Abstract
Continuous flow chemical processes offer several advantages as compared to batch chemistries. These are particularly relevant in the case of heterogeneously catalyzed transformations of biomass-derived platform molecules into valuable chemicals and fuels. This work is aimed to provide an overview of key continuous flow processes developed to date dealing with a series of transformations of platform chemicals including alcohols, furanics, organic acids and polyols using a wide range of heterogeneous catalysts based on supported metals, solid acids and bifunctional (metal + acidic) materials.
1. Introduction
Fossil derived fuels (i.e., coal, natural gas and petroleum) currently supply most of the energy consumed on the planet. This high reliance on fossil resources represents a serious issue in terms of availability of resources, environmental pollution, and future development of society since these natural resources are highly contaminant, unevenly distributed around the world and they are in diminishing supply. These important concerns have stimulated the search for new well-distributed and non-contaminant renewable sources of energy including solar, wind, hydroelectric power, geothermal activity, and biomass. Biomass has attracted a great deal of interest in recent years as the only renewable source of organic carbon currently available on Earth. Consequently, it is considered to be the perfect replacement for petroleum in the production of fuels and chemicals [1].
In any case, the progressive replacement of petroleum with biomass involves a number of challenges in terms of processing approaches since both resources are diametrically opposed chemically speaking (Scheme 1). Petroleum is chemically composed of a mixture of hydrocarbons (e.g., lineal, cyclic, aromatics, alkenes, etc.) as opposed to highly functionalized biomass feedstocks which typically comprise highly-oxygenated compounds embedded in some cases in complex polymeric structures. As a result, the highly-optimised catalytic approaches developed over the past 50 years in the petrochemical industry cannot be directly projected to process biomass feedstocks. For example, while in the petrochemical industry the production of hydrocarbon fuels (e.g., diesel, gasoline and jet fuels) is a relatively simple process involving separation of hydrocarbons (by fractional distillation) and subsequent catalytic upgrading (to control molecular weight and structure), the conversion of biomass into fuels is typically associated with deep chemical changes and multistep processing [2]. The particular composition of biomass has also a decisive effect on catalysts design. Typical petroleum catalysts designed to resist high temperatures and hydrophobic environments might not be effective upon the new conditions required to process biomass.
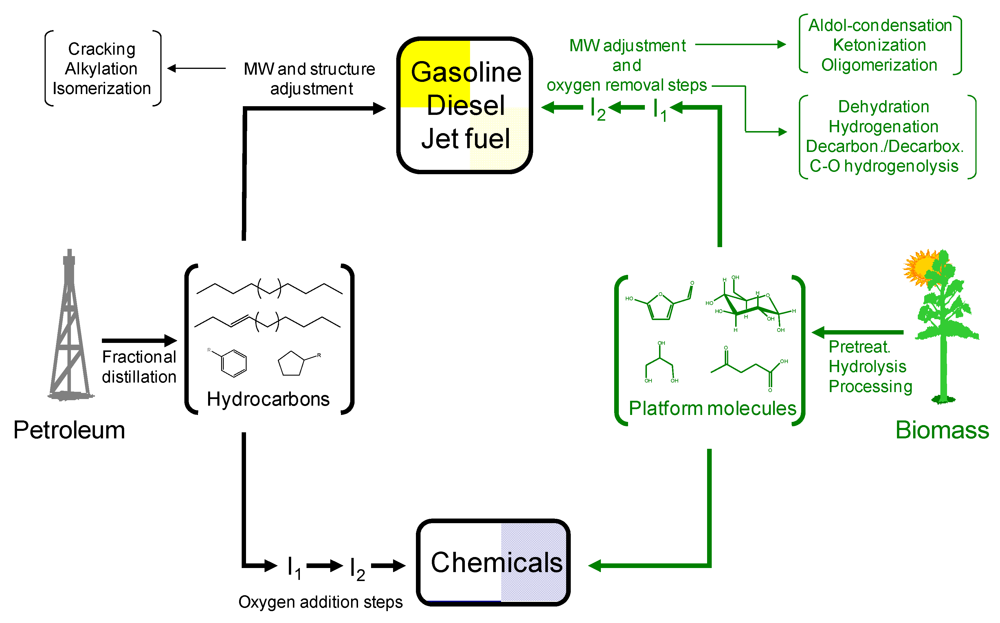
Scheme 1.
Divergences between petroleum and biomass processing to chemicals and liquid hydrocarbon transportation fuels. Reproduced by permission of the Royal Society of Chemistry from [2].
The high level of structural and chemical complexity of biomass is an important issue when envisioning new processes. One potential solution to overcome biomass complexity involves its conversion into simpler fractions which are more easily handled in downstream processes. This approach, resembling that utilized in the petrochemical industry, appropriately facilitates the simultaneous production of fuels, chemicals, and energy in a single facility denoted as biorefinery. As an important part of this biorefinery concept, a small group of biomass-derived molecules have been recently identified to be especially relevant according to a series of indicators [3]. These important biomass derivatives (the so-called platform molecules or building blocks) are relatively simple compounds containing multiple functionalities in their structures which are suitable for a range of chemical transformations to valuable compounds. Platform molecules are carefully selected on the basis of a series of factors including the availability of commercial technologies for their production and their platform potential for the simultaneous production of fuels and chemicals in biorefineries. Relevant examples of biomass platform molecules include sugars (glucose, xylose), polyols (sorbitol, xylitol, glycerol), furans (hydroxymethylfurfural or HMF, furfural), acids (lactic acid, levulinic acid) and alcohols (ethanol).
Controlling chemical reactivity represents an important challenge when operating with biomass derivatives. Overfunctionalization of many of these compounds typically leads to high (and sometimes uncontrolled) reactivity. Additionally, many of these molecules have a natural tendency to decompose with temperature.
New processing approaches are thus required to control reactivity in biomass derivatives thereby directing the conversion to the desired products. In this regard, continuous flow processing has a number of significant and inherent advantages for biomass processing as compared to batch reactor technologies:
- (1) Continuous flow processing allows a better of control of reaction conditions. This is advantageous when dealing with highly reactive feedstocks such as those derived from biomass. Furthermore, as will be shown in some of the examples detailed bellow, continuous approaches offer more flexibility to modify conditions through the course of a reaction thereby allowing an optimum control of intermediates in consecutive A → B → C type of reactions. Double-bed reactors, operating at different temperature conditions, could in principle be designed to achieve the desired and optimum control of reactivity in consecutive reactions involving several intermediates.
- (2) Flow processing also facilitates scaling up which is an important point taking into consideration that many of the biomass processes are still in the lab scale. The development of flow technologies will thus contribute to the commercialization of biomass technologies in the near future.
- (3) Since the chemical composition of biomass feedstocks is normally very different from that of the final products, multiple processing steps are typically required in such transformations, negatively affecting the economy of the process. The utilization of flow processing technologies allows intensification of the chemical processes, thereby significantly contributing to simplify technologies, as detailed in some of the examples of the present review.
- (4) Unlike batch processing, fixed-bed flow technologies do not require catalyst separation after reaction and regeneration, if required, is readily performed over the same catalytic bed. In the case of biomass processing, easy regeneration is crucial since the high reactivity of biomass derivatives typically leads to overreactions generating carbonaceous deposits and tars that poison and deposit on the catalysts surface.
- (5) Many of the biomass processes will require oxygen removal steps to produce the final product. Oxygen is generally removed from biomass molecules in the form of H2O or COx (e.g., CO and CO2). When operating under batch conditions, these gases build up in the reactor leading to increasing pressure and, potentially, new and uncontrolled processes. Flow operation allows continuous removal of these gases which may not interfere in the main catalytic process.
- (6) The microwave-to-flow paradigm, recently highlighted by Kappe’s group [4] is a smart approach to translating batch microwave chemistries to more scalable flow conditions upon mimicking the relatively high pressures and temperatures obtained in a microwave experiment in a continuous flow reactor equipped with a back pressure regulator [4]. The proposed methodology is envisaged to be particularly useful for biomass valorisation practises which could be in principle screened in a quick and efficient manner under microwave batch conditions and then translated to flow chemistry protocols after optimisation of reaction parameters, catalysts and conditions.
In this work, we aim to provide a general overview of the most relevant catalytic flow technologies developed (as well as under development) for the conversion of biomass platform molecules into fuels and chemicals. Important aspects such as the reactions conditions (pressure, temperature, space velocity) and the catalysts used are analyzed, with the platform potential for each biomass derivative discussed in the different sections. It is important to note that most of the processes to convert biomass into platform molecules are based on biological or chemical batch technologies. Consequently, many of the flow processes herein described require a previous batch conversion step. Even though an entire continuous process from biomass to the final product would be highly desirable in terms of scalability, the transformation of batch processes into continuous technologies would involve strong processing challenges, especially for those molecules produced by biological routes.
2. Continuous-Flow Transformations of Biomass Derivatives into Fuels and Chemicals
2.1. Ethanol
Ethanol is the predominant biomass-derived fuel at the present time representing 80% of the world biofuel production. Ethanol is added to conventional gasoline to improve the combustion of the mixture, decreasing the generation of pollutants such as CO, NOx and SOx. A very simple fermentation technology along with a partial compatibility with the existing infrastructure for gasoline have allowed ethanol industry to grow fast, and production reached 87 billion L in 2010 [5]. Ethanol is thus likely to become a cheap and abundant renewable resource taking into consideration that technologies for the conversion of lignocellulosic feedstocks (more abundant that currently used starches and edible sugars) are under extensive investigation and may become economically feasible in the near future [3]. However, ethanol possesses important limitations as a fuel namely, low energy density (23.4 MJ/L vs. 34.4 MJ/L of gasoline, which negatively affects the gas mileage of vehicles), and serious compatibility issues with the gasoline infrastructure (only dilute blends are tolerated by current gasoline vehicles because of corrosiveness and water absorption issues of ethanol).
Apart from its use as a biofuel, ethanol possesses an enormous potential as a chemical feedstock. A number of valuable chemicals can be derived from ethanol by using continuous-flow catalytic technologies (Figure 1). Steam reforming is the most studied continuous catalytic process of ethanol which allows the gasification of aqueous solutions of ethanol into hydrogen-rich mixtures at high temperatures (typically 600–800 °C) and atmospheric pressure using Ni, Co and noble metals (e.g., Pt, Pd, and Rh) supported on stable oxides [6,7]. Hydrogen is co-produced along with CO2 with a maximum yield of 6 moles of gas per mol of ethanol fed into the process, although this theoretical maximum is never reached since by-products including CH4 (produced by hydrogenation of CO and CO2 reforming products over metal catalysts) are typically produced at steam reforming conditions. The main challenge of these technologies lies in a careful control over the chemistry of the process, which is challenging at the high temperatures required to reform ethanol.
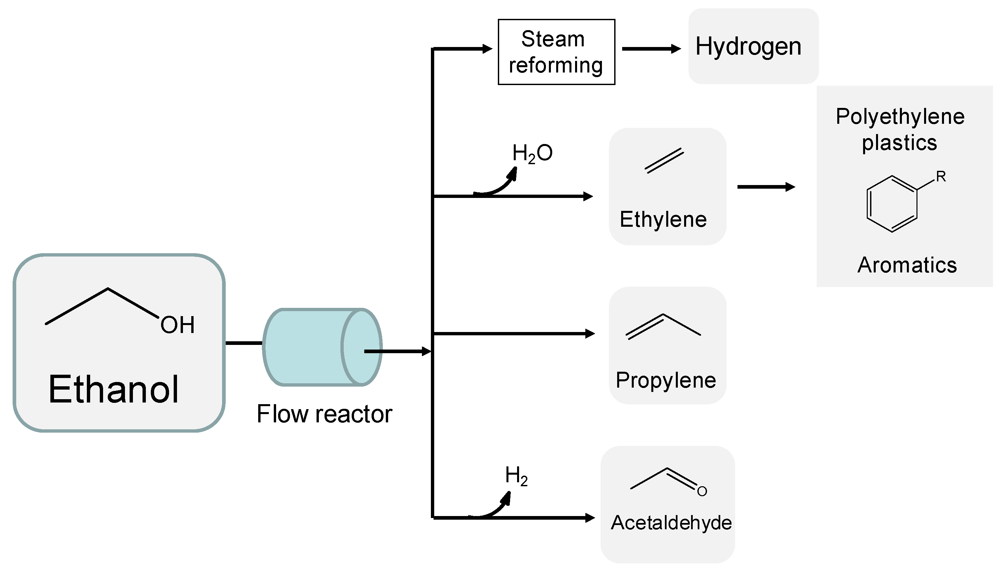
Figure 1.
Production of fuels and high added value chemicals from ethanol by flow catalytic processing. Adapted from [2]. Reproduced by permission of the Royal Society of Chemistry.
A proper control of reaction temperature and space velocity conditions has been found to be crucial to minimise hydrogen-consuming reactions (e.g., methanation) which in turn leads to acceptable hydrogen yields. The catalysts formulation also plays an important role determining the outlet gas composition in steam reforming technologies. For example, metal-oxide supports able to activate water (ZnO, CeO2, and La2O3) are typically employed as supports of metal catalysts to favor water-gas shift processes (WGS, CO + H2O→ CO2 + H2) which lead to purified H2 streams with very low levels of CO to be potentially relevant in fuel cell applications. These materials also possess basic characteristics avoiding coke-forming polymerization reactions (typically catalyzed on acidic sites) and improving the stability of the catalysts. Double-bed continuous approaches are utilized to enhance hydrogen yields in ethanol reforming processes. This proposed strategy allows the use of different catalytic beds operating at fixed optimum conditions which is advantageous to avoid undesirable processes and to increase stability. For example, dehydrogenation of acetaldehyde is favored with respect to ethylene dehydration employing a double-bed catalytic configuration with a first bed of a Cu-containing catalyst operating at low temperatures (e.g., 300–400 °C). The intermediate acetaldehyde species is subsequently passed through a second bed containing a reforming catalysts (e.g., Ni-based material) operating at higher temperatures [8]. In this way, subsequent polymerization of organic species to carbonaceous deposits (coke) is minimized. This concept enhances hydrogen yields while preserving the stability of the catalysts via minimization of coke-generating reactions.
There are a number of emerging processes for the continuous flow conversion of ethanol into valuable chemicals which in any case have been less investigated compared to reforming reactions (Figure 1). Bio-ethanol can be readily dehydrated into bio-ethylene with quantitative yields using acidic catalysts [9]. This process is of significant importance as ethylene is the most produced organic compound worldwide (annual production 120 million tons) and one of the seven primary building blocks of the petrochemical industry. With current growing oil prices, ethanol could potentially serve as a renewable and cheap feedstock for the production of polyethylene plastics, and commercial plants are already operative in Brazil with a capacity to produce 200,000 tons per year of green polyethylene from sugar cane ethanol [10].
Important aromatic compounds including benzene, toluene, and xylene (BTX, base of the petrochemical industry) could potentially be generated from as-produced bio-ethylene in the bioethanol dehydration process. However, the controlled oligomerization of ethylene is quite challenging and a good deal of research will be required to identify suitable catalysts (with optimum acid strength and porosity properties) and reaction conditions to yield BTX products in acceptable quantities. Alternatively, the generated bio-ethylene could be partially dimerized to butene, with the mixture subsequently undergoing methathesis to form propylene, the second most important starting product in the petrochemical industry after ethylene. Interestingly, the process has been recently patented [11]. The main challenge of this technology for the use of bioethanol lies in the extremely high purity ethylene required to perform the methathesis reaction which requires catalysts that are highly-sensitive to nitrogen and sulfur impurities (typically present in bioethanol feeds). This will require an extensive investigation on catalyst design and development under conditions and purities present in bioethanol feedstocks.
Apart from olefins, important C2 commodity chemicals such as acetaldehyde can be also continuously derived from ethanol with 100% selectivity. The process involves the dehydrogenation of ethanol over inexpensive Cu catalysts at mild conditions (low temperatures and ambient pressure) and it represents an attractive route to co-produce a large-scale consumed chemical (acetaldehyde) as well as hydrogen in a single catalytic bed [12]. The process has not been commercially exploited so far, since stability issues associated with Cu sintering still have to be resolved.
2.2. Furans
Biomass-derived sugars, obtained from cellulose, hemicellulose or starches, can be dehydrated to form furan compounds including hydroxymethyl furfural (HMF) and furfural typically in aqueous mineral acids such as HCl or H2SO4 and/or solids acids which allow a facile separation and recycling of the catalysts during the dehydration process. The industrial production of furfural is a well established batch process that benefit from the easy depolymerization of hemicelluloses to C5 sugars (mainly xylose). Furfural production reaches 200,000 tons per year being one of the most common industrial chemicals derived from biomass. Comparatively, HMF requires deconstruction of highly-recalcitrant and crystalline cellulose via an additional glucose isomerization step to fructose, which is more easily converted into the furanic intermediate.
Figure 2 summarizes the most relevant continuous flow catalytic processes for the conversion of furfural and HMF into fuels and chemicals. HMF can be converted into biofuel additives via hydrogenation of the carbonyl group and subsequent C−O hydrogenolysis of the hydroxyl intermediate dihydroxymethyl furan (DHMF) in a cascade-type continuous flow process reported by Dumesic and co-workers [13]. The process utilizes a Cu-based catalyst under hydrogen pressure to achieve removal of OH groups while preserving the C=C bonds in the furanic ring. The final product, dimethylfuran (DMF), possesses excellent energy-density and boiling point characteristics to be used as a transportation liquid fuel 100% compatible with current hydrocarbon-based infrastructure. Continuous flow conditions are finely adjusted to allow carbonyl hydrogenation and OH hydrogenolysis to take place in a single step. Interestingly, the utilization of continuous flow conditions avoids the potential poisoning of the Cu catalysts by the chlorides used to salt out HMF from the aqueous phase. Vapor phase hydrogenolysis can be carried out in a flow mode circumventing NaCl contact with the catalyst bed which would not be possible in the case of batch processing.
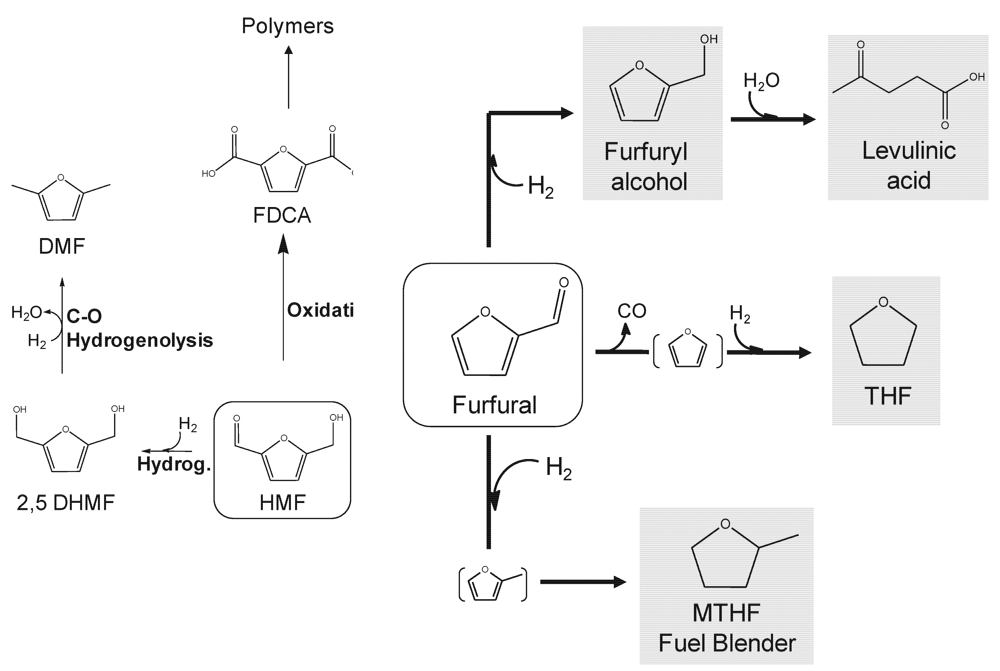
Figure 2.
Hydroxymethylfurfural (HMF) and furfural as platform molecules for the production of high-added value chemicals and biofuels by flow catalytic processing. Adapted from [2]. Reproduced by permission of the Royal Society of Chemistry.
HMF derivatives such as 2,5-furandicarboxylic acid (FDCA) can also find markets in the production of biopolymers as replacement of petroleum‑derived terephtalic acid (Figure 2). FDCA can be synthesized from HMF by catalytic oxidation under pressurized air/oxygen at mild temperature conditions (100–150 °C) using metal catalysts. Even though the majority of studies used batch‑pressurized reactors to perform oxidation, this process has been recently scaled up to bench‑scale flow conditions by Gray and coworkers [14]. These authors found FDCA yields to be highly dependent on the employed oxidant (e.g., air, oxygen), the pH of the HMF aqueous solution used as feed, the nature of the metal support, and the pressure and temperature conditions in the reactor.
Similarly, furfural possesses a great platform potential for the simultaneous production of fuels and important chemicals in biorefineries (Figure 2). Hydrogenation of furfural is able to provide a range of valuable products by a number of technologies with the potential to be developed in a continuous operating mode. In particular, reduction at mild conditions (e.g., 120 °C, atmospheric pressure) using transition metal catalysts based on Cu yields furfuryl alcohol [15], a compound highly-demanded for the production of foundry resins. Furfuryl alcohol can additionally be hydrolyzed under acid conditions producing levulinic acid (4-oxopentanoic acid), an important biomass platform molecule that will be subsequently discussed. Decarbonylation was found to be prevalent over Pd-based catalysts (even at high space velocities) producing tetrahydrofuran (THF) after hydrogenation [16]. THF is a common organic solvent and precursor of polymers with an annual production that reaches 200,000 tons. This reaction was performed under microwave heating and formic acid as a hydrogen source [17], which is currently being translated into more scalable continuous flow processes by mimicking the moderate to high temperature achieved in the sealed microwave vessel using an X-Cube flow reactor [18].
More severe hydrogenation conditions (e.g., higher pressures, utilization of noble metal catalysts) favor a complete hydrogenation of the furanic ring to afford methyl tetrahydrofuran (MTHF). MTHF is a hydrophobic molecule which, unlike ethanol, can be blended with gasoline up to 60% (v/v) without adverse effects on engine performances or gas mileage. The most relevant hydrogenation routes to MTHF employing continuous flow reaction systems have been recently reviewed by Lange and coworkers [19], which explore the potential of furfural for the production of transportation biofuels.
2.3. Organic Acids
Organic acids are common biomass intermediates and some of the most relevant biomass platform molecules [20]. Carboxylic acids are present in certain quantities in biomass liquids (e.g., bio-oils) and many known biological routes are able to convert biomass sugars into carboxylic acids. Among the large number of biomass acids, this section will summarize the potential to produce fuels and chemicals by means of continuous flow catalytic technologies of two of them, namely, lactic acid (2‑hydroxypropanoic acid, LA) and levulinic acid (4-oxopentanoic acid, LVA). These two acids have been selected on the basis of their potential to be exploited as feedstocks under flow conditions.
Lactic acid is the most widely occurring carboxylic acid in nature. It is mainly produced by bacterial fermentation of biomass sugars in batch reactors. Traditional uses of lactic acid in the food industry (as additives) have been complemented by new recently developed applications in the fields of specialty chemicals and polymers for this platform molecule [21]. The improvement of existing fermentation and separation technologies, as well as the development of non-biological routes for the conversion of aqueous sugars into lactic acid, and the aforementioned novel applications of bio-lactic acid have considerably increased the platform potential of this molecule in recent years [22].
The special chemical composition of LA (containing two functional –OH and –COOH groups in a small molecule of only 3 carbon atoms) provides rich and versatile chemistry that allows a variety of flow catalytic transformations to useful products (Figure 3). LA is readily decarbonylated to acetaldehyde using metal catalysts under flow conditions, with the process being reported to accelerate in the presence of acidic catalysts [23,24]. This tendency of LA to acid-catalysed decomposition into acetaldehyde can be utilized to design novel routes to produce advanced biofuels (e.g., higher alcohols). LA readily dehydrates to produce acrylic acid (monomer employed to manufacture acrylic fibers) when strong dehydration conditions are applied [25]. The acidic strength of the catalyst as well as the residence time of LA in the fixed catalytic bed have been reported to be key aspects determining the acrylic/acetaldehyde molar ratio in flow experiments. This continuous flow route allows the production of propanoic acid when operating under hydrogen conditions and in the presence of bifunctional (metal and acid site) catalysts [26]. Propanoic acid is typically used as a preservative in foodstuffs [27].
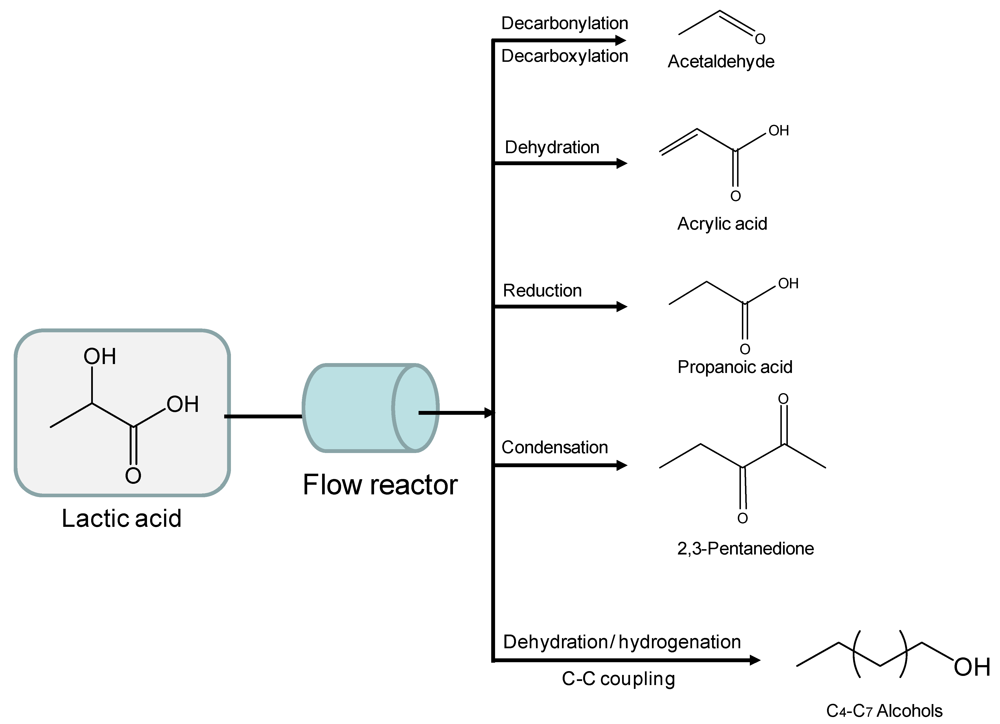
Figure 3.
High-added value chemicals and fuels derived from various transformations of lactic acid. Adapted from [2]. Reproduced by permission of the Royal Society of Chemistry.
Gunter and coworkers detailed a novel route to upgrade LA which involves acid dehydration and subsequent condensation of intermediates on phosphate catalysts at moderate temperatures (e.g., 280–350 °C) [28]. The process produces 2,3 pentanedione which is a high valuable fine chemical currently obtained in limited amounts. These authors found that temperatures up to 320 °C and high residence times favored such condensation product as compared to shorter residence times and higher temperatures that lead to acrylic acid and acetaldehyde as main products. Since equimolar amounts of CO2 and pentadione are generated in the process, a Claysen type condensation mechanism followed by internal molecular restructuring of the adduct is proposed to explain the formation of this interesting product (Scheme 2).
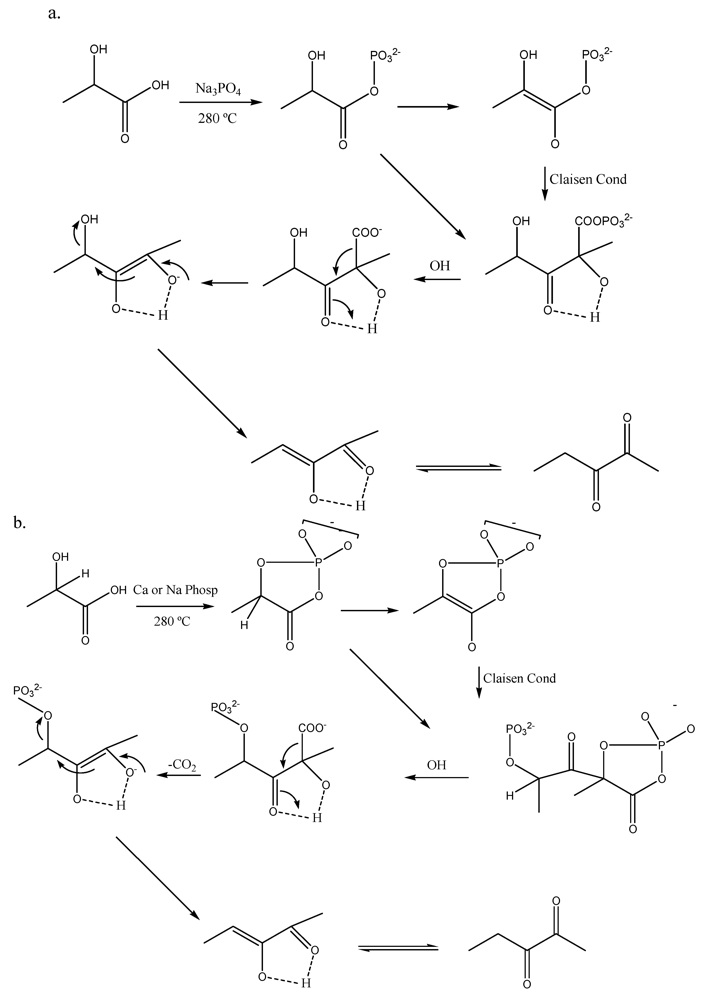
Scheme 2.
Proposed mechanisms for 2,3-pentanedione formation from lactic acid using phosphate catalysts. Adapted from [28].
One of the main operating issues of lactic acid lies in its high and sometimes uncontrolled reactivity which typically lead to a mixture of products upon reaction. Recently, a new flow catalytic approach involving oxygen removal (to control the activity of lactic acid) followed by C−C coupling reactions (e.g., aldol-condensation and ketonization) of intermediates has been proposed to upgrade lactic acid to alcohols in the C4−C7 range suitable as gasoline-compatible biofuels (Figure 3) [29]. The process makes use of a bi-functional Pt/Nb2O5 catalytic bed (with the catalyst containing metal and acidic sites) and moderate temperature and pressures, typically 350 °C and 50 bar. The removal of oxygen from lactic acid is favored by the use of bi-functional catalysts through a cycle of dehydration (on the acid sites of Nb2O5) and hydrogenation (catalyzed by platinum), leading to the production of two monofunctional intermediates, namely acetaldehyde and propanoic acid. These monofunctional compounds, along with gaseous CO and CO2, are the primary products observed when aqueous solutions of lactic acid were converted using Pt/Nb2O5. An increase in conversion (by increasing temperature or decreasing space velocity) leads to the onset of an organic layer that spontaneously separates from water. Analysis of the organic phase showed that it was mainly composed of propanoic acid and ketones in the C4−C7 range. Interestingly, this organic layer was not observed when a control catalyst supported on inert carbon black (Vulcan) was employed in the reaction. Approximately 50% of the carbon in the feed can be stored in this organic layer when optimum reaction conditions and concentrated solutions of lactic acid are used. The chemistry involved in the formation of the higher ketones is explained on the basis of the condensation of acetaldehyde and propanoic acid by aldol‑condensation and ketonization [30] reactions, respectively, both taking place over the niobia support (Scheme 3). As in the case of ethanol steam reforming (Section 2.1), double bed catalytic flow approaches can be combined to improve yields to ketonization products. A second bed of ceria‑zirconia (following that of Pt/Nb2O5) operating at the same conditions of pressure and temperature could achieve a complete ketonization of the remaining unconverted propanoic acid in the first bed [26]. The organic layer obtained in this process can be separated into its components, serving as a source of valuable chemicals. Alternatively, the oil can be treated with Ru/C catalysts to convert the ketones into the corresponding alcohols without any needs of separation. This liquid mixture of alcohols in the C4−C7 range can be used as a high-energy density liquid fuel for the transportation sector. Concentrated aqueous solutions of lactic acid (e.g., 60 wt%) can be efficiently processed over Pt/Nb2O5 with good stability for more than two days on stream under continuous flow conditions.
As indicated in section 2.2, the treatment of biomass-derived hexoses at moderate temperatures under aqueous acid conditions leads to the production of HMF. This compound is, however, highly reactive and readily decomposes into an equimolar mixture of formic and levulinic acids (FA, LVA). Technologies such as the Biofine process [31] make the most of this process for the industrial production of LVA from inexpensive lignocellulosic waste including paper mill sludge, urban waste and agricultural residues. LVA could be also potentially obtained from less-recalcitrant hemicellulose (but less abundant and with a more heterogeneous composition) via dehydration of pentoses to furfural and subsequent hydrogenation to furfuryl alcohol (Figure 2).
A number of recent flow catalytic technologies have been developed to upgrade LVA into liquid transportation fuels (Figure 4). The key intermediate in many of these technologies is γ valerolactone (GVL) which is produced via hydrogenation of LVA over non-acidic metal catalysts such as Ru/C achieving near quantitative yields of GVL at mild temperatures (e.g., 150 °C) [32,33]. Interestingly, the formic acid co-produced in the sugar dehydration process can serve as hydrogen source for LVA reduction to GVL, thereby avoiding utilization of external fossil-fuel derived hydrogen [34]. Several flow catalytic routes have been recently developed to upgrade GVL into advanced biofuels, that is, biofuels with high energy densities and 100% compatible with the current transportation infrastructure. For example, GVL can be readily converted to MTHF via hydrogenation with intermediate formation of 1,4-pentanediol (Figure 4) [35]. MTHF is a highly interesting fuel which can be implemented in gasoline engines without the need of drastic changes in vehicles.
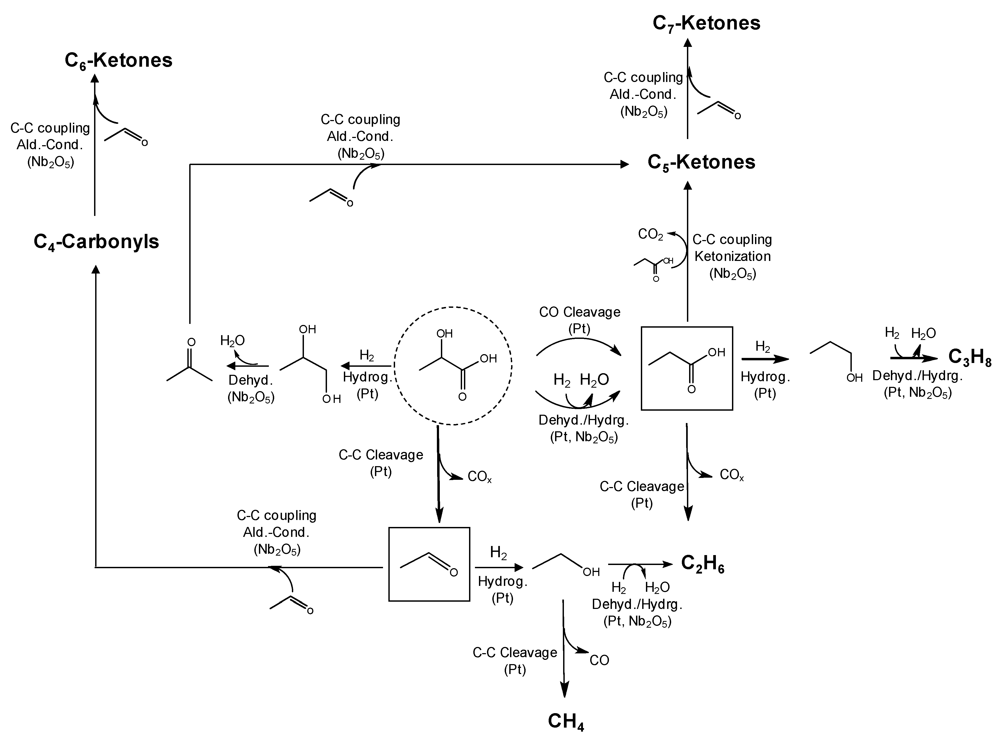
Scheme 3.
Proposed reaction scheme for 2‑hydroxypropanoic acid (LA) catalyzed transformation into C5−C7 ketones using Pt/Nb2O5 as catalyst. Copyright Wiley–VCH Verlag GmbH & Co.KGaA. Reproduced with permission from [29].
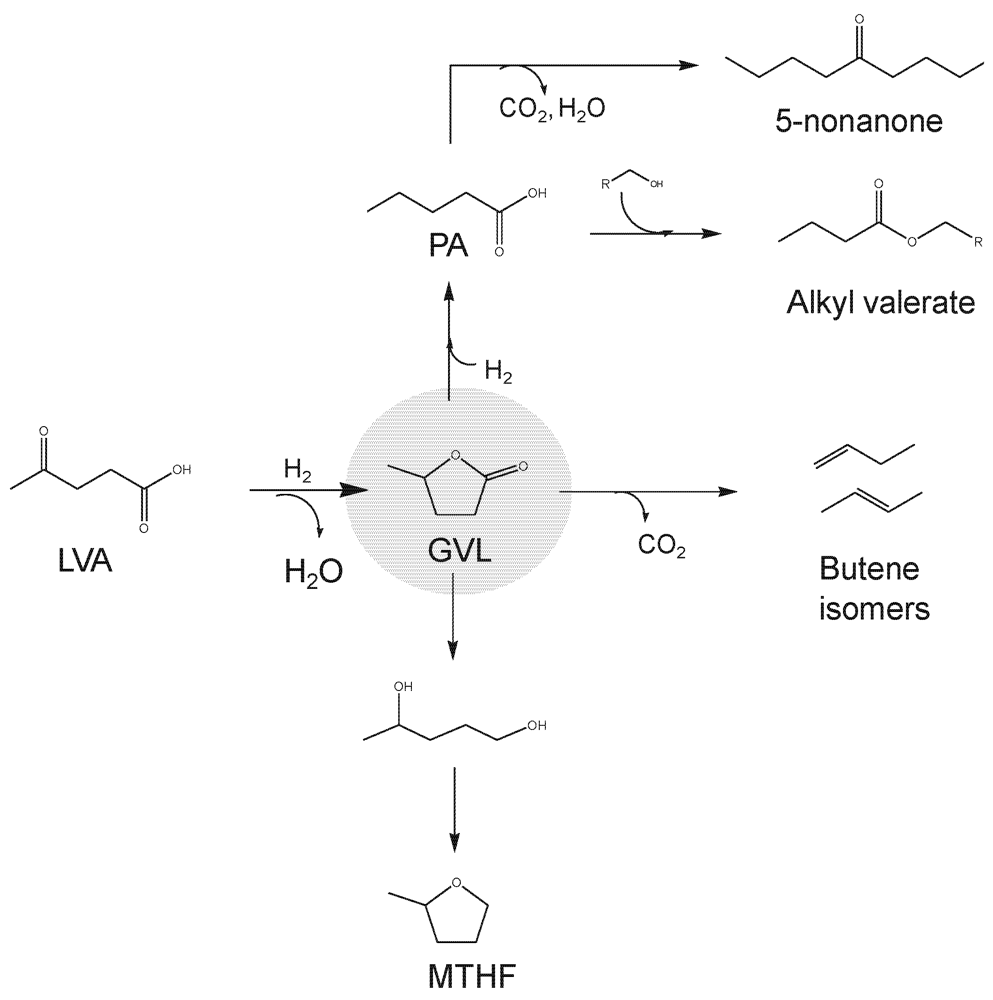
Figure 4.
Levulinic acid and γ valerolactone (GVL) as platform molecules for the production of liquid transportation biofuels. Adapted from reference 2. Reproduced by permission of the Royal Society of Chemistry.
An alternative route that is currently gaining momentum involves the conversion of GVL into pentanoic acid (PA) by means of ring-opening (on acid sites) and hydrogenation (on metal sites) reactions employing bifunctional heterogeneous catalysts at moderate temperatures and pressures. Lange and co-workers have recently exploited this route to continuously produce the so-called valeric biofuels (i.e., alkyl valerates), a novel family of lignocellulosic biofuels with excellent energy density, polarity and volatility ignition properties to be used in conventional engines without any modifications [36]. Excellent yields of PA (92%) can be achieved from aqueous solutions (e.g., 50 wt%) of LVA over Pd/Nb2O5 operating in flow mode [37]. Remarkably, PA was ketonized to 5-nonanone over the niobic support with yields up to 60% when the space velocity in the flow reactor was reduced to the level of WHSV = 0.1 h−1, This yield is however limited by the large number of processes involved which prevent a proper control of intermediate steps (LVA to PA to 5-nonanone). As in the case of lactic acid, one solution to overcome this limitation involves the utilization of double-bed flow reactors in which conditions of each bed are independently adjusted to maximize yields of intermediate steps.
For example, a double-bed configuration of Pd/Nb2O5 (at 275 °C) + Ce0.5 Zr0.5O2 (at 425 °C) allows aqueous solutions of GVL to be transformed into 5-nonanone with yields of 90% [38]. In this configuration, the first bed achieves conversion of PA to GVL which is subsequently converted into 5‑nonanone in the second bed in excellent final yields. Such C9 ketone can serve as a platform molecule for the production of hydrocarbon fuels with molecular weight and structures adequate for gasoline, diesel and jet fuels applications [39].
Similar dual continuous flow reactor approaches have been designed to upgrade aqueous solutions of GVL into jet fuels through the formation of C4 alkenes (Figure 4) [40]. The first flow reactor is loaded with acidic silica-alumina which achieves decarboxylation of GVL to butenes which are subsequently oligomerized to a distribution of alkenes centered at C12 using an acidic Amberlyst catalyst. In this case, the double bed configuration is not feasible since water has to be removed before entering the second reactor to achieve an effective oligomerization of butenes. The flow process is simple and achieves GVL deoxygenation and subsequent C−C coupling in a clean (CO2 can be efficiently sequestrated at the system pressure) and cheap (no external hydrogen required) fashion.
2.4. Polyols and Sugars
Glycerol (1,2,3-propanetriol) is perhaps one of the most relevant biomass derivatives whose importance as a renewable platform molecule has dramatically increased in recent years [41]. It is produced in large amounts in biodiesel facilities (100 kg of glycerol per ton of biodiesel) as byproduct of the transesterification of vegetable oils and animal fats with methanol for biodiesel production. Consequently, novel technologies to upgrade this molecule into fuels and chemicals are highly desirable.
Glycerol possesses a rich chemistry derived from the three hydroxyl groups. With regard to flow catalytic routes, the most relevant processes are depicted in Figure 5. As in the case of ethanol, glycerol can serve as a good source of hydrogen by means of steam reforming processes over metal catalysts [42]. Aqueous glycerol solutions can be gasified to CO, H2 and CO2 at mild temperatures (200−280 °C) and with higher hydrogen yields as compared to ethanol reforming. Additionally, as indicated in Figure 5, the process is more versatile and reaction conditions and catalytic materials can be carefully selected to transform concentrated aqueous solutions of glycerol into either syngas (a valuable mixture of CO and H2) for Fischer-Tropsch (F-T) or methanol syntheses [43]. Alternatively, H2-enriched streams can also be obtained by coupling APR with water gas-shift (WGS) processes [44] using double-bed flow reactors.
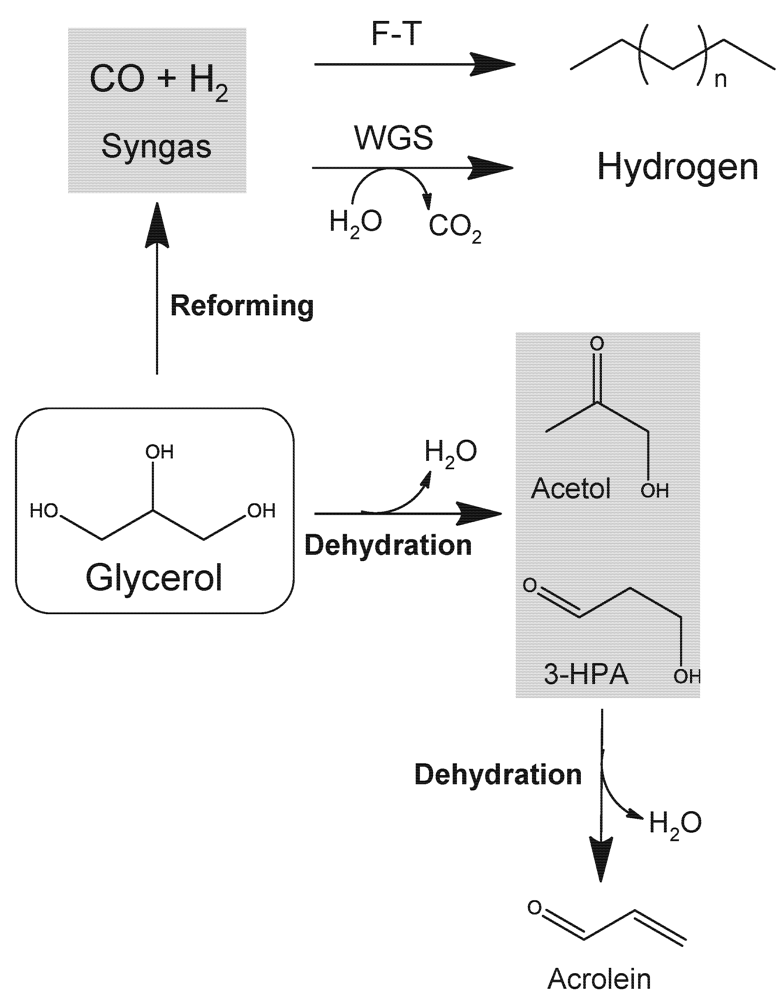
Figure 5.
Main routes for the flow catalytic transformation of glycerol into fuels and chemicals. Adapted from [2]. Reproduced by permission of the Royal Society of Chemistry.
Glycerol can also be dehydrated to acrolein using strong solid acids (ZrO2-WO3) as catalysts [45]. Acrolein is an important intermediate in the production of acrylic acid which is polymerized to acrylic resins used as superabsorber polymers in diapers and related products. Proper control of temperature and space velocity conditions in the flow reactor allows the production of acrolein in acceptable yields, although unwanted reactions including polymerization and cracking (leading to catalyst deactivation) are difficult to avoid at the high-acidic conditions.
A two-step flow catalytic process has been recently designed to convert biomass sugars and polyols in form of aqueous solutions into liquid hydrocarbon fuels of diverse classes [46]. In a first step, sugars and polyols are partially deoxygenated over a Pt-Re/C catalyst at temperatures near 225 °C to yield a mixture of monofunctional hydrocarbons in the C4−C6 range (including acids, alcohols, ketones and heterocycles) which are stored in an organic phase that spontaneously separates from water. The final composition of this organic stream is determined by pressure, temperature and WHSV conditions employed in the flow reactor. Subsequently, the organic stream of monofunctional compounds could be converted into targeted liquid hydrocarbon fuels of different classes through C−C coupling reactions including aldol-condensations and ketonizations.
2.5. Synthesis Gas
As in the case of fossil coal and natural gas, biomass can be gasified under controlled oxidant atmosphere to produce a mixture of CO and H2 denoted as synthesis gas (syngas) which is precursor of fuels and chemicals by well-established continuous flow technologies. The utilization of biomass for syngas generation (in substitution of classical feeds such as coal and natural gas) is associated with a number of processing challenges namely, the control over the H2/CO molar ratio (since biomass is highly oxygenated as compared to fossil fuels), and the strict cleaning standards required for the biomass derived syngas (extremely high purity is required to downstream processing to fuels and chemicals) [47]. Biomass-derived syngas, once cleaned and with appropriate H2/CO ratio, serves as feedstock for liquid hydrocarbon fuels (e.g., gasoline, diesel and jet fuel) by well-known Fischer-Tropsch (F-T) technologies [48]. This route is commonly denoted as biomass to liquids (BTL) and could be considered as the renewable version of coal to liquids (CTL) and gas to liquids (GTL) related processes. Commercialization of BTL technologies is being hindered by the elevated cost of the process, although some commercial technologies such as Primus Green Energy [49] and Sundrop fuelsTM [50] are currently produced fuels on a large scale. Syngas can be also converted into methanol by methanol synthesis approaches [51], and this methanol subsequently transformed into gasoline by the Mobil´s methanol to gasoline process [52] or improved TIGAS technologies from Topsoe [53]. These alternative processes can help to improve the economics of the BTL route which is, by far, the main limitation of the process.
3. Conclusions and Future Prospects
Continuous flow chemical processes for biomass valorization to fuels and chemicals hold significant potential for future development in our aim to drive our chemistries to more efficient and scalable approaches, while being environmentally sound and sustainable at the same time. The highlighted examples demonstrate the potential of a range of transformations of biomass-derived platform molecules under continuous flow conditions and heterogeneous catalysis. Several high added value chemicals and biofuel precursors can be obtained using different continuous flow chemical methodologies which possess already established markets and developed applications to replace fossil-derived commodities. Many of these and related routes to convert platform molecules into valuable end products under continuous flow conditions offer a significant industrial potential, with some already being developed or under development, taking advantage of the important benefits of continuous flow processing. We envisage a series of topics to be part of future key investigations in the implementation of continuous flow chemical processing of biomass feedstocks:
- - Design of novel flow processes for an efficient and effective biomass conversion
- - Design of water-tolerant and stable catalysts able to perform aqueous chemistries in high yields to products controlling the selectivity and reactivity of biomass-derived intermediates
- - Development of low environmental impact technologies based on multi-step reactors, cheap and readily available transition metal (bifunctional) catalysts, mild reaction conditions, etc.
Regardless of the industrial potential benefits of the implementation of continuous flow processes in biomass valorization practices, the environmental advantages of these methodologies in the processing of platform chemicals has to be taken into account. In this regard, continuous flow processing is the future of biomass valorization practices and we hope this manuscript can serve as momentum to both academia and industry to join efforts in order to carry on designing flow processes for biomass processing envisaging their implementation at industrial scale.
Acknowledgments
RL gratefully acknowledges Ministerio de Ciencia e Innovación, Gobierno de España for the concession of a Ramon y Cajal contract (ref. RYC-2009-04199) and funding under projects CTQ–2010-18126 and CTQ2011-28954-C02-02 (MICINN) as well as Consejeria de Ciencia e Innovación, Junta de Andalucía for funding under project P10-FQM-6711.
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar]
- Serrano-Ruiz, J.C.; Luque, R.; Sepúlveda-Escribano, A. Transformations of biomass-derived platform molecules: From high added-value chemicals to fuels via aqueous-phase processing. Chem. Soc. Rev. 2011, 40, 5266–5281. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar]
- Glasnov, T.N.; Kappe, C.O. The microwave-to-flow paradigm: Translating high-temperature batch microwave chemistry to scalable continuous-flow processes. Chem. Eur. J. 2011, 17, 11956–11968. [Google Scholar] [CrossRef]
- Ethanol producer magazine, International Ethanol Report 2010. Available online: http://www.ethanolproducer.com/articles/6696/international-ethanol-report-2010 (accessed on 10 May 2012).
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current status of hydrogen production techniques by steam reforming of ethanol: A review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A review on reforming bio-ethanol for hydrogen production. Int. J. Hydrog. Energy 2007, 32, 3238–3247. [Google Scholar]
- Batista, M.S.; Assaf, E.M.; Assaf, J.M.; Ticianelli, E.A. Double bed reactor for the simultaneous steam reforming of ethanol and water gas shift reactions. Int. J. Hydrog. Energy 2006, 31, 1204–1209. [Google Scholar]
- Bedia, J.; Barrionuevo, R.; Rodríguez-Mirasol, J.; Cordero, T. Ethanol dehydration to ethylene on acid carbon catalysts. Appl. Catal. B 2001, 103, 302–310. [Google Scholar]
- Ethanol producer magazine, Braskem starts up ethanol-to-ethylene plant. Available online: http://www.ethanolproducer.com/articles/7022/braskem-starts-up-ethanol-to-ethylene-plant (accessed on 10 May 2012).
- Takai, T.; Mochizuki, D.; Umeno, M. Method of producing propylene containing biomass-origin carbon. European Patent Application EP1953129 (A1), 2008. Mitsui Chemicals Inc.. [Google Scholar]
- Tu, Y.; Chen, Y. Effects of alkaline-earth oxide additives on silica-supported copper catalysts in ethanol dehydrogenation. Ind. Eng. Chem. Res. 1998, 37, 2618–2622. [Google Scholar]
- Roman-Leshkov, Y.; Barrett, C.J.; Liu, Z.Y.; Dumesic, J.A. Production of dimethylfuran for liquid fuels from biomass-derived carbohydrates. Nature 2007, 447, 982–986. [Google Scholar]
- Lilga, M.A.; Richard, T.H.; Gray, M. Production of Oxidized Derivatives of 5-Hydroxymethylfurfural (HMF). Top. Catal. 2010, 53, 1264–1269. [Google Scholar]
- Surapas, S.; Pham, T.; Prasomsri, T.; Sooknoi, T.; Mallinson, R.G.; Resasco, D.E. Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts. J. Catal. 2011, 280, 17–27. [Google Scholar]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of furfural over supported metal catalysts: A comparative study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- García-Suárez, E.J.; Balu, A.M.; Tristany, M.; García, A.B.; Philippot, K.; Luque, R. Versatile dual hydrogenation–oxidation nanocatalysts for the aqueous transformation of biomass-derived platform molecules. Green Chem. 2012, 14, 1434–1439. [Google Scholar] [CrossRef]
- X-Cube™—Catalysis Made Simple. ThalesNano Nanotechnology Inc: Graphisoft Park, Hungary, 2009. Available online: http://www.thalesnano.com/products/x-cube (accessed on 10 May 2012).
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass. Volume 1—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy: Washington, DC, USA, 2004; pp. 1–67. [Google Scholar]
- Fan, Y.; Zhou, C.; Zhu, X. Selective catalysis of lactic acid to produce commodity chemicals. Catal. Rev. 2009, 51, 293–324. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 2010, 328, 602–605. [Google Scholar]
- Mok, W.S.; Antal, M.J.; Jones, M. Formation of acrylic acid from lactic acid in supercritical water. J. Org. Chem. 1989, 54, 4596–4602. [Google Scholar]
- Gunter, G.C.; Langford, R.H.; Jackson, J.E.; Miller, D.J. Catalysts and supports for conversion of lactic acid to acrylic acid and 2, 3-pentanedione. Ind. Eng. Chem. Res. 1995, 34, 974–980. [Google Scholar]
- Sawicki, R.A. Catalyst for Dehydration of Lactic Acid to Acrylic Acid. U.S. Patent 4729978, 1998. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic upgrading of lactic acid to fuels and chemicals by dehydration/hydrogenation and C–C coupling reactions. Green Chem. 2009, 11, 1101–1104. [Google Scholar]
- Samuel, U.R.; Kohler, W.; Gamer, A.O.; Keuser, U. Propionic acid and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; VCH Publishers Inc: Weinheim, Germany, 1993. [Google Scholar]
- Gunter, G.C.; Miller, D.J.; Jackson, J.E. Formation of 2,3-pentanedione from lactic acid over supported phosphate catalysts. J. Catal. 1994, 148, 252–260. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic processing of lactic acid over Pt/Nb2O5. ChemSusChem 2009, 2, 581–586. [Google Scholar] [CrossRef]
- Renz, M. Ketonization of carboxylic acids by decarboxylation: Mechanism and scope. Eur. J. Org. Chem. 2005, 2005(6), 979–988. [Google Scholar] [CrossRef]
- Fitzpatrick, S.W. Production of levulinic acid from carbohydrate-containing materials. U.S. Patent 5608105, 1997. [Google Scholar]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of γ-Valerolactone by Hydrogenation of Biomass-derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Lai, D.M.; Fu, Y.; Guo, Q.X. Catalytic conversion of biomass-derived carbohydrates into γ-valerolactone without using an external H2 supply. Angew. Chem. Int. Ed. 2009, 48, 6529–6532. [Google Scholar]
- Bozell, J.J.; Moens, L.; Elliott, D.C.; Wang, Y.; Neuenscwander, G.G.; Fritzpatrick, S.W.; Bilski, R.J.; Jarnefeld, J.L. Production of levulinic acid and use as a platform chemical for derived products. Res. Conserv. Recycl. 2000, 28, 227–239. [Google Scholar] [CrossRef]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 2010, 49, 4479–4483. [Google Scholar]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Braden, D.J.; West, R.M.; Dumesic, J.A. Conversion of cellulose to hydrocarbon fuels by progressive removal of oxygen. Appl. Catal. B 2010, 100, 184–189. [Google Scholar]
- Martin-Alonso, D.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar]
- Bond, J.Q.; Martin-Alonso, D.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar]
- Pagliaro, M.; Rossi, M. Future of Glycerol, New Usages for a Versatile Raw Material; RSC publishing: Cambridge, UK, 2008. [Google Scholar]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a source for fuels and chemicals by low-temperature catalytic processing. Angew. Chem. Int. Ed. 2006, 45, 3982–3985. [Google Scholar]
- Simonetti, D.A.; Rass-Hansen, J.; Kunkes, E.L.; Soares, R.R.; Dumesic, J.A. Coupling of glycerol processing with Fischer-Tropsch synthesis for production of liquid fuels. Green Chem. 2007, 9, 1073–1083. [Google Scholar]
- Kunkes, E.L.; Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. An integrated catalytic approach for the production of hydrogen by glycerol reforming coupled with water-gas shift. Appl. Catal. Environ. 2009, 90, 693–698. [Google Scholar]
- Zhou, C.H.C.; Beltramini, J.N.; Fan, Y.X.; Lu, G.Q.M. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2007, 37, 527–549. [Google Scholar]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 2008, 322, 417–421. [Google Scholar]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic routes for the conversion of biomass into liquid hydrocarbon transportation fuels. Energy Environ. Sci. 2011, 4, 83–99. [Google Scholar]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar]
- Biomass to Fuel Conversion; Primus Green Energy Ltd.: Hillsborough, NJ, USA, 2012. Available online: http://www.primusge.com/how-it-works/biomass-to-fuel-conversion/ (accessed on 15 June 2012).
- Sundrop Fuels, Inc. Available online: http://www.sundropfuels.com/ (accessed on 15 June 2012).
- Lange, J.P. Methanol synthesis: A short review of technology improvements. Catal. Today 2001, 64, 3–8. [Google Scholar]
- Exxon Mobil, Research and Engineering, Methanol to gasoline (MTG). Available online: http://www.exxonmobil.com/Apps/RefiningTechnologies/files/sellsheet_09_mtg_brochure.pdf (accessed on 15 June 2012).
- Haldor Topsøe. Available online: http://www.topsoe.com/business_areas/gasification_based/Processes/Gasoline_TIGAS.aspx (accessed on 15 June 2012).
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).