Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies
Abstract
:1. Introduction
2. Environmental and Toxicological Aspects of Carbon Nanotubes
3. CNTs in the Environment
4. Recycling Aspects and Strategies for CNTs
5. Aspects for Incineration Processes
6. Options for Recycling CNTs
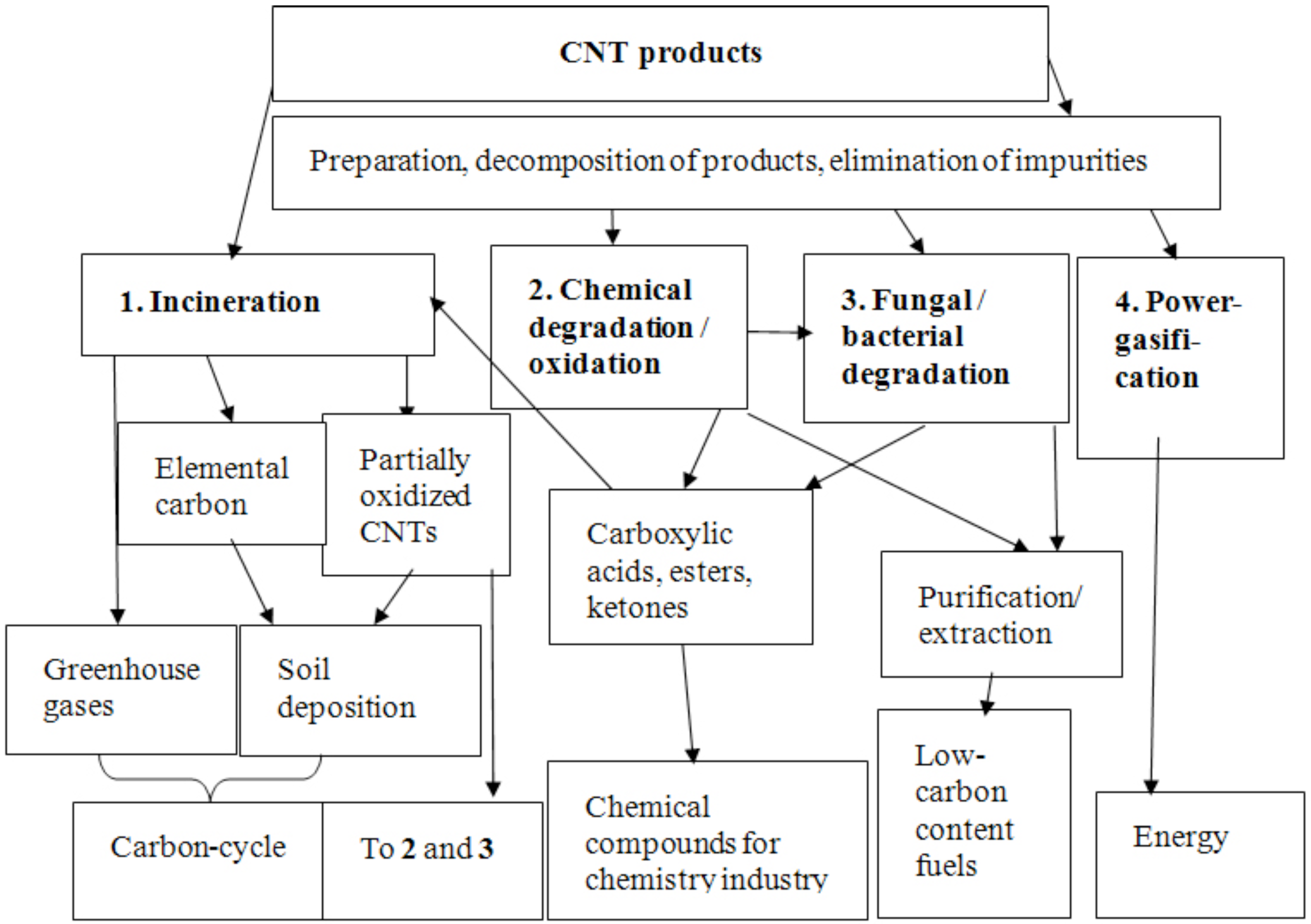
7. Conclusions
References and Notes
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- Ghadiri, M.R.; Granja, J.R.; Milligan, R.A.; McRee, D.E.; Khazanovich, N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 1993, 366, 324–327. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Stephan, O.; Colliex, C.; Trauth, D. Aligned carbon nanotube arrays formed by cutting a polymer resin--nanotube composite. Science 1994, 265, 1212–1214. [Google Scholar]
- Falvo, M.R.; Clary, G.J.; Taylor, R.M., 2nd; Chi, V.; Brooks, F.P.J.; Washburn, S.; Superfine, R. Bending and buckling of carbon nanotubes under large strain. Nature 1997, 389, 582–584. [Google Scholar] [CrossRef]
- Andrae, A.; Andersen, O. Life cycle assessment of integrated circuit packaging technologies. Int. J. Life Cycle Ass. 2011, 16, 258–267. [Google Scholar] [CrossRef]
- Bethune, D.S.; Kiang, C.H.; Devries, M.S.; Gorman, G. Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls. Nature 1993, 363, 605–607. [Google Scholar] [CrossRef]
- Wood, J.R.; Zhao, Q.; Frogley, M.D.; Meurs, E.R.; Prins, A.D.; Peijs, T. Carbon nanotubes: From molecular to macroscopic sensors. Phys. Rev. B 2000, 62, 7571–7575. [Google Scholar] [CrossRef]
- Dillon, A.C.; Heben, M.J. Hydrogen storage using carbon adsorbents: Past, present and future. Appl. Phys. A 2001, 72, 133–142. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.F.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Avouris, P. Carbon nanotube electronics. Chem Phys 2002, 281, 429–445. [Google Scholar] [CrossRef]
- Landi, B.J.; Raffaelle, R.P.; Heben, M.J.; Alleman, J.L.; VanDerveer, W.; Gennett, T. Single-wall carbon nanotube-Nafion composite actuators. Nano Lett. 2002, 2, 1329–1332. [Google Scholar] [CrossRef]
- Li, W.Z.; Liang, C.H.; Qiu, J.S.; Zhou, W.J.; Han, H.M.; Wie, Z.B. Carbon nanotubes as support for cathode catalyst of a direct methanol fuel cell. Carbon 2002, 40, 791–794. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-assisted highly efficient synthesis of impurity-free singlewalled carbon nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- De Villoria, R.; Yamamoto, N.; Miravete, A.; Wardle, B. Multi-physics damage sensing in nano-engineered structural composites. Nanotechnology 2011, 22, 185502. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Wie, T.; Zhi, L.; Wei, F. Nanographene-constructed carbon nanofibers grown on graphene sheets by chemical vapor deposition: High-performance anode materials for lithium ion batteries. ACS Nano 2011, 5, 2787–2794. [Google Scholar] [CrossRef]
- Matyba, P.; Yamaguchi, H.; Eda, G.; Chhowalla, M.; Edman, L.; Robinson, N. Graphene and mobile ions: The key to all-plastic, solution-processed light-emitting devices. ACS Nano 2010, 4, 637–642. [Google Scholar] [CrossRef]
- Eskelinen, A.; Kuzyk, A.; Kaltiaisenaho, T.; Timmermans, M.; Nasibulin, A.; Kaupppinen, E.; Törmä, P. Assembly of single-walled carbon nanotubes on DNA-origami templates through streptavidin-biotin interaction. Small 2011, 7, 746–750. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, X.; Yang, R.; Zhu, Z.; Chen, Y.; Tan, W. Single-walled carbon nanotubes as optical materials for biosensing. Nanoscale 2011, 3, 1949–1956. [Google Scholar] [CrossRef]
- Lu, L.; Chen, W. Supramolecular self-assembly of biopolymers with carbon nanotubes for biomimetic and bio-inspired sensing and actuation. Nanoscale 2011, 3, 2412–2420. [Google Scholar] [CrossRef]
- Colbert, D.; Zhang, J.; McClure, S.; Nikolaev, P.; Chen, Z.; Hafner, J.; Owens, D.; Kotula, P.; Carter, C.; Weaver, J.; Rinzler, A.; Smalley, R. Growth and sintering of fullerene nanotubes. Science 1994, 266, 1218–1222. [Google Scholar]
- Kociak, M.; Suenaga, K.; Hirahara, K.; Saito, Y.; Nakahira, T.; Iijima, S. Linking chiral indices and transport properties of double-walled carbon nanotubes. Phys. Rev. Lett. 2002, 89, 155501–155504. [Google Scholar] [CrossRef]
- Kumar, N.; Shah, V.; Walker, V. Perturbation of an arctic soil microbial community by metal nanoparticles. J. Hazard Mater. 2011, 190, 816–822. [Google Scholar] [CrossRef]
- Doyle, M.; Watson, W.; Bowlin, N.; Sheavly, S. Plastic particles in coastal pelagic ecosystems of the Northeast Pacific ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef]
- Walsh, B. The Perils of Plastic. Time Magazine 2010. [Google Scholar]
- Velzeboer, I.; Kupryianchyk, D.; Peeters, E.; Koelmans, A. Community effects of carbon nanotubes in aquatic sediments. Environ. Int. 2011, 37, 1126–1130. [Google Scholar]
- Atha, D.H.; Wang, H.H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef]
- Nakanishi, J. Risk Assessment of Manufactured Nanomaterials: Carbon nanotubes (CNT); Final report issued on 12 August 2011, NEDO project (P06041); New Energy and Industrial Technology Development Organization: Kawasaki, Japan, 2011. [Google Scholar]
- Lam, C.-W.; James, J.; McCluskey, R.; Arepalli, S.; Hunter, R. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar]
- Shvedova, A.; Kisin, E.; Mercer, R.; Murray, A.; Johnson, V.; Potapovich, A.; Tyurina, Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; Hubbs, A.; Antonini, J.; Evans, D.; Ku, B.; Ramsey, D.; Maynard, A.; Kagan, V.; Castranova, V.; Baron, P. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, 698–708. [Google Scholar] [CrossRef]
- Shvedova, A.; Kisin, E.; Murray, A.; Gorelik, O.; Arepalli, S.; Castranova, V.; Young, S.; Gao, F.; Tyurina, Y.; Oury, T.; Kagan, V. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL⁄6 mice. Toxicol. Appl. Pharmacol. 2007, 221, 339–348. [Google Scholar] [CrossRef]
- Shvedova, A.; Kisin, E.; Murray, A.; Johnson, V.; Gorelik, O.; Arepalli, S.; Hupps, A.; Mercer, R.; Keohavong, P.; Sussman, N.; Jin, J.; Yin, J.; Stone, S.; Chen, B.; Deye, G.; Maynard, A.; Castranova, V.; Baron, P.; Kagan, V. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL⁄6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, 552–565. [Google Scholar]
- Smith, C.; Shaw, B.; Handy, R. Toxicity of single walled carbon nanotubes to rainbow trout, (Oncorhynchus mykiss): Respiratory toxicity, organ pathologies, and other physiological effects. Aquat. Toxicol. 2007, 82, 94–109. [Google Scholar]
- Vogel, S.; Kappes, M.; Hennrich, F.; Richert, C. An unexpected new optimum in the structure space of DNA solubilizing single-walled carbon nanotubes. Chemistry 2007, 13, 1815–1820. [Google Scholar] [CrossRef]
- Kwok, K.; Leung, K.; Flahaut, E.; Cheng, J.; Cheng, S. Chronic toxicity of double-walled carbon nanotubes to three marine organisms: Influence of different dispersion methods. Nanomedicine (London) 2010, 5, 951–961. [Google Scholar]
- Shvedova, A.; Kagan, V. The role of nanotoxicology in realizing the “helping without harm” paradigm of nanomedicine: Lessons from studies of pulmonary effects of single-walled carbon nanotubes. J. Intern. Med. 2010, 267, 106–118. [Google Scholar] [CrossRef]
- Teeguarden, J.; Webb-Robertson, B.; Waters, K.; Murray, A.; Kisin, E.; Varnum, S.; Jacobs, J.; Pounds, J.; Zanger, R.; Shvedova, A. Comparative proteomics and pulmonary toxicity of instilled single-walled carbon nanotubes, crocidolite asbestos, and ultrafine carbon black in mice. Toxicol. Sci. 2011, 120, 123–135. [Google Scholar] [CrossRef]
- Lam, C.-W.; James, J.; McCluskey, R.; Hunter, R. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126. [Google Scholar]
- Kane, A.; Hurt, R. Nanotoxicology: The asbestos analogy revisited. Nat. Nanotechnol. 2008, 3, 378–379. [Google Scholar] [CrossRef]
- Jaurand, M.; Renier, A.; Daubriac, J. Mesothelioma: Do asbestos and carbon nanotubes pose the same health risk? Part. Fibre Toxicol. 2009, 6, 16. [Google Scholar] [CrossRef]
- Allen, B.; Kichambare, P.; Gou, P.; Vlasova, I.; Kapralov, A.; Konduru, N.; Kagan, V.; Star, A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008, 8, 3899–3903. [Google Scholar] [CrossRef]
- Hunt, G. The Labelling “Nano-products”—Update February 2011. In Proceedings of Products, Privacy & People: Regulating at the Nanoscale, House of Lords, London, UK, 28 February 2011; BioCentre: London, UK, 2011. [Google Scholar]
- European Commission, Opinion on the appropriateness of the risk assessment methodology in accordance with the technical guidance documents for new and existing substances for assessing the risks of nanomaterials. Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR), Health & Consumer Protection Directorate-General: Brussels, Belgium, 2011.
- Gottschalk, F.; Sonderer, T.; Scholz, R.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef]
- Murr, L.; Esquivel, E.; Bang, J.; de la Rosa, G.; Gardea-Torresdey, J. Chemistry and nanoparticulate compositions of a 10,000 year-old ice core melt water. Water Res. 2004, 38, 4282–4296. [Google Scholar] [CrossRef]
- Mueller, N.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef]
- Koelmans, A.; Nowack, B.; Wiesner, M. Comparison of manufactured and black carbon nanoparticle concentrations in aquatic sediments. Environ. Pollut. 2009, 157, 1110–1116. [Google Scholar] [CrossRef]
- Salvetat, J.-P.; Bonard, J.-M.; Thomson, N.; Kulik, A.; Forr’o, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, A, 255. [Google Scholar]
- Sanera, B.; Dinça, F.; Yürüm, Y. Utilization of multiple graphene nanosheets in fuel cells: 2. The effect of oxidation process on the characteristics of graphene nanosheets. Fuel 2011, 90, 2609–2616. [Google Scholar] [CrossRef]
- Som, C.; Wick, P.; Krug, H.; Nowack, B. Environmental and health effects of nanomaterials in nanotextiles and façade coatings. Environ. Int. 2011, 37, 1131–1142. [Google Scholar] [CrossRef]
- Sobek, A.; Bucheli, T. Testing the resistance of single- and multi-walled carbon nanotubes to chemothermal oxidation used to isolate soots from environmental samples. Environ. Pollut. 2009, 157, 1065–1071. [Google Scholar] [CrossRef]
- Bom, D.; Andrews, R.; Jacques, D.; Anthony, J.; Bailin Chen, B.; Meier, M.; Selegue, J. Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: Evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett. 2002, 2, 615–619. [Google Scholar] [CrossRef]
- Vignes, A.; Dufaud, O.; Perrin, L.; Thomas, D.; Bouillard, J.; Janès, A.; Vallières, C. Thermal ignition and self-heating of carbon nanotubes: From thermokinetic study to process safety. Chem. Eng. Sci. 2009, 64, 4210–4221. [Google Scholar] [CrossRef]
- Liu, G.; Niu, Z.; Van Niekerk, D.; Xue, J.; Zheng, L. Polycyclic aromatic hydrocarbons (PAHs) from coal combustion: Emissions, analysis, and toxicology. Rev. Environ. Contam. Toxicol. 2008, 192, 1–28. [Google Scholar]
- Modina, H.; Perssona, K.; Andersson, A.; van Praaghe, M. Removal of metals from landfill leachate by sorption to activated carbon, bone meal and iron fines. J. Hazard. Mater. 2011, 189, 749–754. [Google Scholar] [CrossRef]
- Kennedy, A.; Hull, M.; Steevens, J.; Dontsova, K.; Chappell, M.; Gunter, J.; Weiss, C.J. Factors influencing the partitioning and toxicity of nanotubes in the aquatic environment. Environ. Toxicol. Chem. 2008, 27, 1932–1941. [Google Scholar] [CrossRef]
- Barnard, D. Sonochemical degradation of PAH in aqueous solution. Part I: Monocomponent PAH solution. Ultrason. Sonochem. 2009, 16, 260–265. [Google Scholar]
- lvarez, P.; García-Araya, J.; Beltrán, F.; Masa, F.; Medina, F. Ozonation of activated carbons: Effect on the adsorption of selected phenolic compounds from aqueous solutions. J. Colloid Interface Sci. 2005, 283, 503–512. [Google Scholar] [CrossRef]
- Jonsson, S.; Persson, Y.; Frankki, S.; van Bavel, B.; Lundstedt, S.; Haglund, P.; Tysklind, M. Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton’s reagent: A multivariate evaluation of the importance of soil characteristics and PAH properties. J. Hazard. Mater. 2007, 149, 86–96. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Zhu, C.; Sun, S. Microbial PAH-degradation in soil: Degradation pathways and contributing factors. Pedosphere 2006, 16, 555–565. [Google Scholar] [CrossRef]
- Johnsen, A.; Wick, L.; Harms, H. Principles of microbial PAH-degradation in soil. Environ. Pollut. 2005, 133, 71–84. [Google Scholar]
- Bianco, A.; Kostarelos, K.; Prato, M. Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. (Camb). 2011, 47, 10182–10188. [Google Scholar]
- Yan, J.; Cheng, S.; Zhang, X.; Shi, L.; Zhu, J. Effect of four metals on the degradation of purified terephthalic acid wastewater by Phanaerochaete chrysosporium and strain Fhhh. Bull. Environ. Contam. Toxicol. 2004, 72, 387–393. [Google Scholar]
- Koukouzas, N.; Katsiadakis, A.; Karlopoulos, E.; Kakaras, E. Co-gasification of solid waste and lignite—A case study for Western Macedonia. Waste Manag. 2008, 28, 1263–1275. [Google Scholar] [CrossRef]
- Zhao, Y.; Allen, B.; Star, A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A 2011, 115, 9536–9544. [Google Scholar] [CrossRef]
- Meyer, D.; Curran, M.; Gonzalez, M. An examination of existing data for the industrial manufacture and use of nanocomponents and their role in the life cycle impact of nanoproducts. J. Environ. Tech. 2009, 43, 1256–1263. [Google Scholar]
- Pol, V.; Thivagarajan, P. Remediating plastic waste into carbon nanotubes. J. Environ Monit. 2010, 12, 455–459. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manzetti, S.; Andersen, O. Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies. Challenges 2013, 4, 75-85. https://doi.org/10.3390/challe4010075
Manzetti S, Andersen O. Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies. Challenges. 2013; 4(1):75-85. https://doi.org/10.3390/challe4010075
Chicago/Turabian StyleManzetti, Sergio, and Otto Andersen. 2013. "Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies" Challenges 4, no. 1: 75-85. https://doi.org/10.3390/challe4010075
APA StyleManzetti, S., & Andersen, O. (2013). Carbon Nanotubes in Electronics: Background and Discussion for Waste-Handling Strategies. Challenges, 4(1), 75-85. https://doi.org/10.3390/challe4010075



