Abstract
Intrauterine and early life has been accepted as important susceptibility windows for environmental exposure and disease later in life. Emerging evidence suggests that exposure before conception may also influence health in future generations. There has been little research on human data to support this until now. This review gives evidence from epigenetic as well as immunologic research, and from animal as well as human models, supporting the hypothesis that there may be important susceptibility windows before conception in relation to exposure such as obesity, diet, smoking and infections. It is likely that we can identify vulnerability windows in men and women in which interventions may have an impact on several generations in addition to individual health. Establishing vulnerability windows affecting health over future generations, and not only in the now or the near future of the individual, may provide tremendous opportunities for health policy and practice.
1. Introduction
Planetary health, formally defined by the in vivo Planetary Health network, as the interdependent vitality of all natural and anthropogenic ecosystems (social, political and otherwise), is inseparably connected to human health [1]. According to WHO [2], 68% of deaths worldwide in 2012 were caused by chronic, non-communicable diseases (NCDs) such as chronic respiratory diseases, cardiovascular diseases, cancers and diabetes. NCDs are the result of a combination of genetic, physiological, environmental and behavioral factors and one of the major health and development challenges of the 21st century. It is necessary to reduce the global burden of NCDs to obtain a sustainable development [2]. As the global burden of disease shifted from infectious toward chronic, non-communicable diseases (NCDs), a greater need to understand the interplay between environment and planetary ecosystems has emerged [3]. Health is often viewed as a personal responsibility and when it comes to NCDs several of them have strong linkage to human lifestyle behavior and might contribute to stigmatization of certain individuals and groups. However, it is problematic to implement systems that hold individuals responsible for their own health, since diseases and disabilities results from a complex interplay of genetic and environmental factors [4].
There is an increasing understanding on how environmental exposure might have an impact on transmission of disease across generations. Epigenetics are presented as a mechanistic base to explain how exposure in one generation can effect multiple generations. Asthma and allergies are caused by complex interactions between environmental triggers and individual predisposition, and have increased globally during the last decades. Asthma and allergies cause a significant burden of disease from early childhood and throughout the lifespan. Despite decades of research, there are no proven strategies for prevention and a new scientific approach is urgently needed. Asthma and allergies provides us with a showcase to study environmental impact across generations.
1.1. Susceptibility Windows for Environmental Exposures, Policy Meets Biology
In the 1970s Forsdahl [5] found that poor living conditions in childhood and adolescence in Norway in the early 19th century could be an important risk factor for heart disease later in life. In the 1980s Barker and colleagues introduced the fetal origins hypothesis [6]. The Forsdahl/Barker Developmental Origins of Health and Disease (DOHaD) hypothesis evolved from epidemiological studies of infant and adult mortality. It is now well documented that intrauterine and early life are important susceptibility windows for environmental impact on health and disease later in life. This concept has had an enormous impact on health policy and healthcare- programs for mother- and childcare. However, emerging evidence suggests that environment even before conception may influence health and disease (Figure 1).
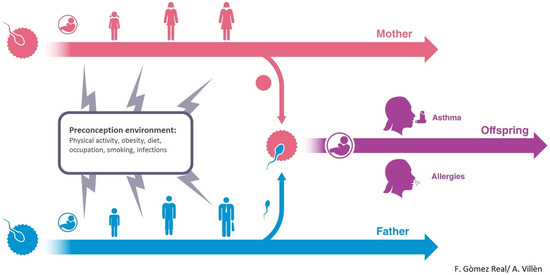
Figure 1.
Maternal and paternal lifeline and possible influence on offspring asthma and allergies.
Furthermore, recent studies suggest that not only mothers’ but also fathers’ health behavior and environment may be of importance for the health of future generations [7,8,9]. With regard to the maternal line, there is a growing awareness of the effects of maternal factors in the programming of offspring immunity [10]. Epidemiological and experimental evidence suggests that maternal immune transfer via nursing can provide offspring with long lasting disease-specific protection [11,12]. With regard to the paternal line, it appears that different environmental factors such as smoking and overweight may cause genetic and epigenetic changes in sperm that are transmissible to offspring [7,13,14,15,16,17,18,19,20]. There are several indications that parental exposure before conception might influence disease risk in offspring differentially through the maternal and paternal lines [7].
The traditional paradigm of dosage—that the dose of exposure determines health effects—is being challenged by a paradigm of timing, i.e., an exposure of little importance in adulthood could have devastating effects if occurring at more susceptible time windows, as in adolescence or in utero. Results from animal research suggest particularly vulnerable susceptibility time-windows in utero, just before puberty, and at each reproductive cycle [7] with regard to future offspring health. There has been little research on human data to support this until now, and the limited research in this field has so far mainly focused on cigarette smoke [21,22,23]. Adolescence is a time window that is relatively under-researched, and an age window that is given limited priority in health policies. A recent publication by Marcon et al., based on over 100,000 persons participating in European cohorts, showed that smoking is on the rise in Europe among boys and girls aged 15 years or younger, despite the reduction observed among older subjects [24]. This worrying fact highlights the failure—or the lack of priority—to reach this age group. Establishing vulnerability windows affecting health over future generations, and not only in the now or the near future of the individual, may have major consequences for health policy and practice.
1.2. Approaches to Studying Environmental Impact across Generations
In the following, we will present (i) Potential epigenetic mechanisms for transfer of environmental effects across generations, (ii) Multi-generational animal models, (iii) How maternal infection provide a long lasting footprint on offspring immunity and (iv) Evidence for transmission across generations in asthma and allergic disease in humans.
2. Epigenetic Research: Building Maps for Predicting and Preventing Disease
The term “epigenetics” has now been applied quite widely in biology to describe a range of biological processes and phenomenon. This includes molecular mechanisms such as DNA methylation and histone modification that control DNA packaging within the nucleus. Epigenetic processes such as these are critical to developmental processes and cellular differentiation, and allow cells to have a memory of ‘state’. For example, a respiratory epithelial cell ‘knows’ it is a lung cell, even when taken out of the lung and cultured over multiple passages in the laboratory. In the reverse of this, fully differentiated cells can be induced to become pluripotent and capable of differentiating into multiple cells types through removal of epigenetic marks [25].
Epigenetics is also used to explain the phenomenon of complex patterns of inheritance such as parent of origin effects and intergenerational inheritance. As a consequence of the fact that in mammals oocytes develop early in the female fetus, environmental exposures may have direct effects on the phenotype of one or two generations (intergenerational inheritance, Figure 2). Finally, epigenetics is used to refer to complex patterns of inheritance that cannot be explained by changes to the primary DNA sequence and that are passed via the germline over >2 generations. Such “transgenerational epigenetics” (Figure 2) is (relatively) common in plants [26], and rare in mammals [27].
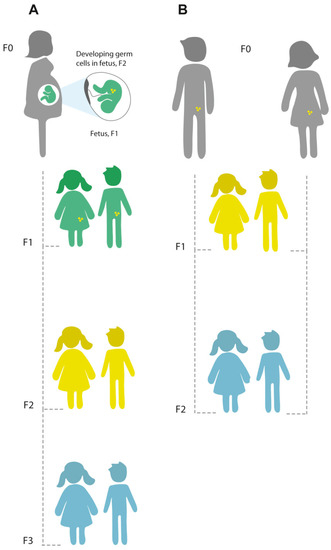
Figure 2.
Principles of intergenerational and transgenerational epigenetic inheritance. (A) If a pregnant woman (F0) is exposed to an environmental stressor, her son or daughter (F1; green), and his or her germ cells that will form the F2 generation (yellow) are also directly exposed, and this might result in intergenerational effects. The third generation (F3; blue) is the first generation that could represent transgenerational epigenetic inheritance. (B) If a man or a woman (F0) and their germ cells to the F1 generation (yellow) are directly exposed to an environmental stressor, the F2 offspring (blue) is the first generation that could represent transgenerational epigenetic inheritance. (Figure reproduced from [28] under the terms of Creative Commons Attribution License (CC BY) https://creativecommons.org/licenses/by/4.0/).
What Are the Mechanisms of Inter and Transgenerational Inheritance?
The mechanisms underlying true transgenerational inheritance in mammals, if indeed they truly occur, are still far from clear. There are two rounds of extensive epigenetic reprogramming in meiosis, in the formation of germ cells and shortly after fertilization [27,29]. The germ cells are believed to be more susceptible to environmental influences during these reprogramming phases. It is hypothesized that transmission of information occurs through epigenetic variation in sperm, oocytes, or both sets of gametes. Several biological mechanisms may transmit epigenetic information from one generation to the next, such as DNA methylation, histone modification, or changes in non-coding RNA (ncRNA) [9,27,29,30].
Due to its stability in stored DNA samples and comparative ease of measurement, DNA methylation has been the most studied epigenetic mechanism in human studies of inter- and transgenerational effects. DNA methylation is the process of covalent addition of a methyl group at the 5-carbon of the cytosine ring resulting in 5-methylcytosine (5-mC) groove of DNA and inhibit transcription. In human DNA, you find 5-methylcytosine in approximately 1.5% of genomic DNA and in somatic cells occurs almost exclusively in the context of paired symmetrical methylation of a 5’-CpG-3’ site, in which a cytosine nucleotide is located adjacent to a guanidine nucleotide. DNA methylation, through interaction with DNA methyl binding proteins, can regulate gene expression. However, as noted above, DNA methylation undergoes two rounds of erasure, in the formation of gametes and shortly after fertilization, and it is unclear whether, or how, memory of CpG site methylation maintains through meiosis. Despite this caveat, there is some evidence that DNA methylation may account for transgenerational effects, possibly through escape of methylation from erasure. For example, differentially methylated regions (DMRs) have been identified in the sperm and male primordial germ cells of the F3 generation of paternal line descendants of rats exposed to the endocrine disruptor vinclozolin [31,32]. In Agouti mice, methyl donor supplementation during pregnancy altered the trajectory of obesity across generations due to altered expression of the agouti gene resulting from changes in DNAm in the offspring [33].
A second mechanism that is potential route for transgenerational inheritance is histone modification. The invertebrate model organism, C. Elegans, whose genome lacks deTectable 5-mC and does not encode a conventional DNA methyl transferase, can impart heritable epigenetic changes, generated from histone modification, to subsequent generations [34].
Thirdly, another possible mechanism for conveying epigenetic information between generations is non-coding RNAs (ncRNAs), such as microRNA (miRNA), small interfering RNA (siRNA), and piwi-interacting RNA (piRNA), which can potentially act as mediators of environmentally induced transgenerational inheritance. These ncRNAs show enhancer-like function and can control chromatin structure. For example, Gapp et al. demonstrated that traumatic stress in early life altered expression of mouse sperm miRNA, and behavioral and metabolic responses in the progeny. The phenotype of the progeny could recapitulate by injection of sperm miRNAs into fertilized oocytes [35]. In humans exposures as diverse as early life stress (including members of the same sperm miRNA family also reduced in mice exposed to traumatic stress) [36], smoking [18], and endurance training [37] have shown to alter sperm miRNA expression. However, a role for this in epigenetic mediated transgenerational exposure remains to be established.
3. Studying Multi-Generational Epigenetic Effects Using Animal Models
Animal models are crucial for the study of multi-generational epigenetic mechanisms, and rodents play an indispensable role in this field. In this case, rodents are proved to be useful due to their early sexual maturity (7 weeks), fast reproduction rate so that several generations can be studied, and finally the accessibility to their pre- and early postnatal stages of development [38,39]. This all provides an important experimental window for the evaluation of epigenetic outcomes [38,39]. In addition to this, animal models can provide insights into molecular mechanisms contributing to multigenerational transmission.
Two well-known mouse models that are widely used for epigenetic studies are; yellow agouti and axin1-fused. The yellow agouti mouse model is widely used to study environmental and nutritional factors affecting the epigenome of the fetus, where the variation of the coat color and obesity propensity of these mice is dependent on the DNA methylation and histone modification status of the agouti promoter [38,40,41]. In case of the axin1-fused mouse models, differences in DNA methylation are correlated with the presence or absence of its characteristic feature, a kinked tail [38,42,43], and this phenotype can be inherited transgenerational [44].
Interestingly, transgenerational transmission through epigenetic factors has been documented in various studies, for example, a study by Ng and colleagues [45] has shown that female offspring of male rats fed a high-fat diet have complications in regulating insulin levels and tend to gain more weight in comparison to female offspring of male rats fed a regular diet [45]. This implies an intergenerational epigenetic transmission of impaired glucose-homeostasis (modified metabolic phenotype) from fathers to female offspring. Furthermore, a recent study demonstrated that an exposure of a maternal grandfather to a high-fat diet, not only disrupted the metabolic phenotype of the mother but also of a grandson pointing to the epigenetic transmission through the founding male germ cells [46]. This disruption of metabolic phenotype is one of several experiences a father can pass to the offspring. In another example, in utero exposure to maternal smoking has been demonstrated to induce transgenerational transmission of asthma phenotype to F3 rat offspring [47] and lead to lung function deficits in the F1 mouse generation [48]. Some other examples include the exposure to stress which is a risk factor for transgenerational transmission of depression; increased stress susceptibility and development of emotional and behavioral disorders in offspring generated from male mouse pups (F0) subjected to unpredictable postnatal separation from the mother [49]. Interestingly, this depressive behavior reversed by anti-depressant treatment [49]. Other studies support the transgenerational transmission of stress vulnerability by demonstrating that adult male mice exposed to chronic social defeat stress generated male offspring (F1) with the depression- and anxiety-like abnormalities [50]. Another study showed that pre-conception exposure of male mice to Bisphenol A (BPA), diminished sperm count and quality in the subsequent F1 generation [51].
Understanding epigenetic mechanisms will shed light on various aspects of developmental and molecular biology as well as on neurodegenerative and metabolic disorders and mice provide valuable animal models to understand these transgenerational epigenetic effects.
4. Maternal Pre-Conception Environment and Long Lasting Influences on Offspring Immunity
Maternal transfer of immunity both in utero and via nursing provides critical sources of early life immune education and is extremely important for protection from many infectious and non-infectious diseases [52,53]. This maternally acquired protection from infection is typically associated with a passive transfer to offspring of maternal innate opsonins and antibodies, which provide a transient, but critical, early life protection from infection [54,55]. This protection provides high levels of effective but often temporary immunity to dangerous infections such as non-typhoidal Salmonella spp. in infants. This mode of protection is typically lost when maternally derived antibodies are degraded, but the maturing offspring immune system’s ability to generate its own effective adaptive immunity replaces it [56]. A wide body of research highlights the fundamental role early-life environment plays in determining the broad transcriptional identity of circulating lymphocytes and showing that the early-life environment has a very strong influence on T-cells [57,58,59,60,61,62].
While both nursing and in utero maternal immune inputs have traditionally been associated with transfer of maternally derived antibody [63] it has been apparent for some time that other immunogenic components of breast milk such as cytokines and non-inherited antigens are also likely to influence offspring immunity [10]. Epidemiological and experimental evidence suggests that maternal immune transfer via nursing can also provide offspring with long lasting disease-specific protection [11]. Such long lasting change to an offspring immune system resulting from an early-life effect can be termed a Predictive Adaptive Response [64]. While the focus of this review is how such effects may mediate via epigenetic influences, other mechanisms can also provide long-term influences on offspring health. For example, while maintenance of maternally transferred products via breastmilk is widely considered temporary, the indirect effects of such transferred protection, such as exposure to antigen and cytokines, could well be longer lasting [12]. However, it is not well defined how these mechanisms mediate any such long-term protection and contributions and maintenance of maternal components in offspring is incompletely understood.
Obviously, the number of potential maternal influences on offspring immunity is going to be diverse. However, key areas in this field that need to be further explored are maternal exposure to micro and macro-organisms before or during pregnancy as well as the essential and dramatic change in maternal physiology to ensure a successful pregnancy. Understanding these influences will also inform as to how other influences may affect offspring health. Maternal exposure to micro/macro-organisms, be they either pathogens or components of the micro/macro-biome, are likely to be key drivers of changing offspring immunity [65]. For example, changes in maternal microbiome due to pathogen exposure or antibiotic treatment has shown to result in significant and lasting consequences for offspring [66,67]. It has been shown that these effects do have ramifications for offspring immunity. Furthermore, it has been shown that transient colonization of germ free mothers with E. coli alter offspring innate immune compartments [68], while oral treatment of mice during pregnancy with vancomycin leads to significant changes in offspring lymphoid cell populations [69]. It is, therefore, evident that the bacterial profile of the maternal intestine has an impact on the subsequent immune development of offspring.
Parasitic helminth infections (an important cause of infection and disease) are mostly chronic and clinically benign yet they still leave a profound immunological footprint on a host. While the effect of helminth infections on the pregnant mother is unclear, the implication for infant immunity from a maternal helminth infection is more understandable. Children whose mothers have been infected with helminths during pregnancy can exhibit populations of B and T cells responsive to helminth antigens [70,71,72,73] or B cells class switched to secrete IgE and IgG4 [74]. The potential effects of this in utero sensitization are likely to be broad and not restricted to homologous influence on subsequent infant helminth infection. For example vaccine efficacy in infants born to schistosome- or filarial-infected mothers can be impaired [75,76] as demonstrated by reduced levels of protective IgG against important pathogens such as Haemophilus influenza type B and Diphtheria [76].
Several human studies show that helminth infections associate with reduced prevalence of atopic disease, for example [77,78,79]. Insights from birth cohort studies in Uganda suggest acquirement of such protection, at least in part, prenatally [80]. While host adapted helminth infections appear to provide some levels of protection against allergy, exposure to zoonotic helminth infections such as Toxocara spp. might be a risk factor for atopic disease. Epidemiological data suggested that this risk might be associated with paternal exposure to these parasites [81].
In addition to exposure to other organisms, it is also important to be cognizant of the requirement for immune regulation for a successful pregnancy. Full term pregnancy requires the maternal endometrium to accept invasion and infiltration of the semi-allogenic fetus. This “antigenic-exposure” typically induces a type 1 immune response i.e., with an IFN gamma biased response. For the maternal immune system to accept the fetus as an allograft and not destroy it by mounting this type 1 cytotoxic response, the maternal-fetal interface suppresses potential maternal inflammatory responses [82]. Achievement of this appears to be in part at least by production of type 2 cytokines by the placenta, which counterbalances the potential pro-inflammatory effects of type 1 cytokines. Such a pro-inflammatory Type 1 response involving cytokines like IFNγ and TNFα could have a range of negative effects on fetal survival, such as promoting expression of the pro-apoptotic transmembrane protein Fas and predispose trophoblast cells to apoptosis. Regulatory cytokines such as IL-10, suppress these type 1 immune effects and allied cytotoxic events such as activation of NK cells, thereby promoting successful implantation and maintenance of the trophoblast [83,84]. Studies in mice support the importance of a strong type 2 immune environment showing that feto-placental tissue spontaneously secretes type 2 and regulatory cytokines, which maintain a type 2-biased cytokine production greater than that of re-stimulated maternal splenic cells [85,86]. Thus, a normal pregnancy depends on a type 2-biased environment to avoid loss of the trophoblast. This regulatory immune imbalance that protects the fetus in utero is passed onto the fetus itself and is maintained into the neonatal period [82]. In the neonate, this maintained Th2 bias, is only recent addressed. To date preclinical work has demonstrated its contribution to the co-ordination of early-life pulmonary development [87], which possibly could be at a cost of enhanced risk of neonatal allergic disease developing [88].
5. Evidence for Transmission across Generations in Asthma and Allergic Disease in Humans
Epidemiological studies have formed an important basis for our emerging understanding of the transmission of susceptibility to health and disease across generations in humans. In the Överkalix cohort, Pembrey and colleagues [89,90,91] found that variation in food supply during the early life of paternal grandparents was associated with variation in mortality rate in their grandchildren. They also found striking sex-specific differences in transmissions, namely that the food supply of the paternal grandfather was associated with the mortality rate of grandsons only, while the early life food supply of the paternal grandmother was associated with the mortality rate of granddaughters only [89,90,91]. This was exclusive seen when exposure occurred before puberty, suggesting that a reprogramming of gametes might be involved. In the UK Avon Longitudinal Study of Parents And Children (ALSPAC) cohort, a correlation of body mass index among sons, with paternal smoking was only observed when paternal smoking took place before puberty [90]. Another study based on survivors of the Dutch famine of 1944 showed differences in obesity in children born from mothers who were pregnant during the famine. Exposures during the first half of the pregnancy were associated with higher rates of obesity. This was associated with hyper methylation of the imprinted insulin-like growth factor receptor gene, pointing to the possibility that DNA-methylation might be involved [92].
In the ECRHS (European Community Respiratory Health Survey) cohort, asthmatic and allergic disease status was measured in the parent generation at three time points over twenty years. In the third study wave, parents reported on offspring allergies. Bertelsen and colleagues found stronger associations of offspring allergies with parental asthmatic and allergic disease assessed before conception as compared to after birth of the child [93]. This indicates that disease activity might induce changes that are transmissible to the next generation, possibly explained by epigenetic inheritance rather than by shared environment or genetics alone [94]. Furthermore, a study by Jogi et al. of helminths and allergies in two generations in Norway found that fathers’ Toxocara exposure was associated with daughters’ allergies, and mother’s Toxocara exposure with sons’ allergies [81]. The sex-specific pattern might be suggestive of epigenetic transmission.
With regard to respiratory health, tobacco smoke in particular has been investigated for a potential role as a transgenerational risk factor for asthma [95,96], since there are animal studies supporting multigenerational effects of nicotine exposure during gestation and lactation on lung development [47]. In human cohorts, it has been shown higher asthma risk in persons whose maternal grandmother smoked when pregnant, independent of maternal smoking [23,97,98,99]. Other studies have found that grandmothers’ smoking was associated with their grandchildren’s respiratory outcomes through the paternal line [21,22]. Investigating pre-conception risks in the RHINE cohort, no effect of maternal smoking prior to conception on offspring asthma could be identified in a study by Svanes et al., while an effect of smoking in pregnancy was confirmed, as in previous studies [21]. However, the father’s preconception smoking was associated with offspring asthma, and the association was particularly strong if the father had started smoking before age 15 years [21,99]. The observation in the ALSPAC study that sons of fathers who smoked before age 11 years had increased body fat also point to early puberty as an important window of susceptibility [100]. In the RHINESSA (Respiratory Health In Northern Europe, Spain and Australia)—cohort, ongoing analyses give further evidence supporting the theory that father’s early puberty might be an important susceptibility window for future offspring health. Accordini et al. showed that lung function was lower among offspring of fathers who smoked before age 15 [101]. Johannessen et al. found that the risk of asthma was higher among offspring of fathers that became overweight at voice break, while no such effect was found for mother’s overweight [20]. Lønnebotn et al. found that high pre-pubertal weight gain in fathers or in offspring themselves was associated with offspring asthma; no effect was identified for weight gain from puberty to adulthood or within adulthood [102] (Table 1).

Table 1.
Summary of intergenerational human studies related to asthma and allergy.
Thus, human findings support the hypothesis that adolescence may be an important susceptibility window. Mechanistically, it is likely that preconception exposure effects on phenotype in later generations might be transmitted through germ cells [7]. Germ cells develop differently in males and females, which could explain the differences in effects through the maternal and paternal lines. In-utero exposure of fathers to smoking could influence primordial germ cell development [13], and a vulnerability window in pre-puberty could be related to de novo DNA methylation occurring during primordial germ cell differentiation to spermatogonia [103].
6. Conclusions
This review presents emerging evidence suggesting that there are important susceptibility windows long before conception, that are likely to affect the health of future generations. Intrauterine and early life has already been accepted as important susceptibility windows for environmental exposure effects on health and disease later in life. It is well documented that a mother’s environment shortly before and during pregnancy is of importance for offspring future health. For instance, both nursing and in utero maternal immune inputs have traditionally been associated with transfer of maternally derived short acting antibodies. However, recent epidemiological and experimental evidence suggests that also maternal pre-conception infection can provide a long lasting footprint on offspring immunity, and that maternal immune transfer via nursing can provide offspring with long lasting disease-specific protection. Recent research also consistently suggests that the environment of future fathers, such as cigarette smoking, is important for offspring health. The concept of inheritable environmental risks in humans is novel, and, by its nature, difficult to study in humans where generations span decades. Recent research shows that several biological mechanisms such as DNA-methylation, histone modification or changes in non-coding RNA may transfer epigenetic information from one generation to the next. Animal models give the possibility to avoid confounding in relation to exposure, and provide insight into molecular mechanisms contributing to multigenerational transmission.
This paper reviews evidence from immunological and epigenetic research, and from human as well as animal models, supporting the hypothesis that there are susceptibility windows to health and disease before conception. Identifying such vulnerability windows in which improved health conditions may have an impact, not only on the individual health but also on several generations, provides a biological basis for sustainable preventive interventions. It is difficult to imagine a more sustainable health care concept, than intervening in specified age groups, with maximal benefits across generations. Adolescence appears to be a susceptibility window of particular importance, for own as well as offspring health—possibly as important as intrauterine life. A recent analysis showing that initiation to smoking during early adolescence is increasing in Europe is an alarming example that this age group needs more focus and higher priority. With regard to mother and child care, public health policies has reasonably successfully given priority to intervening in this key vulnerability window. With regard to early adolescence, there is a need for further research, but the existing evidence is in our view consistent enough to call for action. A change in public health policies is needed, acknowledging this age group as another key vulnerability window and allocating the required resources.
This provides a tremendous opportunity for efficient intervention with regard to improving human health. However, there is a large burden on the individual, to improve own health and now also that of future generations, through personal health achievement. Furthermore, our research usually focuses on risk factors and harm, rather than advantageous behavior and environmental factors. Our life at home, in the community and in the nation, is all part of the planetary environment guided by political and social systems [3]. The society can promote health through the built environment, school systems, pollution control, food and drug safety, health education etc. The scientific community should increase the focus on advantageous behaviors and environmental factors. It is of key importance that intervention towards better health is not a matter of personal achievement, but of the society.
We must consider the future in making our decisions about the present, when aiming for a sustainable development in health.
Author Contributions
Conceptualization: J.W.H., W.H., S.K.-E., F.G.R., C.S.; Methodology: J.W.H., W.H., S.K.-E., F.G.R., C.S.; Writing—original draft preparation: M.L., N.E.-M., J.W.H., W.H., S.K.-E., F.G.R., C.S.; Writing—review & editing: M.L., N.E.-M., J.W.H., W.H., S.K.-E., F.G.R., C.S.; Visualization: M.L., F.G.R.; Supervision: F.G.R., C.S.; Project administration: M.L.
Funding
W.H., J.W.H. and C.S. are supported by World Universities Network (WUN) Research Development Fund and WUN Sustainability Fund. J.W.H. and C.S. are supported by the Ageing Lungs in European Cohorts (ALEC) Study [www.alecstudy.org], which has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No.633212. C.S., J.W.H., S.K.-E and W.H. are supported by Research Council of Norway, FRIPRO toppforsk grant nr.274767. C.S. is supported by Western Norway Regional Health Authorities strategic investment grant nr. 912011; S.K.-E. by Leibniz Competition 2016-2020; J.W.H. by National Institute of Health, USA R01 AI121226; and W.H. supported by NRF Competitive support for Rated Researchers: 111815 and Deutsche Forschungsgemeinschaft (DFG): LA 2746/2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prescott, S.L.; Logan, A.C.; Albrecht, G.; Campbell, D.E.; Crane, J.; Cunsolo, A.; Holloway, J.H.; Kozyrskyj, A.L.; Lowry, C.A.; Penders, J.; et al. The Canmore Declaration: Statement of Principles for Planetary Health. Challenges 2018, 9, 31. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Prescott, S.L.; Logan, A.C. Planetary Health: From the Wellspring of Holistic Medicine to Personal and Public Health Imperative. Explore (NY) 2018. [Google Scholar] [CrossRef] [PubMed]
- Callahan, D.; Jennings, B. Ethics and public health: Forging a strong relationship. Am. J. Public Health 2002, 92, 169–176. [Google Scholar] [CrossRef]
- Forsdahl, A. Are poor living conditions in childhood and adolescence an important risk factor for arteriosclerotic heart disease? Br. J. Prev. Soc. Med. 1977, 31, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. BioEssays News Rev. Mol. Cell. Dev. Biol. 2014, 36, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Sales, V.M.; Ferguson-Smith, A.C.; Patti, M.E. Epigenetic Mechanisms of Transmission of Metabolic Disease across Generations. Cell Metab. 2017, 25, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Marchant, A.; Sadarangani, M.; Garand, M.; Dauby, N.; Verhasselt, V.; Pereira, L.; Bjornson, G.; Jones, C.E.; Halperin, S.A.; Edwards, K.M.; et al. Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 2017, 17, e197–e208. [Google Scholar] [CrossRef]
- Verhasselt, V. Is infant immunization by breastfeeding possible? Philos. Trans. R. Soc. Lond. B. Bio.l Sci. 2015, 370. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Nguyen, V.; Muller, H.K.; Walker, A.M. Maternal Milk T Cells Drive Development of Transgenerational Th1 Immunity in Offspring Thymus. J. Immunol. 2016, 197, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Silfver, K.A.; Stenqvist, A.; Giwercman, A. The Impact of Paternal and Maternal Smoking on Semen Quality of Adolescent Men. PLoS ONE 2013, 8, e66766. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Schmid, T.E.; Baumgartner, A. Male-mediated developmental toxicity. Asian J. Androl. 2014, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Sepaniak, S.; Forges, T.; Gerard, H.; Foliguet, B.; Bene, M.C.; Monnier-Barbarino, P. The influence of cigarette smoking on human sperm quality and DNA fragmentation. Toxicology 2006, 223, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Laubenthal, J.; Zlobinskaya, O.; Poterlowicz, K.; Baumgartner, A.; Gdula, M.R.; Fthenou, E.; Keramarou, M.; Hepworth, S.J.; Kleinjans, J.C.; van Schooten, F.J.; et al. Cigarette smoke-induced transgenerational alterations in genome stability in cord blood of human F1 offspring. FASEB J 2012, 26, 3946–3956. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; Rowan-Carroll, A.; Williams, A.; Polyzos, A.; Berndt-Weis, M.L.; Yauk, C.L. Sidestream tobacco smoke is a male germ cell mutagen. Proc. Natl. Acad. Sci. USA 2011, 108, 12811–12814. [Google Scholar] [CrossRef]
- Marczylo, E.L.; Amoako, A.A.; Konje, J.C.; Gant, T.W.; Marczylo, T.H. Smoking induces differential miRNA expression in human spermatozoa: A potential transgenerational epigenetic concern? Epigenetics 2012, 7, 432–439. [Google Scholar] [CrossRef]
- Rehan, V.K.; Liu, J.; Naeem, E.; Tian, J.; Sakurai, R.; Kwong, K.; Akbari, O.; Torday, J.S. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med. 2012, 10, 129. [Google Scholar] [CrossRef]
- Johannessen, A.; Calciano, L.; Lonnebotn, M.; Bertelsen, R.J.; Braback, L.; Holm, M.; Janson, C.; Jogi, R.; Kirkeleit, J.; Lodge, C.; et al. Late Breaking Abstract—Fathers’ overweight and offspring asthma—An intergenerational perspective. Eur. Respir. J. 2017, 50 (Suppl. 61), PA2615. [Google Scholar]
- Svanes, C.; Koplin, J.; Skulstad, S.M.; Johannessen, A.; Bertelsen, R.J.; Benediktsdottir, B.; Braback, L.; Elie Carsin, A.; Dharmage, S.; Dratva, J.; et al. Father’s environment before conception and asthma risk in his children: A multi-generation analysis of the Respiratory Health In Northern Europe study. Int. J. Epidemiol. 2017, 46, 235–245. [Google Scholar] [CrossRef]
- Miller, L.L.; Henderson, J.; Northstone, K.; Pembrey, M.; Golding, J. Do grandmaternal smoking patterns influence the etiology of childhood asthma? Chest 2014, 145, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Langholz, B.; Salam, M.T.; Gilliland, F.D. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 2005, 127, 1232–1241. [Google Scholar] [CrossRef]
- Marcon, A.; Pesce, G.; Calciano, L.; Bellisario, V.; Dharmage, S.C.; Garcia-Aymerich, J.; Gislasson, T.; Heinrich, J.; Holm, M.; Janson, C.; et al. Trends in smoking initiation in Europe over 40 years: A retrospective cohort study. PLoS ONE 2018, 13, e0201881. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, T.S.; Hanna, J.; Zhang, X.; Ku, M.; Wernig, M.; Schorderet, P.; Bernstein, B.E.; Jaenisch, R.; Lander, E.S.; Meissner, A. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008, 454, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Quadrana, L.; Colot, V. Plant Transgenerational Epigenetics. Annu. Rev. Genet. 2016, 50, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Morkve Knudsen, T.; Rezwan, F.I.; Jiang, Y.; Karmaus, W.; Svanes, C.; Holloway, J.W. Transgenerational and intergenerational epigenetic inheritance in allergic diseases. J. Allergy Clin. Immunol. 2018, 142, 765–772. [Google Scholar] [CrossRef]
- Wu, H.; Hauser, R.; Krawetz, S.A.; Pilsner, J.R. Environmental Susceptibility of the Sperm Epigenome During Windows of Male Germ Cell Development. Curr. Environ. Health Rep. 2015, 2, 356–366. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar] [CrossRef]
- Guerrero-Bosagna, C.; Settles, M.; Lucker, B.; Skinner, M.K. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 2010, 5. [Google Scholar] [CrossRef]
- Skinner, M.K.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8, e66318. [Google Scholar] [CrossRef]
- Wolff, G.L.; Kodell, R.L.; Moore, S.R.; Cooney, C.A. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998, 12, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Dickson, D.A.; Paulus, J.K.; Mensah, V.; Lem, J.; Saavedra-Rodriguez, L.; Gentry, A.; Pagidas, K.; Feig, L.A. Reduced levels of miRNAs 449 and 34 in sperm of mice and men exposed to early life stress. Transl. Psychiatry 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, L.R.; Donkin, I.; Fabre, O.; Versteyhe, S.; Mechta, M.; Pattamaprapanont, P.; Mortensen, B.; Krarup, N.T.; Barres, R. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin. Epigenet. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, C.S. Animal models to study environmental epigenetics. Biol. Reprod. 2010, 82, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Harris, C. Animal models in epigenetic research: Institutional animal care and use committee considerations across the lifespan. ILAR J. 2012, 53, 370–376. [Google Scholar] [CrossRef]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell Biol. 2003, 23, 5293–5300. [Google Scholar] [CrossRef]
- Duhl, D.M.; Vrieling, H.; Miller, K.A.; Wolff, G.L.; Barsh, G.S. Neomorphic agouti mutations in obese yellow mice. Nat. Genet. 1994, 8, 59–65. [Google Scholar] [CrossRef]
- Belyaev, D.K.; Ruvinsky, A.O.; Borodin, P.M. Inheritance of alternative states of the fused gene in mice. J. Hered. 1981, 72, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C. The Inheritance and Expression of Fused, a New Mutation in the House Mouse. Genetics 1937, 22, 1–13. [Google Scholar] [PubMed]
- Rakyan, V.K.; Chong, S.; Champ, M.E.; Cuthbert, P.C.; Morgan, H.D.; Luu, K.V.; Whitelaw, E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc. Natl. Acad. Sci. USA 2003, 100, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.F.; Lin, R.C.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 2010, 467, 963–966. [Google Scholar] [CrossRef] [PubMed]
- Chambers, T.J.; Morgan, M.D.; Heger, A.H.; Sharpe, R.M.; Drake, A.J. High-fat diet disrupts metabolism in two generations of rats in a parent-of-origin specific manner. Sci. Rep. 2016, 6, 31857. [Google Scholar] [CrossRef]
- Rehan, V.K.; Liu, J.; Sakurai, R.; Torday, J.S. Perinatal nicotine-induced transgenerational asthma. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L501–L507. [Google Scholar] [CrossRef] [PubMed]
- Dehmel, S.; Nathan, P.; Bartel, S.; El-Merhie, N.; Scherb, H.; Milger, K.; John-Schuster, G.; Yildirim, A.O.; Hylkema, M.; Irmler, M.; et al. Intrauterine smoke exposure deregulates lung function, pulmonary transcriptomes, and in particular insulin-like growth factor (IGF)-1 in a sex-specific manner. Sci. Rep. 2018, 8, 7547. [Google Scholar] [CrossRef]
- Franklin, T.B.; Russig, H.; Weiss, I.C.; Graff, J.; Linder, N.; Michalon, A.; Vizi, S.; Mansuy, I.M. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 2010, 68, 408–415. [Google Scholar] [CrossRef]
- Dietz, D.M.; Laplant, Q.; Watts, E.L.; Hodes, G.E.; Russo, S.J.; Feng, J.; Oosting, R.S.; Vialou, V.; Nestler, E.J. Paternal transmission of stress-induced pathologies. Biol. Psychiatry 2011, 70, 408–414. [Google Scholar] [CrossRef]
- Dobrzynska, M.M.; Gajowik, A.; Radzikowska, J.; Tyrkiel, E.J.; Jankowska-Steifer, E.A. Male-mediated F1 effects in mice exposed to bisphenol A, either alone or in combination with X-irradiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2015, 789–790, 36–45. [Google Scholar] [CrossRef]
- Jones, G.; Steketee, R.W.; Black, R.E.; Bhutta, Z.A.; Morris, S.S.; Bellagio Child Survival Study Group. How many child deaths can we prevent this year? Lancet 2003, 362, 65–71. [Google Scholar] [CrossRef]
- Wolf, J.H. Low breastfeeding rates and public health in the United States. Am. J. Public Health 2003, 93, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, K.M.; Mellen, B.G.; Wright, P.F.; Mitchel, E.F., Jr.; Griffin, M.R. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N. Engl. J. Med. 2000, 342, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.A.F.; Cherian, T.; Chow, J.; Shahid-Salles, S.A.; Laxminarayan, R.; John, T.J. Acute Respiratory Infections in Children. In Disease Control Priorities in Developing Countries, 2nd ed.; Jamison, D.T., Breman, J.G., Measham, A.R., Alleyne, G., Claeson, M., Evans, D.B., Jha, P., Mills, A., Musgrove, P., Eds.; The World Bank: Washington, DC, USA, 2006. [Google Scholar]
- MacLennan, C.A.; Gondwe, E.N.; Msefula, C.L.; Kingsley, R.A.; Thomson, N.R.; White, S.A.; Goodall, M.; Pickard, D.J.; Graham, S.M.; Dougan, G.; et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 2008, 118, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.K.; Chen, E.; Ross, K.M.; McEwen, L.M.; Maclsaac, J.L.; Kobor, M.S.; Miller, G.E. Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology 2018, 97, 131–134. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Shalowitz, M.U.; Story, R.E.; Leigh, A.K.K.; Ham, P.; Arevalo, J.M.G.; Cole, S.W. Divergent transcriptional profiles in pediatric asthma patients of low and high socioeconomic status. Pediatr. Pulmonol. 2018, 53, 710–719. [Google Scholar] [CrossRef]
- Turner, J.D. Holistic, personalized, immunology? The effects of socioeconomic status on the transcriptional milieu of immune cells. Pediatr. Pulmonol. 2018, 53, 696–697. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Hengesch, X.; Leenen, F.A.D.; Schritz, A.; Sias, K.; Schaan, V.K.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Schachinger, H.; et al. Proinflammatory T Cell Status Associated with Early Life Adversity. J. Immunol. 2017, 199, 4046–4055. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Sias, K.; Hengesch, X.; Schaan, V.K.; Leenen, F.A.D.; Adams, P.; Meriaux, S.B.; Schmitz, S.; Bonnemberger, F.; Ewen, A.; et al. T Cell Immunosenescence after Early Life Adversity: Association with Cytomegalovirus Infection. Front. Immunol. 2017, 8, 1263. [Google Scholar] [CrossRef]
- Elwenspoek, M.M.C.; Kuehn, A.; Muller, C.P.; Turner, J.D. The effects of early life adversity on the immune system. Psychoneuroendocrinology 2017, 82, 140–154. [Google Scholar] [CrossRef]
- Heath, P.T.; Culley, F.J.; Jones, C.E.; Kampmann, B.; Le Doare, K.; Nunes, M.C.; Sadarangani, M.; Chaudhry, Z.; Baker, C.J.; Openshaw, P.J.M. Group B streptococcus and respiratory syncytial virus immunisation during pregnancy: A landscape analysis. Lancet Infect. Dis. 2017, 17, e223–e234. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Thomson, C.A. The Impact of Maternal Microbes and Microbial Colonization in Early Life on Hematopoiesis. J. Immunol. 2018, 200, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, L.; Relman, D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4554–4561. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, H.E.; Abrahamsson, T.R.; Jenmalm, M.C.; Harris, K.; Quince, C.; Jernberg, C.; Bjorksten, B.; Engstrand, L.; Andersson, A.F. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 2014, 63, 559–566. [Google Scholar] [CrossRef]
- Gomez de Aguero, M.; Ganal-Vonarburg, S.C.; Fuhrer, T.; Rupp, S.; Uchimura, Y.; Li, H.; Steinert, A.; Heikenwalder, M.; Hapfelmeier, S.; Sauer, U.; et al. The maternal microbiota drives early postnatal innate immune development. Science 2016, 351, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Nyangahu, D.D.; Lennard, K.S.; Brown, B.P.; Darby, M.G.; Wendoh, J.M.; Havyarimana, E.; Smith, P.; Butcher, J.; Stintzi, A.; Mulder, N.; et al. Disruption of maternal gut microbiota during gestation alters offspring microbiota and immunity. Microbiome 2018, 6, 124. [Google Scholar] [CrossRef]
- Guadalupe, I.; Mitre, E.; Benitez, S.; Chico, M.E.; Nutman, T.B.; Cooper, P.J. Evidence for in utero sensitization to Ascaris lumbricoides in newborns of mothers with ascariasis. J. Infect. Dis. 2009, 199, 1846–1850. [Google Scholar] [CrossRef]
- Malhotra, I.; Mungai, P.; Wamachi, A.; Kioko, J.; Ouma, J.H.; Kazura, J.W.; King, C.L. Helminth- and Bacillus Calmette-Guerin-induced immunity in children sensitized in utero to filariasis and schistosomiasis. J. Immunol. 1999, 162, 6843–6848. [Google Scholar]
- Malhotra, I.; Ouma, J.H.; Wamachi, A.; Kioko, J.; Mungai, P.; Njzovu, M.; Kazura, J.W.; King, C.L. Influence of maternal filariasis on childhood infection and immunity to Wuchereria bancrofti in Kenya. Infect. Immun. 2003, 71, 5231–5237. [Google Scholar] [CrossRef]
- King, C.L.; Malhotra, I.; Mungai, P.; Wamachi, A.; Kioko, J.; Ouma, J.H.; Kazura, J.W. B cell sensitization to helminthic infection develops in utero in humans. J. Immunol. 1998, 160, 3578–3584. [Google Scholar]
- Seydel, L.S.; Petelski, A.; van Dam, G.J.; van der Kleij, D.; Kruize-Hoeksma, Y.C.; Luty, A.J.; Yazdanbakhsh, M.; Kremsner, P.G. Association of in utero sensitization to Schistosoma haematobium with enhanced cord blood IgE and increased frequencies of CD5- B cells in African newborns. Am. J. Trop. Med. Hyg. 2012, 86, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, I.; LaBeaud, A.D.; Morris, N.; McKibben, M.; Mungai, P.; Muchiri, E.; King, C.L.; King, C.H. Cord Blood Anti-Parasite IL-10 as Risk Marker for Compromised Vaccine Immunogenicity in Early Childhood. J. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, I.; McKibben, M.; Mungai, P.; McKibben, E.; Wang, X.; Sutherland, L.J.; Muchiri, E.M.; King, C.H.; King, C.L.; LaBeaud, A.D. Effect of antenatal parasitic infections on anti-vaccine IgG levels in children: A prospective birth cohort study in Kenya. PLoS Negl. Trop. Dis. 2015, 9, e0003466. [Google Scholar] [CrossRef] [PubMed]
- van den Biggelaar, A.H.; van Ree, R.; Rodrigues, L.C.; Lell, B.; Deelder, A.M.; Kremsner, P.G.; Yazdanbakhsh, M. Decreased atopy in children infected with Schistosoma haematobium: A role for parasite-induced interleukin-10. Lancet 2000, 356, 1723–1727. [Google Scholar] [CrossRef]
- Obeng, B.B.; Amoah, A.S.; Larbi, I.A.; de Souza, D.K.; Uh, H.W.; Fernandez-Rivas, M.; van Ree, R.; Rodrigues, L.C.; Boakye, D.A.; Yazdanbakhsh, M.; et al. Schistosoma infection is negatively associated with mite atopy, but not wheeze and asthma in Ghanaian Schoolchildren. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Djuardi, Y.; Supali, T.; Wibowo, H.; Kruize, Y.C.; Versteeg, S.A.; van Ree, R.; Sartono, E.; Yazdanbakhsh, M. The development of TH2 responses from infancy to 4 years of age and atopic sensitization in areas endemic for helminth infections. Allergy Asthma Clin. Immunol. 2013, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Mpairwe, H.; Ndibazza, J.; Webb, E.L.; Nampijja, M.; Muhangi, L.; Apule, B.; Lule, S.; Akurut, H.; Kizito, D.; Kakande, M.; et al. Maternal hookworm modifies risk factors for childhood eczema: Results from a birth cohort in Uganda. Pediatr. Allergy Immunol. 2014, 25, 481–488. [Google Scholar] [CrossRef]
- Jogi, N.O.; Svanes, C.; Siiak, S.P.; Logan, E.; Holloway, J.W.; Igland, J.; Johannessen, A.; Levin, M.; Real, F.G.; Schlunssen, V.; et al. Zoonotic helminth exposure and risk of allergic diseases: A study of two generations in Norway. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 66–77. [Google Scholar] [CrossRef]
- Morein, B.; Blomqvist, G.; Hu, K. Immune responsiveness in the neonatal period. J. Comp. Pathol. 2007, 137 (Suppl. 1), S27–S31. [Google Scholar] [CrossRef]
- Aschkenazi, S.; Straszewski, S.; Verwer, K.M.; Foellmer, H.; Rutherford, T.; Mor, G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol. Reprod. 2002, 66, 1853–1861. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; Burnett, T.G. Uterine leukocytes: Key players in pregnancy. Semin. Cell Dev. Biol. 2000, 11, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Abelius, M.S.; Janefjord, C.; Ernerudh, J.; Berg, G.; Matthiesen, L.; Duchen, K.; Nilsson, L.J.; Jenmalm, M.C. The placental immune milieu is characterized by a Th2- and anti-inflammatory transcription profile, regardless of maternal allergy, and associates with neonatal immunity. Am. J. Reprod. Immunol. 2015, 73, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Raghupathy, R. Pregnancy: Success and failure within the Th1/Th2/Th3 paradigm. Semin. Immunol. 2001, 13, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.V.; Williams, T.M.; Walker, K.A.; Dickinson, H.; Sakkal, S.; Rumballe, B.A.; Little, M.H.; Jenkin, G.; Ricardo, S.D. M2 macrophage polarisation is associated with alveolar formation during postnatal lung development. Respir. Res. 2013, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- de Kleer, I.M.; Kool, M.; de Bruijn, M.J.; Willart, M.; van Moorleghem, J.; Schuijs, M.J.; Plantinga, M.; Beyaert, R.; Hams, E.; Fallon, P.G.; et al. Perinatal Activation of the Interleukin-33 Pathway Promotes Type 2 Immunity in the Developing Lung. Immunity 2016, 45, 1285–1298. [Google Scholar] [CrossRef]
- Szyf, M. Nongenetic inheritance and transgenerational epigenetics. Trends Mol. Med. 2015, 21, 134–144. [Google Scholar] [CrossRef]
- Pembrey, M.E. Male-line transgenerational responses in humans. Hum. Fertil. 2010, 13, 268–271. [Google Scholar] [CrossRef]
- Pembrey, M.E.; Bygren, L.O.; Kaati, G.; Edvinsson, S.; Northstone, K.; Sjostrom, M.; Golding, J.; Team, A.S. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. EJHG 2006, 14, 159–166. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Bertelsen, R.J.; Rava, M.; Carsin, A.E.; Accordini, S.; Benediktsdottir, B.; Dratva, J.; Franklin, K.A.; Heinrich, J.; Holm, M.; Janson, C.; et al. Clinical markers of asthma and IgE assessed in parents before conception predict asthma and hayfever in the offspring. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Arshad, S.H.; Karmaus, W.; Zhang, H.; Holloway, J.W. Multigenerational cohorts in patients with asthma and allergy. J. Allergy Clin. Immunol 2017, 139, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Pembrey, M.; Saffery, R.; Bygren, L.O.; Network in Epigenetic Epidemiology. Human transgenerational responses to early-life experience: Potential impact on development, health and biomedical research. J. Med. Genet. 2014, 51, 563–572. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.; Vercelli, D. Epigenetic Mechanisms in Asthma. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. 1), S48–S50. [Google Scholar] [CrossRef]
- Magnus, M.C.; Haberg, S.E.; Karlstad, O.; Nafstad, P.; London, S.J.; Nystad, W. Grandmother’s smoking when pregnant with the mother and asthma in the grandchild: The Norwegian Mother and Child Cohort Study. Thorax 2015, 70, 237–243. [Google Scholar] [CrossRef]
- Lodge, C.J.; Braback, L.; Lowe, A.J.; Dharmage, S.C.; Olsson, D.; Forsberg, B. Grandmaternal smoking increases asthma risk in grandchildren: A nationwide Swedish cohort. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2018, 48, 167–174. [Google Scholar] [CrossRef]
- Accordini, S.; Calciano, L.; Johannessen, A.; Portas, L.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Carsin, A.E.; Dharmage, S.C.; Dratva, J.; et al. A three-generation study on the association of tobacco smoking with asthma. Int. J. Epidemiol. 2018. [Google Scholar] [CrossRef]
- Northstone, K.; Golding, J.; Davey Smith, G.; Miller, L.L.; Pembrey, M. Prepubertal start of father’s smoking and increased body fat in his sons: Further characterisation of paternal transgenerational responses. Eur. J. Hum. Genet. EJHG 2014, 22, 1382–1386. [Google Scholar] [CrossRef]
- Accordini, S.; Johannessen, A.; Calciano, L.; Jogi, R.; Martinez-Moratalla Rovira, J.; Benediktsdottir, B.; Bertelsen, R.J.; Braback, L.; Dharmage, S.; Gomez Real, F.; et al. Three-generation effects of tobacco smoking on lung function within the paternal line. Eur. Respir. J. 2017, 50, PA1178. [Google Scholar] [CrossRef]
- Lønnebotn, M.; Nilsen, R.M.; Dharmage, S.; Franklin, K.A.; Holm, M.; Janson, C.; Jarvis, D.; Johannessen, A.; Kirkeleit, J.; Malinovschi, A.; et al. Associations of fathers and their offsprings weight gain with non-allergic asthma. Eur. Respir. J. 2018. [Google Scholar]
- Kobayashi, H.; Sakurai, T.; Miura, F.; Imai, M.; Mochiduki, K.; Yanagisawa, E.; Sakashita, A.; Wakai, T.; Suzuki, Y.; Ito, T.; et al. High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome Res. 2013, 23, 616–627. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).