Abstract
Antibiotic resistance is a pressing global, one health and planetary health challenge. Links between climate change, antibiotic use, and the emergence of antibiotic resistance have been well documented, but less attention has been given to the impact(s) of earth systems on specific bacterial livestock diseases at a more granular level. Understanding the precise impacts of climate change on livestock health—and in turn the use of antibiotics to address that ill-health—is important in providing an evidence base from which to tackle such impacts and to develop practical, implementable, and locally acceptable solutions within and beyond current antibiotic stewardship programs. In this paper, we set out the case for better integration of earth scientists and their specific disciplinary skill set (specifically, problem-solving with incomplete/fragmentary data; the ability to work across four dimensions and at the interface between the present and deep/geological time) into planetary health research. Then, using a case study from our own research, we discuss a methodology that makes use of risk mapping, a common methodology in earth science but less frequently used in health science, to map disease risk against changing climatic conditions at a granular level. The aim of this exercise is to argue that, by enabling livestock farmers, veterinarians, and animal health observatories to better predict future disease risk and risk impacts based on predicted future climate conditions, earth science can help to provide an evidence base from which to influence policy and develop mitigations. Our example—of climate conditions’ impact on livestock health in Karnataka, India—clearly evidences the benefit of integrating earth scientists into planetary health research.
1. Introduction
In this paper we highlight the need for more flexible and iterative research agendas to address the climate-change-related root drivers of antimicrobial resistance (AMR). The recent addition of the United Nations Environment Program (UNEP) to the Quadripartite Joint Secretariat on Antimicrobial Resistance between WOAH, FOA, WHO, and now UNEP, is welcomed [1], but we argue that there needs to be further bridging between the work of this group and the United Nations Framework Convention on Climate Change (UNFCCC). Climate change and disease risks, including AMR, are two of the most pressing challenges of the Anthropocene and cannot be considered in isolation [2]. Planetary health is already deeply invested in identifying the complex links between climate change and zoonotic disease [3], to raising awareness of the intersection of Anthropocene risks in general [4,5,6], and argues for addressing global and intergenerational risks from AMR through a lens of planetary health ethics [7]. Other fields have also made such links explicit [8,9,10]. There is growing interest in the risks posed to human and animal health by antibiotic resistant bacteria such as Escherichia coli, Staphylococcus aureus, Pseudomonas, Acinetobacters and Enterobacteriaceae in the environment [11,12,13]. However, fewer commentators focus on specific ways in which earth scientists, environmental scientists and infectious disease researchers can work together to evidence the exact conditions that drive emergence and transmission so that this knowledge can more readily inform both climate change and AMR policy and identify implementable solutions.
Thus far, for example, neither Challenges nor The Lancet Planetary Health, the planetary health field’s two most prominent journals, have published a single original research paper evidencing links between antibiotic resistance and climate change explicitly. Only two short commentary papers [14,15], the latter of which is by one of the authors of this paper, touch on the specifics of the issue.
Earth scientists’ work has deeply influenced the field of planetary health—not least the work of those involved in determining the earth systems trends of the Great Acceleration [16] and the planetary boundaries of a safe and just operating space for humanity [17] but it is less common to see earth scientists and health scientists working side-by-side on AMR within a single project team.
We present a case study based on our own research [18,19] which we believe shows the value in allowing space for transdisciplinary research that more holistically and iteratively integrates earth scientists’ discipline-specific skills into planetary health’s conceptual framework. These skills include problem-solving with incomplete/fragmentary data [20,21,22], the ability to think across four dimensions [23] and at the interface between the present and deep/geological time [24]. This, we argue, enables the development of more compelling evidence on changing climate conditions’ direct harms to the prevalence and spread of animal bacterial disease, the use of antibiotics to treat it, and thus the emergence of antibiotic resistance.
2. Transdisciplinary Research: Iterative, Agile and Adaptive
In the process of conducting research into the drivers of antibiotic use and poor antibiotic stewardship in the Indian livestock sector as part of a cross-disciplinary team comprising microbiologists, veterinarians, anthropologists and economists [15,25,26], we listened to farmers and veterinarians in regions of India as far apart as Karnataka in the south and Assam in North-East India, who spoke, openly and implicitly during ethnographic observations of the pressures that climate change places on their livelihoods. The changing climate has already pushed these farmers from crop farming to livestock raising and now stresses the health of their animals [15]. These observations pushed us to consider a closer examination of the environmental drivers of ill-health in order to understand the root causes of antibiotic use intended to treat that ill-health; to consider not only which bacteria were present in the environment but why and how they are there. Whilst a focus on climate change was technically outside of the original remit of our funding and of the project intentions, COVID-19 travel restrictions pushed us into desk-based research using secondary data, and then enabled the replacement of ethnographic researchers, who left the project when they were unable to undertake further fieldwork, with earth scientists who were able to explore climate impacts more deeply.
3. The Value of More Granular Integration of Earth Science with Planetary Health
Understanding the impact of climate change on human and livestock health is critical to safeguarding global food supplies and economies and to plan global recovery from the COVID-19 pandemic [2] as well as in maintaining the efficacy of antibiotics. This raises a unique challenge for planetary and one health researchers and practitioners, as they will need to explore new (and perhaps even yet-to-be-developed) methodologies, knowledge, skills and networks in order to enhance environmental awareness. At least in the short term, such researchers are likely to be working with incomplete and fragmented data, as the regions of the world most affected by climate change are also those where surveillance is less robust [19]. Earth scientists, however, are more than familiar with the challenges of such data [20,22]. Furthermore, AMR and other wicked problems of the Anthropocene are not only made visible by the earth system trends of the Great Acceleration graphs [16] but are likely to need additional international policies and treaties to address them, which will need to be underpinned by robust evidence from outside of health science. It is often the impact on health—rather than the environment per se—that attracts political interest and helps treaties to be ratified. The Stockholm Convention on Persistent Organic Pollutants (POPs) [27] and the Montreal Protocol that protects the ozone layer [28], both limit the use of chemicals detrimental to the environment, but in each case the impact on (human) health was a key driver for their adoption and implementation. Future policies and treaties may be even more successful if they foreground the risks to health, evidenced by the well-understood risks imposed by earth system changes. Such international treaties should be considered key planetary health documents but are less familiar to health systems researchers than to earth scientists. Human health is a strong lever for international agreement [29]: working together, earth scientists and health scientists can speak with a collective voice that will be harder for policymakers to ignore.
Recognizing that the root drivers of antibiotic use lie outside the (traditional) power of health systems to address takes an important first step. For example, while microbiologists are able to quantify the levels of bacteria in the environment and their susceptibility (or not) to antibiotics [30] and genomics can map which genes they carry, how closely they are related to other strains and where else those strains are found [31], those same microbiologists will need to reach out to earth scientists to understand, map and predict the meteorological conditions that are most conducive to disease emergence and spread; and to soil and water chemists to understand which pollutants may help to drive antibiotic resistance [32]. The ground set by these collaborative relationships will be even more critical in the later stages of such work: after the mapping processes and meteorological relationships have been founded, the expertise of the microbiologists and veterinarians will be needed to divulge the true impact of the spatial–temporal mapping by carrying out disease surveillance and diagnosis that proves the model as the predicted climatic conditions unfold and disease incidence does (or does not) increase. Experts from both fields will then need to communicate the results of their observations to relevant stakeholders, including commercial farms, governmental bodies, local research institutes, etc., for true transdisciplinarity to be realized [33].
4. Mapping the Spatial Distribution of the Conditions That Drive Ill-Health
Earth scientists may in turn need to work with modelling specialists to build and automate the production of climate-related risk maps [19]. The input of veterinarians and farmers will be needed to ensure such models are utilized as widely as possible. Animal health observatories will need to share epidemiological data from regions at risk (including where and when the prevalence of cases and outbreaks changes) while earth science brings to the table different ways of interpreting risk and of working with fragmentary and incomplete data [21,24] over longer timescales, into both the deep past and longer-term future [34]. Geographers are needed too, to map the topography and topology of regions in which those cases occur, and to consider how farmer’s livelihoods, access to veterinary services and patterns of sector transformation are intersecting with climate changes and local development agendas; for example, whilst environmentally-controlled chicken sheds might on the surface appear to be a sufficient mitigation to the risks of heat-stress-induced dysbiosis and thus reduced immune response that drives higher use of antibiotics in Indian poultry farms, the current failings of rural energy infrastructure prevent this being a practical solution [15]. Looking instead for regions which at present may be cooler than the ideal conditions for livestock rearing, but which may be warming and likely to reach such thresholds in future, so that expansion into such regions can be planned, or which currently favor poultry rearing but are becoming more suited to aquaculture, are alternative options. Such integrated methodologies can also work in other fields, e.g., at the intersection of the spatial distribution of cases of human disease with distributions of social deprivation [35], location of healthcare infrastructure [36] and access to blue and green space [37]. There is growing interest in human health fields in ensuring the integration of air quality and health [38], soil pollution and health [39] etc., on increasingly granular spatial scales. The UK’s National Health Service [40] is a world-leader in setting a Green Agenda for Health [41], aiming towards net-zero carbon operations by 2040. This approach has been as dependent on earth science as it has on approaches that have come from inside health systems and medical science. Figure 1 demonstrates the sizable, shared space between health and climate science, seen through the skillset of an earth scientist, as well as the way in which this overlap is manifest across different stakeholders and geographical scales.
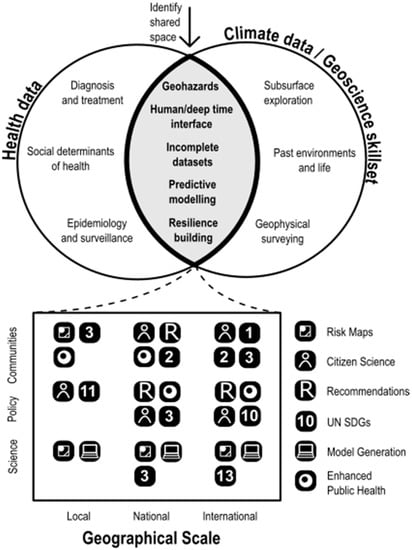
Figure 1.
Conceptualization of the shared space between health and climate/earth science, together with benefits to different stakeholders across different scales. The numbered icons (1, 2, 3, 10, 11, 13) refer to the UN Sustainable Development Goal most relevant to the sector/geographic scale indicated.
Practitioners of one health (defined by the One Health Commission [42] as “an integrated, unifying approach that aims to sustainably balance and optimize the health of people, animals and ecosystems [that] recognizes the health of humans, domestic and wild animals, plants, and the wider environment, including ecosystems that are closely linked and interdependent”) are quick to point out that animal health and human health cannot and should not be considered independently [43]. There is also benefit from greater integration of citizen science, another approach earth science has long embraced [44].
5. Scaling from the Microbiome to Exposome
To understand what is making animals ill, a systematic and systemwide approach is needed to the holistic environment and the conditions within it that create disease ‘situations’ (where conditions such as overcrowding and poor welfare stress animals’ immune responses and causes otherwise commensal bacteria to become pathogenic [45]), so that the ‘exposome’, the ecosystem of external risk exposures can be mapped [46]. How these risk exposures combine (with or without antibiotic exposure) to influence, support or challenge equally complex animal and human microbiomes under different climatic conditions is still poorly understood, despite the considerable work of the decade-long Human Microbiome Project [47]. Tellingly, this has included neither earth science nor a consideration of how climate drivers such as recent and rapid increases in the magnitude and severity of geohazards (e.g., heatwaves and monsoon rainfall) may impact microbiomes.
Because of the complexity that is now developing, systems thinkers [48] and modellers need to be able to combine multiple insights to model not only the risks that have already been identified and predict where they may increase (or decrease) in future, but also how the system-of-systems those risks inhabit are configured. Even then, ethnographers and economists will need to work with communities to determine what can be done to mitigate the predicted risks in a manner that is practical, acceptable and affordable; changing people’s behavior towards making more rational choices regarding the use of antibiotics, let alone for the overall health of themselves or the planet, is far from being a trivial exercise [49].
6. A New Methodology for Mapping the Climate/Disease Risk Interface
Having set the scene for why closer integration of earth scientists into planetary health research teams has scientific value, our recent work in southern India [19] offers a first step towards the future integration of researchers from interrelated and overlapping fields. Our work emerged from two Newton-Bhabha Fund projects that aimed to address drivers of antimicrobial resistance (AMR) in India [30,31,50,51] and to understand the behavioral drivers influencing the use of antibiotics by farmers [15,26] and vets [25].
Farmers’ insights and lived experiences [15,25,26], observed during a rapid ethnographic assessment of livestock systems and recorded in semi-structured interviews, focus groups and transect walks through peri-urban farming communities, led us to consider the role of climate change on animal ill-health as a trigger for antibiotic use. This in turn led us to develop a risk classification tool that assesses how disease risk varies in Karnataka in the present and in possible future scenarios. Despite a relatively limited epidemiological dataset (from the NADRES-v2 database [52]), clear relationships between bacterial disease and high-risk zones were defined using time-series data over a period of 33 years (1987–2020). By constructing risk maps, which are common across geoscientific (e.g., for volcanic hazard and flood risk) and epidemiological research, we used a physics-based statistical approach to define risk thresholds based on the inferred relationships between climate and disease data. The maps were constructed using open-source climate data (Climate Research Unit (CRU) TS 4.5 dataset). Thresholds for risk were defined by using the inferred relationships between the climate data and disease data after statistically investigating the spatio-temporal relations between the two, first with correlative statistical analysis (Spearman’s rank) followed by principal component analysis (PCA). Through this methodology (which is described in more detail in the full scientific research paper version [19]) it is possible to interpret the individual climate variable contribution to risk in each grid box, providing insight into the varying climatic controls for higher and lower risk across the areas. Although there are far more socio-economic factors that also play a role in predicting disease outbreak risk (farm locations, population density, sanitation standards, food standards, veterinary access, vaccination campaigns etc.), these are typically more granular controls whilst climate-associated risk is useful for a ‘bigger picture’ perspective—identifying complete regions of higher and lower risk, which can then be investigated in more detail using the aforementioned socio-economic factors.
Outputs from this modelling work are captured in Figure 2, where each case study has the raw data presented in time-series graphs, followed by the statistical correlative results, then finally the risk maps themselves. While our research in India was primarily interested in drivers of AMR, and thus bacterial diseases, this methodology can be replicated to investigate other diseases and other regions, or even climatic conditions that impact crop yields, if the climate and epidemiological/harvesting data cover similar time periods.
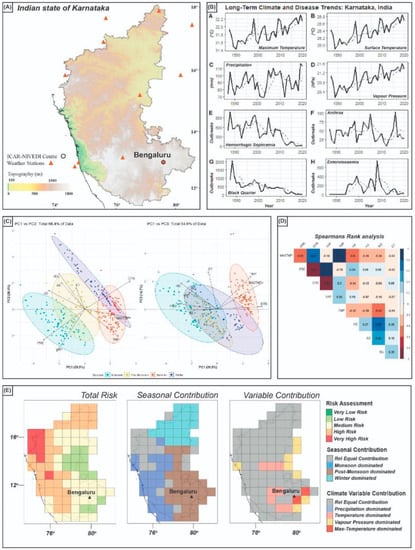
Figure 2.
Generalized output for risk-mapping model, using state of Karnataka as an example: (A) Location map for Karnataka; (B) time-series graphs for both climate variable data and disease outbreak data; (C) PCA results for combined data; (D) Spearman’s rank correlation statistics between climate and disease data; (E) final risk map output with seasonal contribution to risk, and individual variable contribution to risk also mapped in grid-box format. Data presented is modified from [19].
Our transdisciplinary approach led us to identify that hitherto unconsidered changes in the key climate variables of precipitation and vapor pressure (i.e., humidity) are the most important factor governing outbreaks of hemorrhagic septicemia (HS), anthrax (AX), and black quarter (BQ) in livestock across the Indian state of Karnataka. Unaddressed, such outbreaks risk economic damage to the farming community, food security and, in turn, poorer livelihoods for those dependent on both the farming economy and the food it produces but addressing them needs more granular data on precisely which climate conditions are likely to impact which specific diseases, in which species, in which regions and over what timescales, ensuring that those informed by the data will have sufficient time to act.
We intend to continue working with this methodology, improving the robustness of the risk maps by defining more quantitative thresholds upon which disease outbreaks may relate to specific climate variable change, i.e., at what average rainfall, at what average temperatures, and at what average vapor pressure does risk increase. We will need to work across more disciplines, for example with computational modellers, livestock and human disease experts, ethnographers and local data collectors, to achieve these aims. We hope to provide a new platform through which planetary health researchers and earth scientists can come together in new transdisciplinary spaces. To achieve this, we seek more robust, long-term disease data across a variety of global case studies (currently Nepal, Egypt, Kenya, South America), preferably with diverse meteorological conditions to provide the best range of test scenarios—data that other planetary health researchers may hold or be encouraged to gather.
7. Towards Climate Models for Social Justice
Beyond animal health, once earth scientists’ skill sets are embedded into research investigating the underlying drivers of bacterial disease, antibiotic use and thus the emergence of resistance to antibiotics, they can be cascaded out to human health research more widely. Increasing unpredictability and magnitudes of annual hydrological budgets (inflows, outflows and storage of water), greater temperature and wet-bulb humidity extremes, as well as the effects of this on the environmental realm (such as exacerbating the magnitude of air and water pollution) increases the risks associated with human health conditions such as obesity, diabetes and hypertension, which in turn increases susceptibility to more severe symptoms of respiratory diseases, including COVID-19 [53], particularly during heatwaves [54]. On 7 October 2022, a joint report published by the Office for National Statistics and UK Health Security Agency indicated there had been 3000 more deaths in England and Wales than would usually be expected during the year’s unusually hot summer [55].
The impact of climate factors is known to intersect strongly with socioeconomic deprivation [56]: evidencing this impact may help to drive policy to tackle underlying socioeconomic drivers at source and thus help to deliver justice to the most vulnerable pockets of society, speaking to planetary health’s strong ethical focus on championing equity and social justice [57]. Short of relocating agricultural operations to regions of the world less impacted by climate stress, and human populations to regions where their livelihoods will be made less precarious by climate change, developing a methodology for identifying, at very precise granular resolutions, where the areas of highest risk are found—today and in the short- to mid-term future—so that limited resources for intervention can be prioritized to where they are needed most acutely provides a practical mid-term intervention strategy.
Thus, only by taking a system-of-systems approach to health, working simultaneously across all the societal systems and earth systems implicated in the Great Acceleration [16], will we be able to address the real underlying drivers that place pressure on those systems. For all planetary health’s lauding of the conceptual framework of the Great Acceleration and planetary boundaries [16,17], truly integrated, evidence-producing projects between earth scientists, health systems scientists and social scientists remain scarce. This is in spite of strong evidence that earth systems change profoundly challenges human, animal and plant health directly e.g., through ill-health caused by heat-stress [58,59] and crop failure [60,61], and indirectly, e.g., through increased incidence of biological disease caused by pathogens that proliferate more in warmer conditions [62]; or food shortages [63] that cause malnutrition and reduce the immune response. In short, we argue that research on the drivers of antibiotic resistance can no longer afford not to embrace earth scientists, wider environmental considerations, and earth systems science more fully.
Health researchers need to go further than just referring to the current climate science literature by meaningfully integrating earth systems scientists into their ongoing research across the entire lifecycle of a research project, from problem conception/definition to co-development of data collection and analysis methods, to the dissemination of data/information to relevant stakeholders. This in turn leads on to other considerations: once engaged, earth scientists might look to develop enhanced process understanding of, e.g., the monsoon; health scientists might want to determine under what specific climate conditions disease transmission or severity of cases increases, and if the relationship is linear, logarithmic or if it reaches a tipping point aligned to regime change [64]. Local communities will be able to use the evidence earth scientists provide to invest in or implement new ways to de-risk their livestock falling ill and thus safeguard their future livelihoods; government stakeholders will be able to use the same data to protect their population and economies. True earth/health collaboration would satisfy all of these stakeholder needs and would involve an equitable and fair balance of resources and time, which goes far beyond just having a token health scientist on a largely earth science program or vice versa.
8. Conclusions
The evidence produced by the work of the earth scientists in our research project [18,19], evidenced important links between not only climate change in general, but specific aspects of climate change—namely between average and maximum surface temperature, precipitation and vapor pressure, and several livestock bacterial diseases including, but unlikely to be limited to, hemorrhagic septicemia, anthrax, and black quarter. We identified that the north-western coast of Karnataka is at highest risk for outbreaks of these diseases under future climate change prediction models, while the central-eastern and south-eastern regions are at low-risk (for full results, see [19]). The results show that the risk profile of each region is likely to be stable over the next five decades even if temperature increases further, but this may not be true for other regions of the world.
This highlights not only the value of earth science to the immediate challenge at hand, but also the benefit of enabling research projects to break out of their original silos when there is clear value in doing so. The recent addition of UNEP to the (now) Quadripartite Secretariat on AMR will hopefully act as a rallying call to other human, animal, and planetary health researchers to take an even wider, even more transdisciplinary approach to AMR (and to other health challenges of the Anthropocene) and to other earth and environmental scientists to consider how they too might bring their skills to the table.
There are already frameworks into which these more complex collaborations can fit. For example, the UNICEF-led Integrated Outbreak Analytics program [18,65] acts as not only a platform for researchers from diverse fields working with disease outbreak data but also as a network through which collaborative researchers can connect, disseminate their work, share methodologies, and seek out future collaborators. We urge more planetary health researchers to connect and collaborate with them.
Successful transdisciplinary research projects such as the one we have described in this paper have the potential to tackle larger, international and complex issues that affect global communities and speak directly to planetary health’s willingness to face up to even the most complex and challenging ‘wicked problems’ of the Anthropocene [66,67]. The evidence our research provides, of granular links between specific diseases and specific climate conditions, highlights the need for greater synergies between earth scientists, climate change science, planetary and one health research and policy formation. In the short-term, we argue this puts forward a(n even) strong(er) case for greater alignment between the Quadripartite Agreement (between WOAH, FOA, WHO, and UNEP) on antimicrobial resistance and the United Nations Framework Convention on Climate Change (UNFCCC), as these two pressing challenges of the Anthropocene cannot be considered in isolation.
Author Contributions
Conceptualization, J.C., A.E. and J.D.P.; methodology, A.E. and J.D.P.; software, A.E.; validation, A.E.; formal analysis, J.C., A.E. and J.D.P.; investigation, J.C., A.E. and J.D.P.; resources, J.C., A.E. and J.D.P.; data curation, A.E.; writing—original draft preparation, J.C., A.E. and J.D.P.; writing—review and editing, J.C., A.E. and J.D.P.; visualization, A.E.; supervision, J.C.; project administration, J.C.; funding acquisition, J.C. and J.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
J.C. and A.E. were funded by a Newton-Bhabha awards co-funded by the UK Economic and Social Research Council grant number ES/S000216/1 and the India Department of Biotechnology grant number BT/IN/Indo-UK/AMR/05/NH/2018-19. J.D.P. received no funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Royal Holloway, University of London (10/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study. Farmers and veterinarians interviewed were given information sheets and asked to sign a written consent form; the forms were read to illiterate farmers, who then gave verbal consent. Participants were made aware that they were free to withdraw from the study at any time.
Data Availability Statement
Data used in this study were obtained from the publicly available Climate Research Unit (CRU) TS 4.5 dataset and NADRES-2 dataset. Cleaned and formatted data are available from the authors on request.
Acknowledgments
The authors acknowledge the contribution of farmers and veterinarians in Bengalaru, Karnataka, and Guwahati, Assam, who gave us their time during the original project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ferreira, J.P.; Battaglia, D.; García, A.D.; Tempelman, K.; Bullon, C.; Motriuc, N.; Caudell, M.; Cahill, S.; Song, J.; LeJeune, J. Achieving Antimicrobial Stewardship on the Global Scale: Challenges and Opportunities. Microorganisms 2022, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Dodds, K. Unhealthy geopolitics: Can the response to COVID-19 reform climate change policy? Bull. World Health Organ. 2020, 99, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Oladunjoye, I.O.; Mustapha, M.O.; Mustapha, S.T.; Abdullahi, A.A.; Yusuf, R.O.; Abimbola, S.O.; Adebayo, A.O.; Ikebuaso, J.G.; et al. Preventing the Next Pandemic through a Planetary Health Approach: A Focus on Key Drivers of Zoonosis. Challenges 2022, 13, 50. [Google Scholar] [CrossRef]
- Salk, J.D. Planetary Health: A New Reality. Challenges 2019, 10, 7. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C. Down to Earth: Planetary Health and Biophilosophy in the Symbiocene Epoch. Challenges 2017, 8, 19. [Google Scholar] [CrossRef]
- Logan, A.; Berman, S.; Scott, R.; Berman, B.; Prescott, S. Catalyst Twenty-Twenty: Post-Traumatic Growth at Scales of Person, Place and Planet. Challenges 2021, 12, 9. [Google Scholar] [CrossRef]
- Abimbola, S.O.; Otieno, M.A.; Cole, J. Reducing the Use of Antimicrobials as a Solution to the Challenge of Antimicrobial Resistance (AMR): Approaching an Ethical Dilemma through the Lens of Planetary Health. Challenges 2021, 12, 23. [Google Scholar] [CrossRef]
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374. [Google Scholar] [CrossRef]
- Fouladkhah, A.C.; Thompson, B.; Camp, J.S. The Threat of Antibiotic Resistance in Changing Climate. Microorganisms 2020, 8, 748. [Google Scholar] [CrossRef]
- MacFadden, D.R.; McGough, S.F.; Fisman, D.; Santillana, M.; Brownstein, J.S. Antibiotic resistance increases with local temperature. Nat. Clim. Chang. 2018, 8, 510–514. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Zdaniewicz, M. Antibiotic Resistance of Escherichia coli Isolated from Processing of Brewery Waste with the Addition of Bulking Agents. Sustainability 2021, 13, 10174. [Google Scholar] [CrossRef]
- Serwecińska, L. Antimicrobials and Antibiotic-Resistant Bacteria: A Risk to the Environment and to Public Health. Water 2020, 12, 3313. [Google Scholar] [CrossRef]
- Cave, R.; Cole, J.; Mkrtchyan, H.V. Surveillance and prevalence of antimicrobial resistant bacteria from public settings within urban built environments: Challenges and opportunities for hygiene and infection control. Environ. Int. 2021, 157, 106836. [Google Scholar] [CrossRef]
- Asaduzzaman, M. Antimicrobial resistance: An urgent need for a planetary and ecosystem approach. Lancet Planet. Health 2018, 2, e99–e100. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.; Desphande, J. Poultry farming, climate change, and drivers of antimicrobial resistance in India. Lancet Planet. Health 2019, 3, e494–e495. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Broadgate, W.; Deutsch, L.; Gaffney, O.; Ludwig, C. The trajectory of the Anthropocene: The Great Acceleration. Anthr. Rev. 2015, 2, 81–98. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, I.I.I.F.S.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 32. [Google Scholar] [CrossRef]
- Eskdale, A. Combining Ethnographic, Epidemiological and Meteorological Data to Predict Long-Term Disease Outbreak Risk In Karnataka, India. IOA Field Exch. 2022, 3, 6–7. Available online: https://www.corecommitments.unicef.org/kp/integrated-oubtreak-analytics---field-exchange-volume-3---july-2022.pdf (accessed on 1 September 2022).
- Eskdale, A.; El Tholth, M.; Paul, J.D.; Deshphande, J.; Cole, J. Climate stress impacts on livestock health: Implications for farming livelihoods and animal disease in Karnataka, India. CABI one health. CABI Int. 2022. [Google Scholar] [CrossRef]
- Mariethoz, G.; Renard, P. Reconstruction of Incomplete Data Sets or Images Using Direct Sampling. Math. Geosci. 2010, 42, 245–268. [Google Scholar] [CrossRef]
- Manduca, C.A.; Baer, E.; Hancock, G.; Macdonald, R.H.; Patterson, S.; Savina, M.; Wenner, J. Making Undergraduate Geoscience Quantitative. Eos Trans. Am. Geophys. Union 2008, 89, 149–150. [Google Scholar] [CrossRef]
- Oreskes, N.; Shrader-Frechette, K.; Belitz, K. Verification, Validation, and Confirmation of Numerical Models in the Earth Sciences. Science 1994, 263, 641–646. [Google Scholar] [CrossRef] [PubMed]
- De Paor, D.G.; Whitmeyer, S.J. Innovation and obsolescence in geoscience field courses: Past experiences and proposals for the future. Field Geol. Educ. Hist. Perspect. Mod. Approaches 2009, 461, 45. [Google Scholar]
- Manduca, C.A.; Kastens, K.A. Geoscience and geoscientists: Uniquely equipped to study Earth. Geol. Soc. Am. Spec. Pap. 2012, 486, 1–2. [Google Scholar] [CrossRef]
- Eltholth, M.; Govindaraj, G.; Das, B.; Shanabhoga, M.B.; Swamy, H.M.; Thomas, A.; Cole, J.; Shome, B.R.; Holmes, M.A.; Moran, D. Factors Influencing Antibiotic Prescribing Behavior and Understanding of Antimicrobial Resistance Among Veterinarians in Assam, India. Front. Vet.-Sci. 2022, 9, 864813. [Google Scholar] [CrossRef]
- Greru, C.; Thompson, R.; Gowthaman, V.; Shanmugasundaram, S.; Ganesan, N.; Murthy, T.R.G.K.; Eltholth, M.; Cole, J.; Joshi, J.; Runjala, R.; et al. A visualisation tool to understand disease prevention and control practices of stakeholders working along the poultry supply chain in southern India. Humanit. Soc. Sci. Commun. 2022, 9, 1–10. [Google Scholar] [CrossRef]
- Godduhn, A.; Duffy, L.K. Multi-generation health risks of persistent organic pollution in the far north: Use of the precautionary approach in the Stockholm Convention. Environ. Sci. Policy 2003, 6, 341–353. [Google Scholar] [CrossRef]
- Gareau, B.J. A critical review of the successful CFC phase-out versus the delayed methyl bromide phase-out in the Montreal Protocol. Int. Environ. Agreem. Politics Law Econ. 2010, 10, 209–231. [Google Scholar] [CrossRef]
- Morrisette, P.M. The Montreal Protocol. Lessons for Formulating Policies for Global Warming. Policy Stud. J. 1991, 19, 152–161. [Google Scholar] [CrossRef]
- Sivaraman, G.; Sudha, S.; Muneeb, K.; Shome, B.; Holmes, M.; Cole, J. Molecular assessment of antimicrobial resistance and virulence in multi drug resistant ESBL-producing Escherichia coli and Klebsiella pneumoniae from food fishes, Assam, India. Microb. Pathog. 2020, 149, 104581. [Google Scholar] [CrossRef]
- Sivaraman, G.; Muneeb, K.; Sudha, S.; Shome, B.; Holmes, M.; Cole, J. Fish-borne methicillin resistant Staphylococcus haemolyticus carrying atypical staphylococcal cassette chromosome mec (SCCmec) elements. Gene Rep. 2021, 22, 100982. [Google Scholar] [CrossRef]
- Rad, A.K.; Astaykina, A.; Streletskii, R.; Afsharyzad, Y.; Etesami, H.; Zarei, M.; Balasundram, S.K. An Overview of Antibiotic Resistance and Abiotic Stresses Affecting Antimicrobial Resistance in Agricultural Soils. Int. J. Environ. Res. Public Health 2022, 19, 4666. [Google Scholar] [CrossRef]
- Zinsstag, J.; Crump, L. Advancing integrated approaches to health through the new transdisciplinary CABI one health resources. CABI One Health 2022. [Google Scholar] [CrossRef]
- Stewart, I.S.; Nield, T. Earth stories: Context and narrative in the communication of popular geoscience. Proc. Geol. Assoc. 2012, 124, 699–712. [Google Scholar] [CrossRef]
- Schuurman, N.; Bell, N.; Dunn, J.R.; Oliver, L. Deprivation Indices, Population Health and Geography: An Evaluation of the Spatial Effectiveness of Indices at Multiple Scales. J. Urban Health 2007, 84, 591–603. [Google Scholar] [CrossRef]
- Gao, S.; Mioc, D.; Anton, F.; Yi, X.; Coleman, D.J. Online GIS services for mapping and sharing disease information. Int. J. Health Geogr. 2008, 7, 8. [Google Scholar] [CrossRef]
- Chen, W.; Huang, H.; Dong, J.; Zhang, Y.; Tian, Y.; Yang, Z. Social functional mapping of urban green space using remote sensing and social sensing data. ISPRS J. Photogramm. Remote Sens. 2018, 146, 436–452. [Google Scholar] [CrossRef]
- Holloway, T.; Miller, D.; Anenberg, S.; Diao, M.; Duncan, B.; Fiore, A.M.; Henze, D.K.; Hess, J.; Kinney, P.L.; Liu, Y.; et al. Satellite Monitoring for Air Quality and Health. Annu. Rev. Biomed. Data Sci. 2021, 4, 417–447. [Google Scholar] [CrossRef]
- Zeng, S.; Ma, J.; Yang, Y.; Zhang, S.; Liu, G.-J.; Chen, F. Spatial assessment of farmland soil pollution and its potential human health risks in China. Sci. Total. Environ. 2019, 687, 642–653. [Google Scholar] [CrossRef]
- NHS England. Delivering a Net Zero National Health Service. 2022. Available online: https://www.england.nhs.uk/greenernhs/wp-content/uploads/sites/51/2022/07/B1728-delivering-a-net-zero-nhs-july-2022.pdf (accessed on 11 August 2022).
- Audhali, N.; Moore, A.; Martin, T.; Munro, C.; Wedmore, F. 181 Harnessing staff values to catalyse workplace change. Abstracts 2020, 4 (Suppl. S1), A68. [Google Scholar] [CrossRef]
- One Health Commissions. What Is OneHealth? Available online: https://www.onehealthcommission.org/en/why_one_health/what_is_one_health/ (accessed on 26 October 2022).
- Zinsstag, J.; Schelling, E.; Bonfoh, B.; Fooks, A.R.; Kasymbekov, J.; Waltner-Toews, D.; Tanner, M. Towards a ‘One Health’ research and application tool box. Vet. Ital. 2010, 45, 121–133. [Google Scholar]
- Paul, J.D. Breathing Fresh Life into Geoscience: Involving Non-Scientists in Research has a Long Pedigree in other Fields, but Uptake is Slow and Cautious in the Earth Sciences. With Experience from Western Nepal, Jonathan Paul Describes how Citizen Science Projects Can be Done. Geoscientist. 31 May 2021. Available online: https://geoscientist.online/sections/features/breathing-fresh-life-into-geoscience/ (accessed on 31 October 2022).
- Hinchliffe, S.; Bingham, N.; Allen, J.; Carter, S. Pathological Lives: Disease, Space and Biopolitics; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Siroux, V.; Agier, L.; Slama, R. The exposome concept: A challenge and a potential driver for environmental health research. Eur. Respir. Rev. 2016, 25, 124–129. [Google Scholar] [CrossRef] [PubMed]
- The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Iyer, H.S.; DeVille, N.V.; Stoddard, O.; Cole, J.; Myers, S.S.; Li, H.; Elliott, E.G.; Jimenez, M.P.; James, P.; Golden, C.D. Sustaining planetary health through systems thinking: Public health’s critical role. SSM-Popul. Health 2021, 15, 100844. [Google Scholar] [CrossRef]
- Hallsworth, M. Rethinking public health using behavioural science. Nat. Hum. Behav. 2017, 1, 612. [Google Scholar] [CrossRef]
- Sivaraman, G.; Muneeb, K.; Sudha, S.; Shome, B.; Cole, J.; Holmes, M. Prevalence of virulent and biofilm forming ST88-IV-t2526 methicillin-resistant Staphylococcus aureus clones circulating in local retail fish markets in Assam, India. Food Control 2021, 127, 108098. [Google Scholar] [CrossRef]
- Muneeb, K.H.; Sudha, S.; Sivaraman, G.K.; Shome, B.; Cole, J.; Holmes, M. Virulence and intermediate resistance to high-end antibiotic (teicoplanin) among coagulase-negative staphylococci sourced from retail market fish. Arch. Microbiol. 2021, 203, 5695–5702. [Google Scholar] [CrossRef]
- NADRES-2. Available online: https://nivedi.res.in/Nadres_v2/index.php (accessed on 26 October 2022).
- Shah, H.; Khan, S.H.; Dhurandhar, N.V.; Hegde, V. The triumvirate: Why hypertension, obesity, and diabetes are risk factors for adverse effects in patients with COVID-19. Acta Diabetol. 2021, 58, 831–843. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Geladari, E.V.; Kounatidis, D.; Geladari, C.V.; Stratigou, T.; Dourakis, S.P.; Andreadis, E.A.; Dalamaga, M. Diabetes mellitus in the era of climate change. Diabetes Metab. 2020, 47, 101205. [Google Scholar] [CrossRef]
- ONS. Excess Mortality during Heat-Periods: 1 June to 31 August 2022. 7 October 2022. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/excessmortalityduringheatperiods/englandandwales1juneto31august2022 (accessed on 31 October 2022).
- Volaco, A.; Cavalcanti, A.M.; Filho, R.P.; Precoma, D.B. Socioeconomic Status: The Missing Link Between Obesity and Diabetes Mellitus? Curr. Diabetes Rev. 2018, 14, 321–326. [Google Scholar] [CrossRef]
- Foster, A.; Cole, J.; Farlow, A.; Petrikova, I. Planetary Health Ethics: Beyond First Principles. Challenges 2019, 10, 14. [Google Scholar] [CrossRef]
- Lundgren, K.; Kuklane, K.; Gao, C.; Holmer, I. Effects of heat stress on working populations when facing climate change. Ind. Health 2013, 51, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Kjellstrom, T.; Butler, A.J.; Lucas, R.M.; Bonita, R. Public health impact of global heating due to climate change: Potential effects on chronic non-communicable diseases. Int. J. Public Health 2010, 55, 97–103. [Google Scholar] [CrossRef]
- Webber, H.; Gaiser, T.; Oomen, R.; Teixeira, E.; Zhao, G.; Wallach, D.; Zimmermann, A.; Ewert, F. Uncertainty in future irrigation water demand and risk of crop failure for maize in Europe. Environ. Res. Lett. 2016, 11, 074007. [Google Scholar] [CrossRef]
- Goulart, H.M.D.; van der Wiel, K.; Folberth, C.; Balkovic, J.; Hurk, B.V.D. Storylines of weather-induced crop failure events under climate change. Earth Syst. Dyn. 2021, 12, 1503–1527. [Google Scholar] [CrossRef]
- Lake, I.R.; Gillespie, I.A.; Bentham, G.; Nichols, G.L.; Lane, C.; Adak, G.K.; Threlfall, E.J. A re-evaluation of the impact of temperature and climate change on foodborne illness. Epidemiol. Infect. 2009, 137, 1538–1547. [Google Scholar] [CrossRef]
- Wheeler, T.; Von Braun, J. Climate Change Impacts on Global Food Security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Galaz, V.; Österblom, H.; Bodin, Ö.; Crona, B. Global networks and global change-induced tipping points. Int. Environ. Agreem. Politics Law Econ. 2014, 16, 189–221. [Google Scholar] [CrossRef]
- E Carter, S.; Ahuka-Mundeke, S.; Zambruni, J.P.; Colorado, C.N.; van Kleef, E.; Lissouba, P.; Meakin, S.; Waroux, O.L.P.D.; Jombart, T.; Mossoko, M.; et al. How to improve outbreak response: A case study of integrated outbreak analytics from Ebola in Eastern Democratic Republic of the Congo. BMJ Glob. Health 2021, 6, e006736. [Google Scholar] [CrossRef]
- Moran, D. Antimicrobial use and planetary health: Developing a framework for priorities. Lancet Planet. Health 2018, 2, e277–e278. [Google Scholar] [CrossRef]
- Frumkin, H. Sustaining Life: Human Health–Planetary Health Linkages. In Health of People, Health of Planet and Our Responsibility; Springer: Cham, Switzerland, 2020; pp. 21–37. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).