Abstract
Information about unsafe foods or feeds must be exchanged between European Union (EU) member states as quickly as possible. This is why the EU’s Rapid Alert System for Food and Feed (RASFF) exists. It helps to ensure that products that may be harmful to health do not enter the market or can be specifically withdrawn from the market. Different notifications are used depending on the risk and urgency. This article provides an overview of the 61 notifications in the RASFF between 2020 and 2022 on the Δ9-tetrahydrocannabinol (Δ9-THC) content in cannabidiol (CBD) oils and CBD food supplements. These products are available on the EU market despite the lack of novel food approval. Δ9-THC is a naturally occurring psychotropic compound extracted from the hemp plant Cannabis sativa that can have adverse effects on consumers (such as drowsiness, dizziness, tachycardia, or changes in blood pressure). In a previous German national survey, 23 of the 125 products tested (18%) exceeded the lowest observed adverse effect level (LOAEL) of Δ9-THC. In comparison, for products identified as a serious risk in the RASFF, the Δ9-THC concentrations were generally higher (up to 2410 mg/kg) and 14 of 34 products (41%) exceeded the LOAEL. Considering these data, a threshold of 500 mg/kg (0.05%) may be proposed to define a serious risk, as the LOAEL would not be exceeded in typical consumption scenarios below this level and serious risks, as well as narcotic effects in the product group of food supplements, could be excluded. This threshold could be used in the interim until the full toxicological assessment is available within the novel food approval procedure.
1. Introduction
Cannabinoid-containing foods are currently being widely advertised and sold in increasing quantities internationally. Products currently marketed with cannabidiol (CBD) include edibles, food supplements, flavorings, mouth sprays, and various non-food products such as tobacco substitutes and liquids for electronic cigarettes [1]. A typical example is the so-called CBD oil, which is marketed as a food supplement in liquid form or in capsules [2]. There is still some uncertainty about the demarcation of CBD products as narcotics, medicines, or foods [3]. However, the Higher Administrative Court of the German Federal State Baden-Württemberg has recently assumed that CBD products have the property of foodstuffs, for which there is now a well-established commercial expectation [3]. Additionally, the Higher Administrative Court also confirmed the novel food status of these products [3], so that all available CBD oils and CBD-containing food supplements are currently placed on the European Union (EU) market with infringement of food laws.
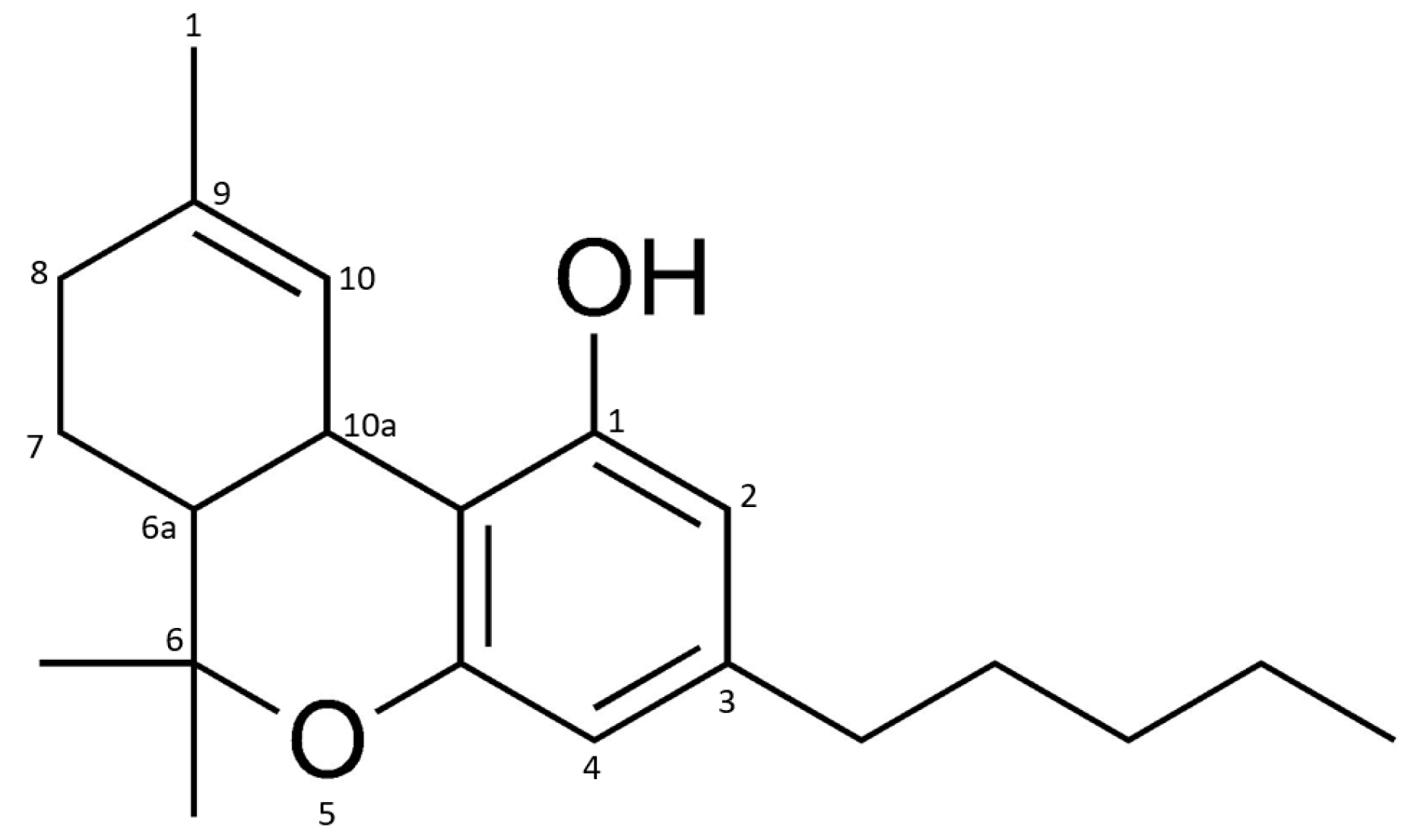
These products are usually made with extracts of the leaves and flowers of the hemp plant (Cannabis sativa L.). The special attraction of this plant is due to the resin that is produced in the glands located on the leaves and flowers. It mostly contains cannabinoids, phytochemicals found only in the hemp plant [2]. The most prominent representative of this class of compounds is Δ9-tetrahydrocannabinol (Δ9-THC, Figure 1), which is hydrogenated at positions 6a and 7 [4].
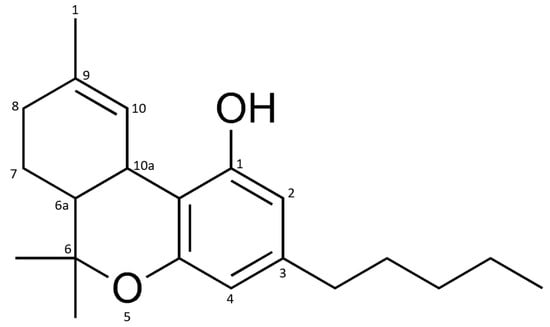
Figure 1.
Chemical structure of Δ9-tetrahydrocannabinol (Δ9-THC).
There are currently no uniform EU maximum levels available for Δ9-THC in foods. Taking into account toxicological assessments based on human data (effects on the central nervous system and heart rate increase), the European Food Safety Authority (EFSA) set a dose of 2.5 mg of Δ9-THC per day as the LOAEL (Lowest observed adverse effect level; lowest dose with observed toxic effect). Taking safety factors into account, an acute reference dose (ARfD) of 1 µg Δ9-THC per kg body weight (bw) was derived from this (assuming a person with a body weight of 70 kg) [5].
Following an opinion of the Standing Committee on Foodstuffs, the European Commission is currently in the process of implementing maximum limits for Δ9-THC in foodstuffs made from hemp seeds on the basis of Regulation (EC) No. 1881/2006: 3.0 mg/kg for dry products (flour, proteins, seeds) and 7.5 mg/kg for hemp seed oil [6].
The currently proposed maximum levels of Regulation (EC) No. 1881/2006 only concern products containing Δ9-THC as a contaminant. These are products derived from hemp seeds, that is, hemp seeds as such, ground hemp seeds, defatted or partially defatted hemp seeds, and other products derived from hemp seeds as well as hemp seed oil. The hemp seed is basically cannabinoid-free at first and is contaminated with cannabinoids from the leaves and flowers in variable degrees during harvesting, depending on the care with which the processing is carried out. Δ9-THC is thus not intentionally added to hemp seed products but is a residue caused by the treatment methods used in farming, and thus meets the definition of a contaminant in Regulation (EEC) No. 315/93 laying down Community procedures for contaminants in foodstuffs.
In other hemp products, such as teas made from leaves or flowers, but also in full spectrum hemp extracts, which provide the basis for many CBD products such as CBD oils, the cannabinoids are not present as contaminants but are already naturally present or are intentionally added to the food, for example, to adjust certain CBD content.
It should also be considered that flowers, leaves, and extracts sometimes contain 100–1000 times more Δ9-THC than seeds. Only by using these materials as ingredients is a harmfully high Δ9-THC intake possible in individual cases, which exceeds the threshold of the LOAEL. In this context, note that the Higher Administrative Court of the German Federal State Baden-Württemberg [3] recently fully confirmed our expert opinions. If it is demonstrated that the Δ9-THC content of a food product exceeds the LOAEL value (2.5 mg) issued by EFSA in 2015, it is harmful to health due to its Δ9-THC content and therefore unsafe (Article 14(1) and (2) (a) of the Basic Regulation [7]) and, therefore, it cannot be placed on the market.
The downside of this is that toxicological expert opinions in individual cases must be conducted considering not only the Δ9-THC content in the food product but also the expected human exposure, that is, the typical daily consumption. It would be much easier to set maximum limits in mg/kg for the group of food supplements as well.
In this article, the authors will focus on the challenges surrounding the derivation of maximum limits for Δ9-THC in food supplements in the European Union (EU).
2. Materials and Methods
This study refers to the increase in the reporting of foods with high Δ9-THC content and the resulting warnings of possible serious risks to consumers due to the psychotropic effects associated with Δ9-THC as well as the chronic toxic effects of Δ9-THC and CBD such as hepatotoxicity.
All data used in this work were retrieved from the EU’s publicly available Rapid Alert System for Food and Feed (RASFF) portal [8]. From the EU RASFF database, notifications with serious hazards for CBD oils and CBD food supplements were investigated. The hazard category THC was selected from the RASFF database for the investigation, as the total of serious notifications of this hazard category together represents more than 75% of all notifications of the hazard category in the EU RASFF database.
The search was performed in the EU RASFF portal database using the following search terms: Product “dietetic foods, food supplements and fortified foods” AND Type “food” AND risk decision “serious” AND Subject “THC”. In addition, risk decisions “not serious” and “undecided” were also examined and compared. Data are for the period of 01 January 2020 till 27 April 2022.
The available data include information on the year, country of origin, product group and matrix of the sample, Δ9-THC content, and content of other cannabinoids.
For hemp food supplements, no consumption data have yet been published as part of an official consumption study. Therefore, an average consumption of 5 g/day for common hemp food supplements was used for the assessment [2].
Note that according to the guidelines of the German Federal Institute for Risk Assessment (BfR), this evaluation is specific for Δ9-THC and not for total-THC, which is a sum of Δ9-THC and non-psychotropic tetrahydrocannabinolic acid (THCA). The BfR suggests that for products, such as food supplements, that will not undergo thermal treatment (which might lead to decarboxylation of the acid form of THC), the risk assessment on an individual basis should not include THCA [9]. It is also of note that apart from the possible decarboxylation of THCA during thermal processes, there is currently no evidence that THCA might be decarboxylated in vivo, for example in the human gastrointestinal tract [10]. Hence, the inclusion of THCA in the risk assessment of food supplements would overestimate exposure and also overestimate psychotropic effects by including the nonpsychotropic acid in total-THC.
Daily Δ9-THC intake was compared with the LOAEL (2.5 mg THC/day) of Δ9-THC [5]. Then the exhaustion of this toxicological evaluation value was calculated. The mean, median, maximum, 90th percentile, and 95th percentile were also calculated. The Δ9-THC levels of all individual samples and the calculations can be found in Supplementary Tables S1–S5. Descriptive statistical calculations described above were performed using Microsoft Excel version 2016 (Microsoft, Redmond, WA, USA). The data were further evaluated using Origin Pro v7.5 (OriginLab Corporation, Northampton, MA, USA). Statistical significance was assumed at below the 0.05 probability level. One-way ANOVA was used to test whether the cases had the same mean, including the Bonferroni post hoc means comparison.
3. Results and Discussion
In total, the dataset retrieved from RASFF contains 61 readings for cannabinoids in various hemp foods. For the subsequent evaluation, only the values for the cannabinoid Δ9-THC from the product group CBD oils and food supplements were considered.
The main country of origin of the notifications was Ireland (38%). Some samples were produced in Germany (31%) or the Czech Republic (9%), while the rest (22%) came from countries such as Poland, Slovenia, Luxembourg, Austria, or Switzerland.
Table 1 shows an overview of the levels of Δ9-THC, which were classified as serious risk in the product group CBD oils and food supplements and compared with all samples of the product group CBD oils and food supplements. The values were also compared with literature values of Δ9-THC analyzed in food supplements between 2018 and 2021. These were generated within the framework of official food control in Germany according to validated and externally accredited methods [11].

Table 1.
Distribution of Δ9-tetrahydrocannabinol in food supplements.
The median Δ9-THC intake (serious risk) of 2.8 mg/d leads to LOAEL exhaustion of 110%. The mean value of the daily Δ9-THC intake of 3.3 mg/d leads to exhaustion of the LOAEL of 129%. Based on the highest determined Δ9-THC content, the results show a maximum daily THC intake of 12 mg/d, which corresponds to an exceedance of LOAEL by more than four times (exhaustion of LOAEL = 478%). This shows that the consumption of certain food supplements could lead to an excess, even of the minimum intoxication dose of 5 mg [12].
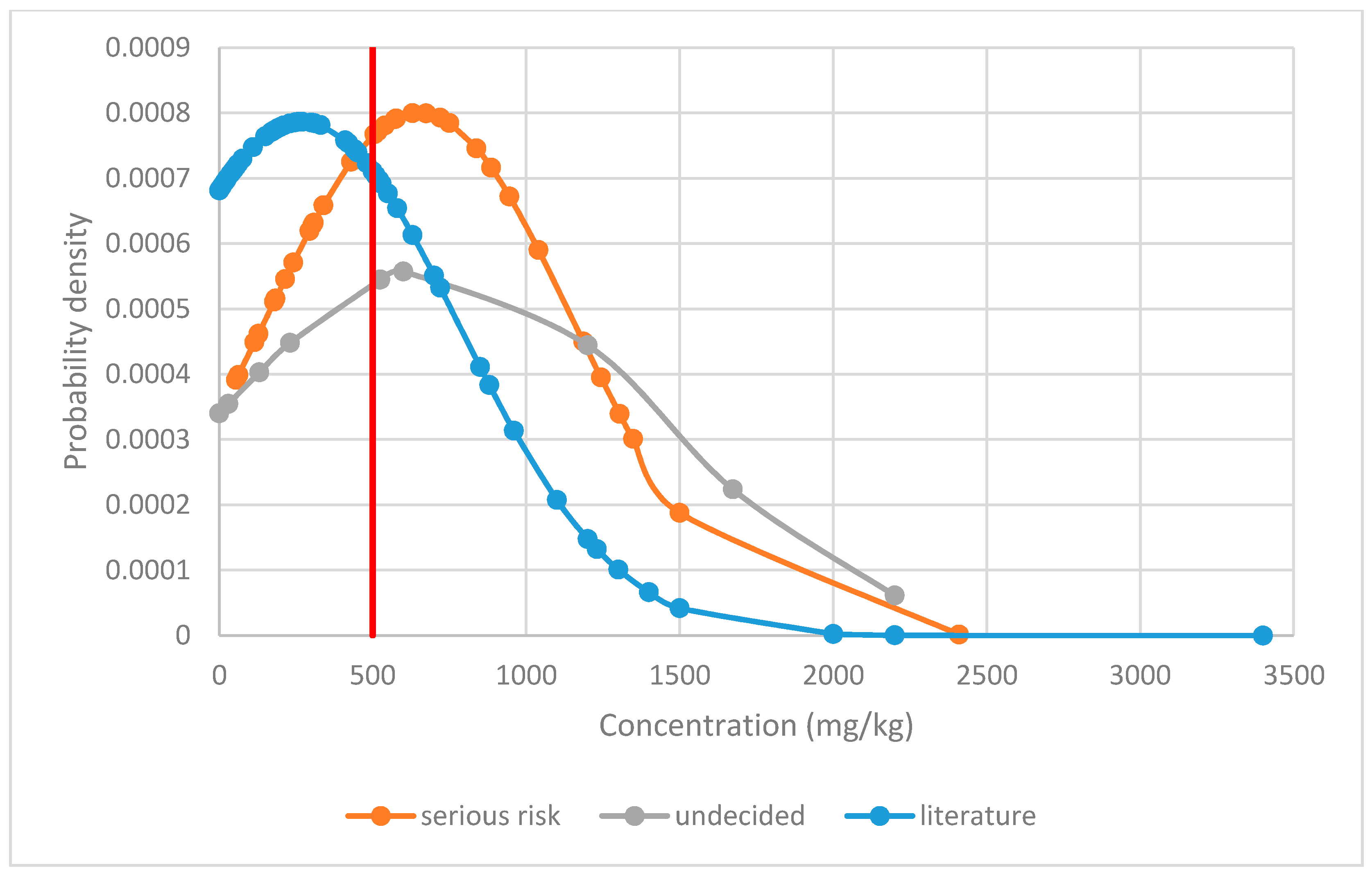
Figure 2 shows the distribution of Δ9-THC content in food supplements, comparing data from the RASFF system classified as serious risk and undetermined risk level and to literature data of a general survey. The three distributions were significantly different (ANOVA p = 0.00014), with a Bonferroni post-hoc comparison showing that both RASFF datasets had significantly (p < 0.05) higher levels than the general literature survey.
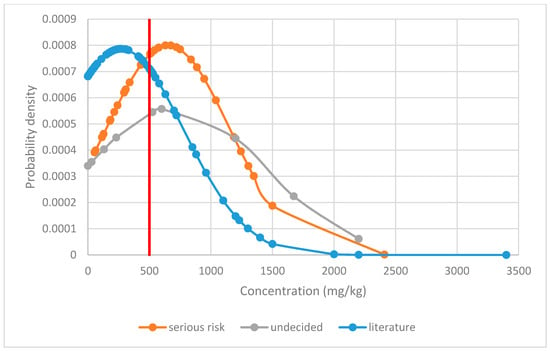
Figure 2.
Normal probability distribution of Δ9-tetrahydrocannabinol levels in food supplements comparing data from the RASFF system assessed as being of serious risk and undecided risk level with literature data [11]. The red line marks the suggested level above which a serious risk can be typically assumed.
Based on the evaluation of the results, a threshold value of 500 mg/kg Δ9-THC in food supplements may be proposed to define a serious risk since up to this limit the LOAEL would not be typically exhausted. This would be a pragmatic approach to more easily identify serious risks in the product group of food supplements. This would also prevent the potential of abuse for intoxication, as required by the German Federal Court of Justice to exclude the demarcation of cannabis products as narcotics [13]. Even in a worst-case scenario of an intake of 5 g, the LOAEL is not fully exhausted. Only in cases of levels below 500 mg/kg, a case-by-case toxicological assessment would need to be conducted, for example, considering intake scenarios in more detail, such as for foods with high consumption amounts.
A study by Steinmetz et al. [14] examined the current safety limit and possible concerns based on available analytical data. Here, a safety-based limit was established at 420 mg/kg for food supplements, which is even more conservative than our assessment, but still in principle well comparable, despite being derived using a different, independent approach.
The UK Advisory Council on the Misuse of Drugs (ACMD) has proposed a maximum level of 50 µg per unit of consumption for Δ9-THC in CBD products. A unit of consumption represents the typical amount of a CBD product consumed on one occasion [15]. This presents a challenge in terms of control, as a unit of consumption is not legally regulated and can vary between different manufacturers of CBD products. The manufacturers of hemp products also often set the daily intake unrealistically low, for example, even down to only one drop of oil per day. A current market investigation by the Swiss cantons shows that many hemp products (especially CBD oils) contain unacceptably high levels of the psychotropic Δ9-THC and effects are to be expected [16].
There is a great deal of uncertainty about the risk assessment of Δ9-THC in food, so work should be done to establish limits within the context of the novel food approval procedure currently being conducted for the various CBD products. These should also consider the chronic effects of both CBD and THC, which are not currently adequately covered due to a lack of data. In the current opinion of EFSA in June 2022, the panel identified several hazards associated with CBD intake and pointed to insufficient data from animal studies and humans on any health risks. Animal studies, for example, show adverse effects, particularly in relation to reproduction. Whether the same is true in humans is still under investigation. The evaluation of CBD as a novel food has been halted for the time being until the applicants provide further data, as the safety of CBD as a novel food cannot be demonstrated at this time [17].
The evaluation of CBD also goes beyond the EU. Currently, the use of CBD in dietary supplements is also banned in the United States by the Food and Drug Administration (FDA) [18].
4. Conclusions
Maximum levels for both Δ9-THC and CBD are expected to be implemented in the specifications of the novel food approvals for CBD products, provided they are positively decided by the EFSA and the EU Commission. Until then, the application of a threshold of 500 mg/kg (0.05%) for Δ9-THC could be a pragmatic approach to exclude serious risks in the product group of food supplements. This would be much easier to implement than the maximum limits per unit of consumption, which is highly variable and a rather vague concept.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/challe13020032/s1, Table S1: Calculations of THC from literature for all hemp food supplement samples between 2018 and 2021; Table S2: Calculations of THC of all CBD oils and supplements from the RASFF portal that have been classified as serious risk; Table S3: Calculations of THC of all CBD oils and supplements from the RASFF portal that have been classified as undecided risk; Table S4: Summary for all samples; Table S5: Distribution of Δ9-tetrahydrocannabinol levels in food supplements (data for Figure 2).
Author Contributions
Conceptualization, D.W.L.; methodology, D.W.L.; software, D.W.L.; validation, S.S.; formal analysis, S.S.; investigation, S.S.; resources, S.G.W.; data curation, S.S.; writing—original draft preparation, S.S.; writing—review and editing, D.W.L., P.G., C.S. and S.G.W.; visualization, S.S.; supervision, D.W.L.; project administration, D.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 27 April 2022). The derivative calculations presented in this study are available in the supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kraft, K.; Thomsen, M.; Schmidt, M. Cannabidiol: Food or drug? A positioning. J. Mod. Med. Chem. 2021, 9, 17–24. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Habel, S.; Fischer, B.; Herbi, F.; Zerbe, Y.; Bock, V.; Rajcic de Rezende, T.; Walch, S.G.; Sproll, C. Are adverse effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000 Res. 2021, 8, 1394. [Google Scholar] [CrossRef] [PubMed]
- Administrative Court (Verwaltungsgerichtshof, VGH) Baden-Württemberg (2022): Ruling of 9 March 2022, Az. 9 S 3426/21. ECLI:DE:VGHBW:2022:0309.9S3426.21.00.
- Golombek, P.; Müller, M.; Barthlott, I.; Sproll, C.; Lachenmeier, D.W. Conversion of cannabidiol (CBD) into psychotropic cannabinoids including tetrahydrocannabinol (THC): A controversy in the scientific literature. Toxics 2020, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Arcella, D.; Cascio, C.; Mackay, K. Acute human exposure assessment to tetrahydrocannabinol (Δ9-THC). EFSA J. 2019, 18, 5953. [Google Scholar] [CrossRef] [Green Version]
- Southey, F. EU-Wide Adoption of Max THC Limits in Hemp Seed Foods a ‘Significant Win for the Sector’. Available online: https://www.foodnavigator.com/Article/2022/03/16/eu-wide-adoption-of-max-thc-limits-in-hemp-seed-foods-a-significant-win-for-sector (accessed on 2 June 2022).
- European Parliament and Council. Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. EC 2002, L031, 1–24. [Google Scholar]
- RASFF Portal. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 27 April 2022).
- BfR. Opinion No. 006/2021 issued 17 February 2021. The BfR Recommends Acute Reference Dose as Basis for Assessing Hemp-Containing Foodstuff; Bundesinstitut für Risikobewertung (BfR): Berlin, Germany, 2021. [CrossRef]
- Moreno-Sanz, G. Can you pass the acid test? Critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannab. Res. 2016, 1, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dräger, H.; Barthlott, I.; Golombek, P.; Walch, S.G.; Lachenmeier, D.W. Time trends of tetrahydrocannabinol (THC) in a 2008-2021 German national survey of hemp food products. Foods 2022, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.; Rehm, J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci. Rep. 2015, 5, 8126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federal Court of Justice (Bundesgerichtshof, BGH). Ruling of 24 March 2021, Az. 6 StR 240/20. ECLI:DE:BGH:2021:240321U6STR240.20.0.
- Steinmetz, F.P.; Nahler, G.; Wakefield, J.C. How safe are hemp-based food products? A review and risk assessment of analytical data from Germany. Nutr. Food Sci. 2022. [Google Scholar] [CrossRef]
- Advisory Council on the Misuse of Drugs (ACMD). Consumer Cannabidiol (CBD) Products. 2021. Available online: https://www.gov.uk/government/publications/acmd-advice-on-consumer-cannabidiol-cbd-products/consumer-cannabidiol-cbd-products-report-accessible-version (accessed on 2 June 2022).
- Bundesamt für Lebensmittelsicherheit und Veterinärwesen (BLV). Risikobewertung. Briefing Letter Cannabidiol (CBD) in Lebensmitteln und Lebereffekte, 2021. Available online: https://www.blv.admin.ch/dam/blv/de/dokumente/lebensmittel-und-ernaehrung/publikationen-forschung/briefing-letter-lebensmittel-lebereffekte.pdf.download.pdf/Briefing%20Letter%20Cannabidiol%20in%20Lebensmitteln%20und%20Lebereffekte%20DE.pdf (accessed on 2 June 2022).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Statement on safety of cannabidiol as a novel food: Data gaps and uncertainties. EFSA J. 2022, 20, 7322. [Google Scholar] [CrossRef]
- Nyland, C.; Moyer, D.C. Regulating for safety: Cannabidiol dose in food. J. Food Protect. 2022. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).