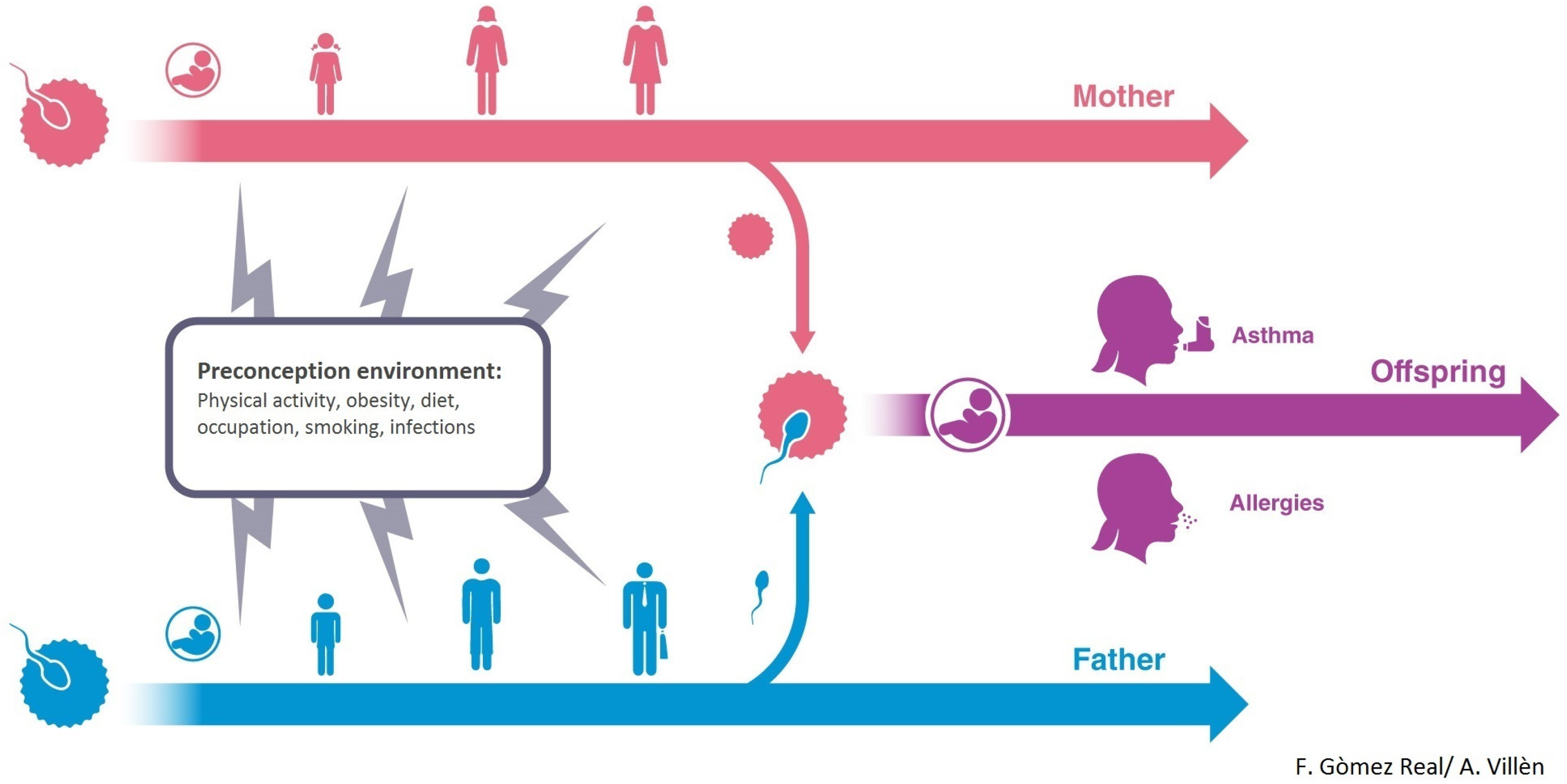
4. Maternal Pre-Conception Environment and Long Lasting Influences on Offspring Immunity
5. Evidence for Transmission across Generations in Asthma and Allergic Disease in Humans
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prescott, S.L.; Logan, A.C.; Albrecht, G.; Campbell, D.E.; Crane, J.; Cunsolo, A.; Holloway, J.H.; Kozyrskyj, A.L.; Lowry, C.A.; Penders, J.; et al. The Canmore Declaration: Statement of Principles for Planetary Health. Challenges 2018, 9, 31. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Prescott, S.L.; Logan, A.C. Planetary Health: From the Wellspring of Holistic Medicine to Personal and Public Health Imperative. Explore (NY) 2018. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.; Jennings, B. Ethics and public health: Forging a strong relationship. Am. J. Public Health 2002, 92, 169–176. [Google Scholar] [CrossRef]
- Forsdahl, A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br. J. Prev. Soc. Med. 1977, 31, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. BioEssays News Rev. Mol. Cell. Dev. Biol. 2014, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Verhasselt, V. Is infant immunization by breastfeeding possible? Philos. Trans. R. Soc. Lond. B. Bio.l Sci. 2015, 370. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Nguyen, V.; Muller, H.K.; Walker, A.M. Maternal Milk T Cells Drive Development of Transgenerational Th1 Immunity in Offspring Thymus. J. Immunol. 2016, 197, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Silfver, K.A.; Stenqvist, A.; Giwercman, A. The Impact of Paternal and Maternal Smoking on Semen Quality of Adolescent Men. PLoS ONE 2013, 8, e66766. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Schmid, T.E.; Baumgartner, A. Male-mediated developmental toxicity. Asian J. Androl. 2014, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sepaniak, S.; Forges, T.; Gerard, H.; Foliguet, B.; Bene, M.C.; Monnier-Barbarino, P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology 2006, 223, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Laubenthal, J.; Zlobinskaya, O.; Poterlowicz, K.; Baumgartner, A.; Gdula, M.R.; Fthenou, E.; Keramarou, M.; Hepworth, S.J.; Kleinjans, J.C.; van Schooten, F.J.; et al. Cigarette smoke-induced transgenerational alterations in genome stability in cord blood of human F1 offspring. FASEB J 2012, 26, 3946–3956. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Rowan-Carroll, A.; Williams, A.; Polyzos, A.; Berndt-Weis, M.L.; Yauk, C.L. Sidestream tobacco smoke is a male germ cell mutagen. Proc. Natl. Acad. Sci. USA 2011, 108, 12811–12814. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Amoako, A.A.; Konje, J.C.; Gant, T.W.; Marczylo, T.H. Smoking induces differential miRNA expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenetics 2012, 7, 432–439. [Google Scholar] [CrossRef]
- Rehan, V.K.; Liu, J.; Naeem, E.; Tian, J.; Sakurai, R.; Kwong, K.; Akbari, O.; Torday, J.S. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012, 10, 129. [Google Scholar] [CrossRef]
- Johannessen, A.; Calciano, L.; Lonnebotn, M.; Bertelsen, R.J.; Braback, L.; Holm, M.; Janson, C.; Jogi, R.; Kirkeleit, J.; Lodge, C.; et al. Late Breaking Abstract—Fathers’ overweight and offspring asthma—An intergenerational perspective. Eur. Respir. J. 2017, 50 (Suppl. 61), PA2615. [Google Scholar]
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Braback, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245. [Google Scholar] [CrossRef]
- Miller, L.L.; Henderson, J.; Northstone, K.; Pembrey, M.; Golding, J. Do grandmaternal smoking patterns influence the etiology of childhood asthma? Chest 2014, 145, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Langholz, B.; Salam, M.T.; Gilliland, F.D. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005, 127, 1232–1241. [Google Scholar] [CrossRef]
- Marcon, A.; Pesce, G.; Calciano, L.; Bellisario, V.; Dharmage, S.C.; Garcia-Aymerich, J.; Gislasson, T.; Heinrich, J.; Holm, M.; Janson, C.; et al. Trends in smoking initiation in Europe over 40 years: A retrospective cohort study. PLoS ONE 2018, 13, e0201881. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Hanna, J.; Zhang, X.; Ku, M.; Wernig, M.; Schorderet, P.; Bernstein, B.E.; Jaenisch, R.; Lander, E.S.; Meissner, A. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008, 454, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Colot, V. Plant Transgenerational Epigenetics. Annu. Rev. Genet. 2016, 50, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Morkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772. [Google Scholar] [CrossRef]
- Wu, H.; Hauser, R.; Krawetz, S.A.; Pilsner, J.R. Environmental Susceptibility of the Sperm Epigenome During Windows of Male Germ Cell Development. Curr. Environ. Health Rep. 2015, 2, 356–366. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Skinner, M.K.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8, e66318. [Google Scholar] [CrossRef]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.A.; Paulus, J.K.; Mensah, V.; Lem, J.; Saavedra-Rodriguez, L.; Gentry, A.; Pagidas, K.; Feig, L.A. Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Transl. Psychiatry 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, L.R.; Donkin, I.; Fabre, O.; Versteyhe, S.; Mechta, M.; Pattamaprapanont, P.; Mortensen, B.; Krarup, N.T.; Barres, R. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin. Epigenet. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Animal models to study environmental epigenetics. Biol. Reprod. 2010, 82, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Harris, C. Animal models in epigenetic research: Institutional animal care and use committee considerations across the lifespan. ILAR J. 2012, 53, 370–376. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Duhl, D.M.; Vrieling, H.; Miller, K.A.; Wolff, G.L.; Barsh, G.S. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 1994, 8, 59–65. [Google Scholar] [CrossRef]
- Belyaev, D.K.; Ruvinsky, A.O.; Borodin, P.M. Inheritance of alternative states of the fused gene in mice. J. Hered. 1981, 72, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C. The Inheritance and Expression of Fused, a New Mutation in the House Mouse. Genetics 1937, 22, 1–13. [Google Scholar] [PubMed]
- Rakyan, V.K.; Chong, S.; Champ, M.E.; Cuthbert, P.C.; Morgan, H.D.; Luu, K.V.; Whitelaw, E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc. Natl. Acad. Sci. USA 2003, 100, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Morgan, M.D.; Heger, A.H.; Sharpe, R.M.; Drake, A.J. High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci. Rep. 2016, 6, 31857. [Google Scholar] [CrossRef]
- Rehan, V.K.; Liu, J.; Sakurai, R.; Torday, J.S. Perinatal nicotine-induced transgenerational asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L501–L507. [Google Scholar] [CrossRef] [PubMed]
- Dehmel, S.; Nathan, P.; Bartel, S.; El-Merhie, N.; Scherb, H.; Milger, K.; John-Schuster, G.; Yildirim, A.O.; Hylkema, M.; Irmler, M.; et al. Intrauterine smoke exposure deregulates lung function, pulmonary transcriptomes, and in particular insulin-like growth factor (IGF)-1 in a sex-specific manner. Sci. Rep. 2018, 8, 7547. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Graff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]
- Dietz, D.M.; Laplant, Q.; Watts, E.L.; Hodes, G.E.; Russo, S.J.; Feng, J.; Oosting, R.S.; Vialou, V.; Nestler, E.J. Paternal transmission of stress-induced pathologies. Biol. Psychiatry 2011, 70, 408–414. [Google Scholar] [CrossRef]
- Dobrzynska, M.M.; Gajowik, A.; Radzikowska, J.; Tyrkiel, E.J.; Jankowska-Steifer, E.A. Male-mediated F1 effects in mice exposed to bisphenol A, either alone or in combination with X-irradiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2015, 789–790, 36–45. [Google Scholar] [CrossRef]
- Jones, G.; Steketee, R.W.; Black, R.E.; Bhutta, Z.A.; Morris, S.S.; Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet 2003, 362, 65–71. [Google Scholar] [CrossRef]
- Wolf, J.H. Low breastfeeding rates and public health in the United States. Am. J. Public Health 2003, 93, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Mellen, B.G.; Wright, P.F.; Mitchel, E.F., Jr.; Griffin, M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000, 342, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.F.; Cherian, T.; Chow, J.; Shahid-Salles, S.A.; Laxminarayan, R.; John, T.J. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- MacLennan, C.A.; Gondwe, E.N.; Msefula, C.L.; Kingsley, R.A.; Thomson, N.R.; White, S.A.; Goodall, M.; Pickard, D.J.; Graham, S.M.; Dougan, G.; et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 2008, 118, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.K.; Chen, E.; Ross, K.M.; McEwen, L.M.; Maclsaac, J.L.; Kobor, M.S.; Miller, G.E. Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology 2018, 97, 131–134. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Shalowitz, M.U.; Story, R.E.; Leigh, A.K.K.; Ham, P.; Arevalo, J.M.G.; Cole, S.W. Divergent transcriptional profiles in pediatric asthma patients of low and high socioeconomic status. Pediatr. Pulmonol. 2018, 53, 710–719. [Google Scholar] [CrossRef]
- Turner, J.D. Holistic, personalized, immunology? The effects of socioeconomic status on the transcriptional milieu of immune cells. Pediatr. Pulmonol. 2018, 53, 696–697. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Hengesch, X.; Leenen, F.A.D.; Schritz, A.; Sias, K.; Schaan, V.K.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Schachinger, H.; et al. Proinflammatory T Cell Status Associated with Early Life Adversity. J. Immunol. 2017, 199, 4046–4055. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Sias, K.; Hengesch, X.; Schaan, V.K.; Leenen, F.A.D.; Adams, P.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Ewen, A.; et al. T Cell Immunosenescence after Early Life Adversity: Association with Cytomegalovirus Infection. Front. Immunol. 2017, 8, 1263. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef]
- Heath, P.T.; Culley, F.J.; Jones, C.E.; Kampmann, B.; Le Doare, K.; Nunes, M.C.; Sadarangani, M.; Chaudhry, Z.; Baker, C.J.; Openshaw, P.J.M. Group B streptococcus and respiratory syncytial virus immunisation during pregnancy: A landscape analysis. Lancet Infect. Dis. 2017, 17, e223–e234. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Thomson, C.A. The Impact of Maternal Microbes and Microbial Colonization in Early Life on Hematopoiesis. J. Immunol. 2018, 200, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Bjorksten, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Guadalupe, I.; Mitre, E.; Benitez, S.; Chico, M.E.; Nutman, T.B.; Cooper, P.J. Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. J. Infect. Dis. 2009, 199, 1846–1850. [Google Scholar] [CrossRef]
- Malhotra, I.; Mungai, P.; Wamachi, A.; Kioko, J.; Ouma, J.H.; Kazura, J.W.; King, C.L. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 1999, 162, 6843–6848. [Google Scholar]
- Malhotra, I.; Ouma, J.H.; Wamachi, A.; Kioko, J.; Mungai, P.; Njzovu, M.; Kazura, J.W.; King, C.L. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect. Immun. 2003, 71, 5231–5237. [Google Scholar] [CrossRef]
- King, C.L.; Malhotra, I.; Mungai, P.; Wamachi, A.; Kioko, J.; Ouma, J.H.; Kazura, J.W. B cell sensitization to helminthic infection develops in utero in humans. J. Immunol. 1998, 160, 3578–3584. [Google Scholar]
- Seydel, L.S.; Petelski, A.; van Dam, G.J.; van der Kleij, D.; Kruize-Hoeksma, Y.C.; Luty, A.J.; Yazdanbakhsh, M.; Kremsner, P.G. Association of in utero sensitization to Schistosoma haematobium with enhanced cord blood IgE and increased frequencies of CD5- B cells in African newborns. Am. J. Trop. Med. Hyg. 2012, 86, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, I.; LaBeaud, A.D.; Morris, N.; McKibben, M.; Mungai, P.; Muchiri, E.; King, C.L.; King, C.H. Cord Blood Anti-Parasite IL-10 as Risk Marker for Compromised Vaccine Immunogenicity in Early Childhood. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, I.; McKibben, M.; Mungai, P.; McKibben, E.; Wang, X.; Sutherland, L.J.; Muchiri, E.M.; King, C.H.; King, C.L.; LaBeaud, A.D. Effect of antenatal parasitic infections on anti-vaccine IgG levels in children: A prospective birth cohort study in Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003466. [Google Scholar] [CrossRef] [PubMed]
- van den Biggelaar, A.H.; van Ree, R.; Rodrigues, L.C.; Lell, B.; Deelder, A.M.; Kremsner, P.G.; Yazdanbakhsh, M. Decreased atopy in children infected with Schistosoma haematobium: A role for parasite-induced interleukin-10. Lancet 2000, 356, 1723–1727. [Google Scholar] [CrossRef]
- Obeng, B.B.; Amoah, A.S.; Larbi, I.A.; de Souza, D.K.; Uh, H.W.; Fernandez-Rivas, M.; van Ree, R.; Rodrigues, L.C.; Boakye, D.A.; Yazdanbakhsh, M.; et al. Schistosoma infection is negatively associated with mite atopy, but not wheeze and asthma in Ghanaian Schoolchildren. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Djuardi, Y.; Supali, T.; Wibowo, H.; Kruize, Y.C.; Versteeg, S.A.; van Ree, R.; Sartono, E.; Yazdanbakhsh, M. The development of TH2 responses from infancy to 4 years of age and atopic sensitization in areas endemic for helminth infections. Allergy Asthma Clin. Immunol. 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Mpairwe, H.; Ndibazza, J.; Webb, E.L.; Nampijja, M.; Muhangi, L.; Apule, B.; Lule, S.; Akurut, H.; Kizito, D.; Kakande, M.; et al. Maternal hookworm modifies risk factors for childhood eczema: Results from a birth cohort in Uganda. Pediatr. Allergy Immunol. 2014, 25, 481–488. [Google Scholar] [CrossRef]
- Jogi, N.O.; Svanes, C.; Siiak, S.P.; Logan, E.; Holloway, J.W.; Igland, J.; Johannessen, A.; Levin, M.; Real, F.G.; Schlunssen, V.; et al. Zoonotic helminth exposure and risk of allergic diseases: A study of two generations in Norway. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 66–77. [Google Scholar] [CrossRef]
- Morein, B.; Blomqvist, G.; Hu, K. Immune responsiveness in the neonatal period. J. Comp. Pathol. 2007, 137 (Suppl. 1), S27–S31. [Google Scholar] [CrossRef]
- Aschkenazi, S.; Straszewski, S.; Verwer, K.M.; Foellmer, H.; Rutherford, T.; Mor, G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol. Reprod. 2002, 66, 1853–1861. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; Burnett, T.G. Uterine leukocytes: Key players in pregnancy. Semin. Cell Dev. Biol. 2000, 11, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Abelius, M.S.; Janefjord, C.; Ernerudh, J.; Berg, G.; Matthiesen, L.; Duchen, K.; Nilsson, L.J.; Jenmalm, M.C. The placental immune milieu is characterized by a Th2- and anti-inflammatory transcription profile, regardless of maternal allergy, and associates with neonatal immunity. Am. J. Reprod. Immunol. 2015, 73, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Pregnancy: Success and failure within the Th1/Th2/Th3 paradigm. Semin. Immunol. 2001, 13, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.V.; Williams, T.M.; Walker, K.A.; Dickinson, H.; Sakkal, S.; Rumballe, B.A.; Little, M.H.; Jenkin, G.; Ricardo, S.D. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir. Res. 2013, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Szyf, M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol. Med. 2015, 21, 134–144. [Google Scholar] [CrossRef]
- Pembrey, M.E. Male-line transgenerational responses in humans. Hum. Fertil. 2010, 13, 268–271. [Google Scholar] [CrossRef]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjostrom, M.; Golding, J.; Team, A.S. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. EJHG 2006, 14, 159–166. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Rava, M.; Carsin, A.E.; Accordini, S.; Benediktsdottir, B.; Dratva, J.; Franklin, K.A.; Heinrich, J.; Holm, M.; Janson, C.; et al. Clinical markers of asthma and IgE assessed in parents before conception predict asthma and hayfever in the offspring. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Karmaus, W.; Zhang, H.; Holloway, J.W. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol 2017, 139, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Pembrey, M.; Saffery, R.; Bygren, L.O.; Network in Epigenetic Epidemiology. Human transgenerational responses to early-life experience: Potential impact on development, health and biomedical research. J. Med. Genet. 2014, 51, 563–572. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.; Vercelli, D. Epigenetic Mechanisms in Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S48–S50. [Google Scholar] [CrossRef]
- Magnus, M.C.; Haberg, S.E.; Karlstad, O.; Nafstad, P.; London, S.J.; Nystad, W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax 2015, 70, 237–243. [Google Scholar] [CrossRef]
- Lodge, C.J.; Braback, L.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 167–174. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018. [Google Scholar] [CrossRef]
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. EJHG 2014, 22, 1382–1386. [Google Scholar] [CrossRef]
- Accordini, S.; Johannessen, A.; Calciano, L.; Jogi, R.; Martinez-Moratalla Rovira, J.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.; Gomez Real, F.; et al. Three-generation effects of tobacco smoking on lung function within the paternal line. Eur. Respir. J. 2017, 50, PA1178. [Google Scholar] [CrossRef]
- Lønnebotn, M.; Nilsen, R.M.; Dharmage, S.; Franklin, K.A.; Holm, M.; Janson, C.; Jarvis, D.; Johannessen, A.; Kirkeleit, J.; Malinovschi, A.; et al. Associations of fathers and their offsprings weight gain with non-allergic asthma. Eur. Respir. J. 2018. [Google Scholar]
- Kobayashi, H.; Sakurai, T.; Miura, F.; Imai, M.; Mochiduki, K.; Yanagisawa, E.; Sakashita, A.; Wakai, T.; Suzuki, Y.; Ito, T.; et al. High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome Res. 2013, 23, 616–627. [Google Scholar] [CrossRef] [PubMed]
| Reference | Main Findings and Study Cohorts | Susceptibility Window |
|---|---|---|
| Accordini et al. Int J Epidemiol, 2018 [99] | Increased asthma risk in offspring if father smoked during early adolescence, or if grandmothers or mothers smoked during pregnancy, independently. European Community Respiratory Health Survey (ECRHS). | Paternal puberty Pregnancy |
| Accordini et al. Eur Respir J, 2017 [101] | Lung function across three generations affected by grand-maternal and paternal smoking. ECRHS/Respiratory Health In Northern Europe Spain and Australia (RHINESSA). | Paternal puberty Pregnancy |
| Svanes et al. Int J Epidemiol, 2017 [21] | Non-allergic asthma in the offspring was associated with paternal smoking and welding before conception. Respiratory Health In Northern Europe (RHINE) study. | Paternal puberty |
| Johannessen et al. Eur Respir J, 2017 | Risk of asthma higher in offspring of fathers (not mothers) who became overweight at voice-break. ECRHS/RHINE/RHINESSA. | Paternal puberty |
| Lønnebotn et al. Eur Respir J, 2018 [102] | Pre-pubertal weight gain in fathers (not mothers) or offspring themselves associated with offspring asthma. ECRHS/RHINE/RHINESSA. | Paternal puberty |
| Lodge et al. Clin Exp Allergy, 2018 [98] | Increased childhood asthma risk in grandchildren of grandmothers smoking during early pregnancy, independent of maternal smoking. Nationwide Swedish cohort. | Pregnancy |
| Magnus et al. Thorax, 2015 [97] | Increased asthma risk in grandchildren caused by grand-maternal smoking during pregnancy, independent of the mother’s smoking status. Norwegian Mother and Child Cohort Study (MoBa). | Pregnancy |
| Miller et al. Chest, 2014 [22] | Suggestive evidence for association of paternal (not maternal) prenatal exposure to mothers’ smoking with asthma and wheezing in paternal daughters. Avon Longitudinal Study of Parents and Children. | Pregnancy |
| Li et al. Chest, 2005 [23] | Maternal and grand-maternal smoking during pregnancy may increase the risk of childhood asthma. Children’s Health study in southern California (CHS). | Pregnancy |
| Bertelsen et al. Clin Exp Allergy, 2017 [93] | Offspring asthma and hay fever more strongly associated with parental asthmatic and allergic disease measured before conception as compared to after birth of the child. ECRHS. | Before conception of child |
| Jogi et al. Clin Exp Allergy, 2018 [81] | Parental Toxocara seropositivity was associated with allergic outcomes in their offspring but not in themselves. ECRHS/RHINESSA | Not applicable (NA) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lønnebotn, M.; El-Merhie, N.; Holloway, J.W.; Horsnell, W.; Krauss-Etschmann, S.; Gómez Real, F.; Svanes, C. Environmental Impact on Health across Generations: Policy Meets Biology. A Review of Animal and Human Models. Challenges 2018, 9, 42. https://doi.org/10.3390/challe9020042
Lønnebotn M, El-Merhie N, Holloway JW, Horsnell W, Krauss-Etschmann S, Gómez Real F, Svanes C. Environmental Impact on Health across Generations: Policy Meets Biology. A Review of Animal and Human Models. Challenges. 2018; 9(2):42. https://doi.org/10.3390/challe9020042
Chicago/Turabian StyleLønnebotn, Marianne, Natalia El-Merhie, John W. Holloway, William Horsnell, Susanne Krauss-Etschmann, Francisco Gómez Real, and Cecilie Svanes. 2018. "Environmental Impact on Health across Generations: Policy Meets Biology. A Review of Animal and Human Models" Challenges 9, no. 2: 42. https://doi.org/10.3390/challe9020042
APA StyleLønnebotn, M., El-Merhie, N., Holloway, J. W., Horsnell, W., Krauss-Etschmann, S., Gómez Real, F., & Svanes, C. (2018). Environmental Impact on Health across Generations: Policy Meets Biology. A Review of Animal and Human Models. Challenges, 9(2), 42. https://doi.org/10.3390/challe9020042