First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea
Abstract
1. Introduction
2. Methods
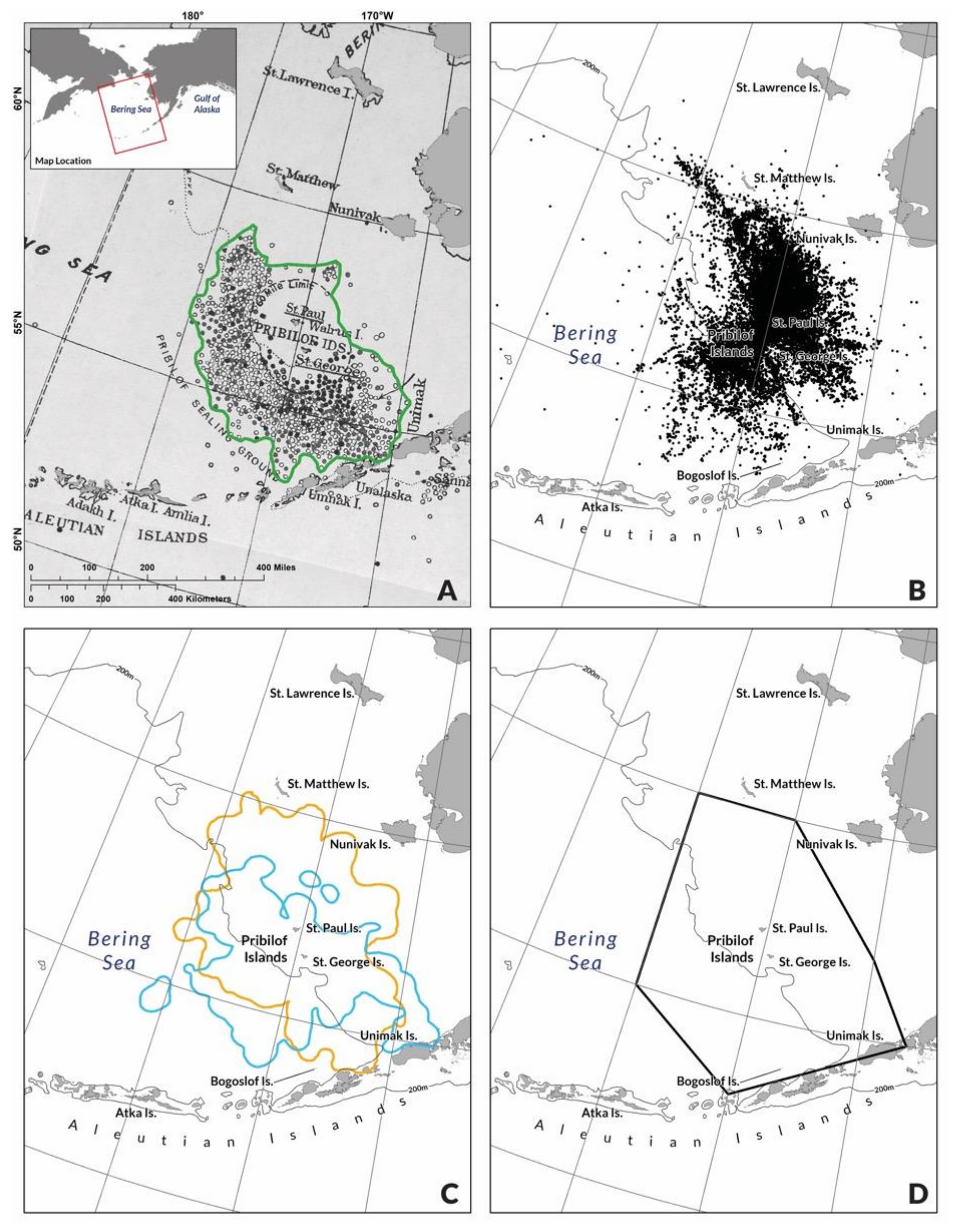
2.1. Pribilof Fur Seal Habitat, Life History and Data Sources
2.2. Bering Sea Pollock Fishery and Data Sources
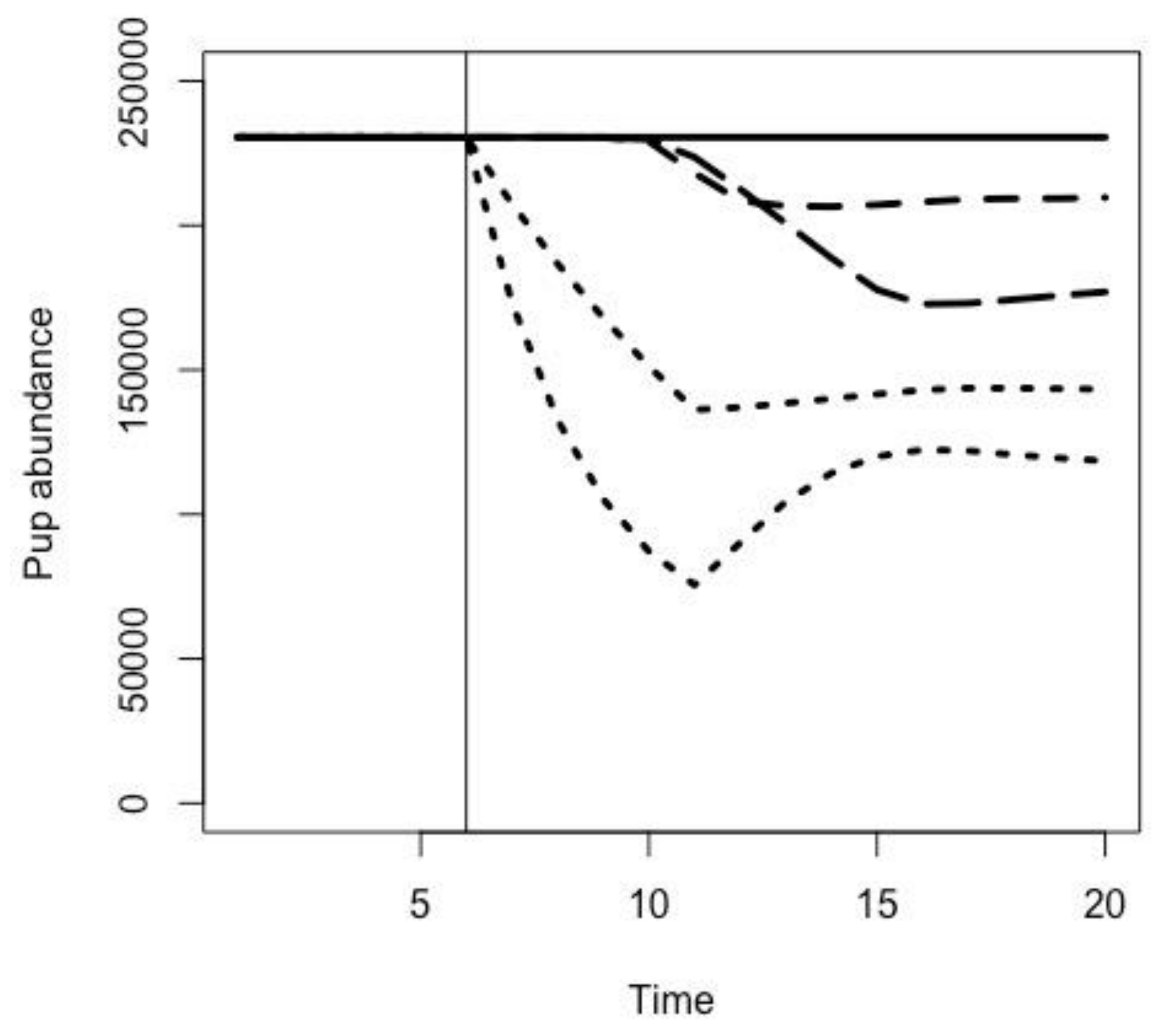
2.3. Effects of Fur Seal Recruitment Perturbations
2.4. Pup Survival Perturbation Models
2.5. Bayesian Estimates of St. Paul Fur Seal Survival as a Function of Pollock Catch
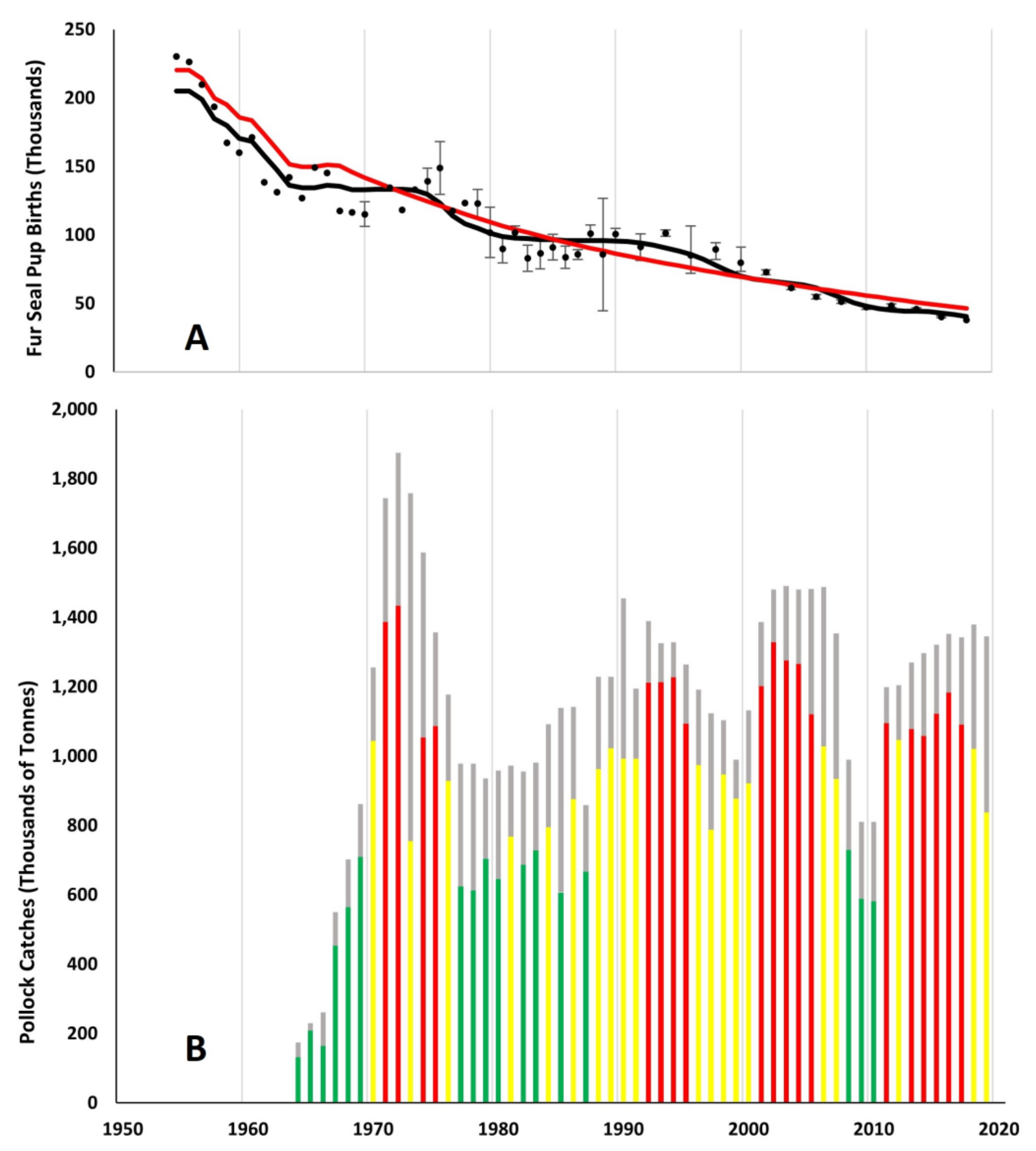
3. Results
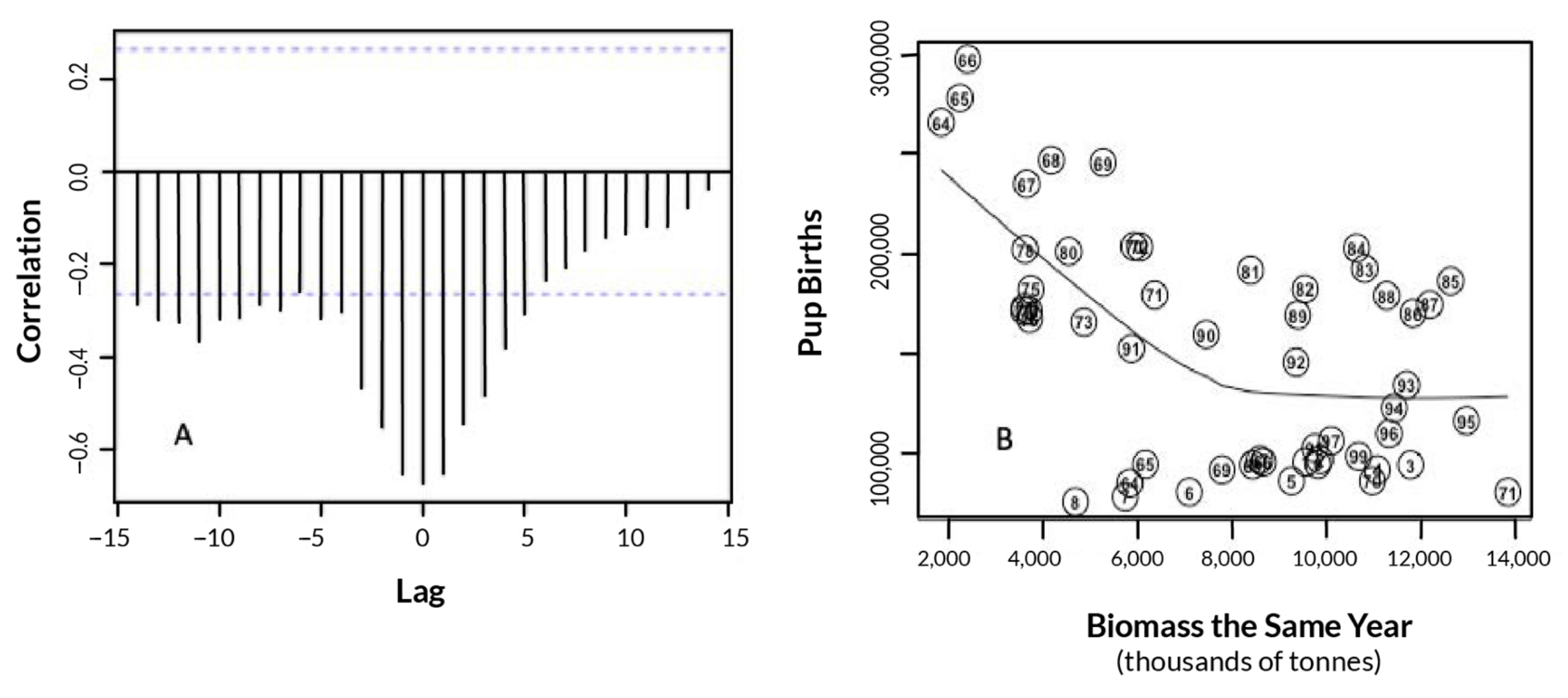
3.1. Effects of Fur Seal Recruitment Perturbations
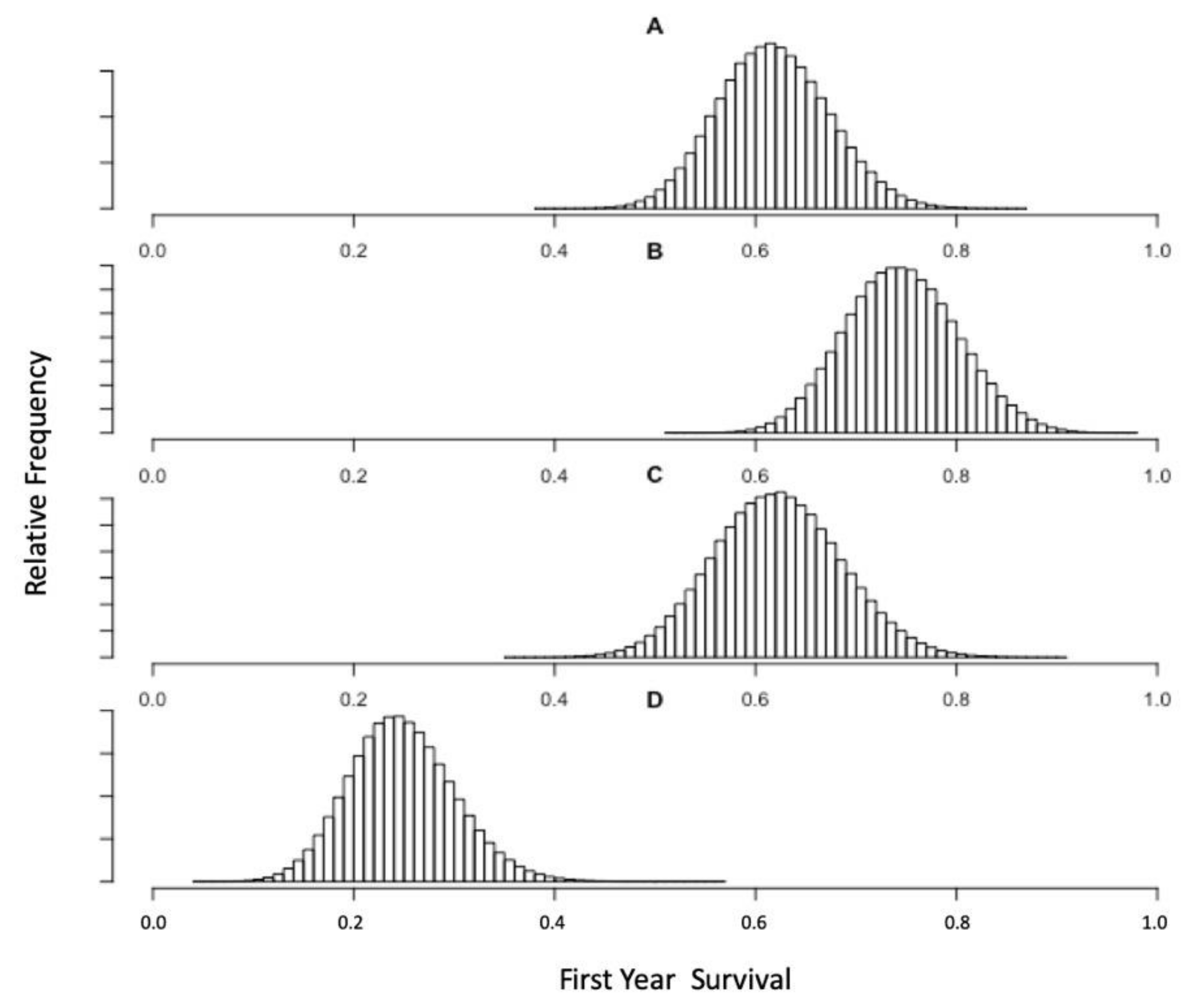
3.2. Pup Survival Perturbation Models
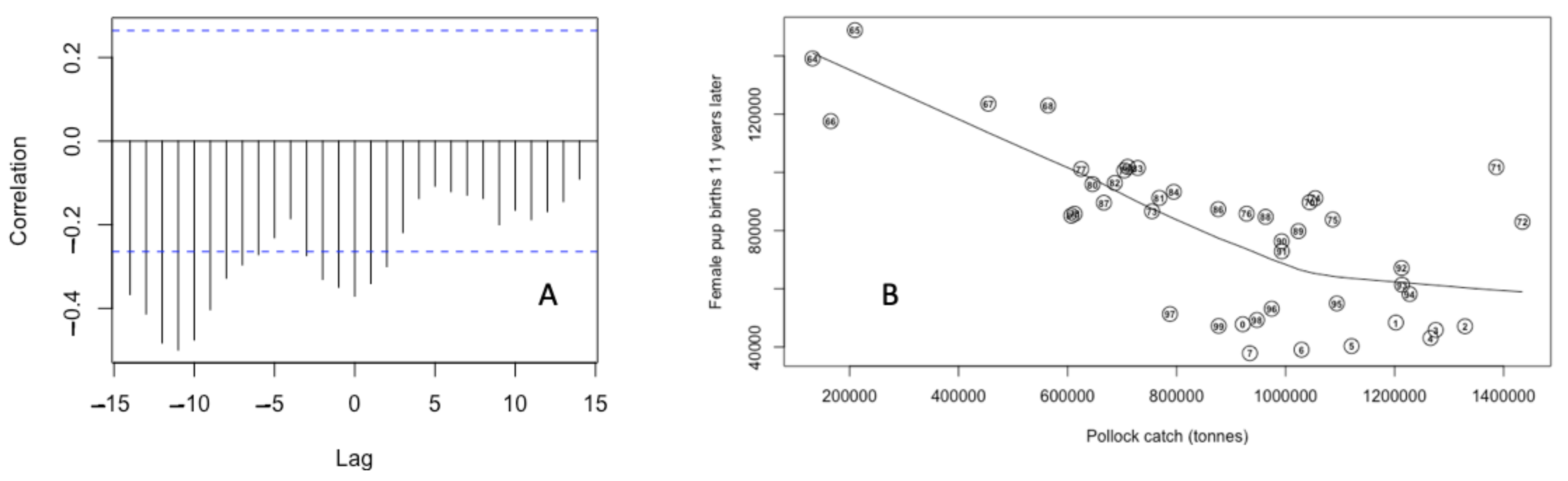
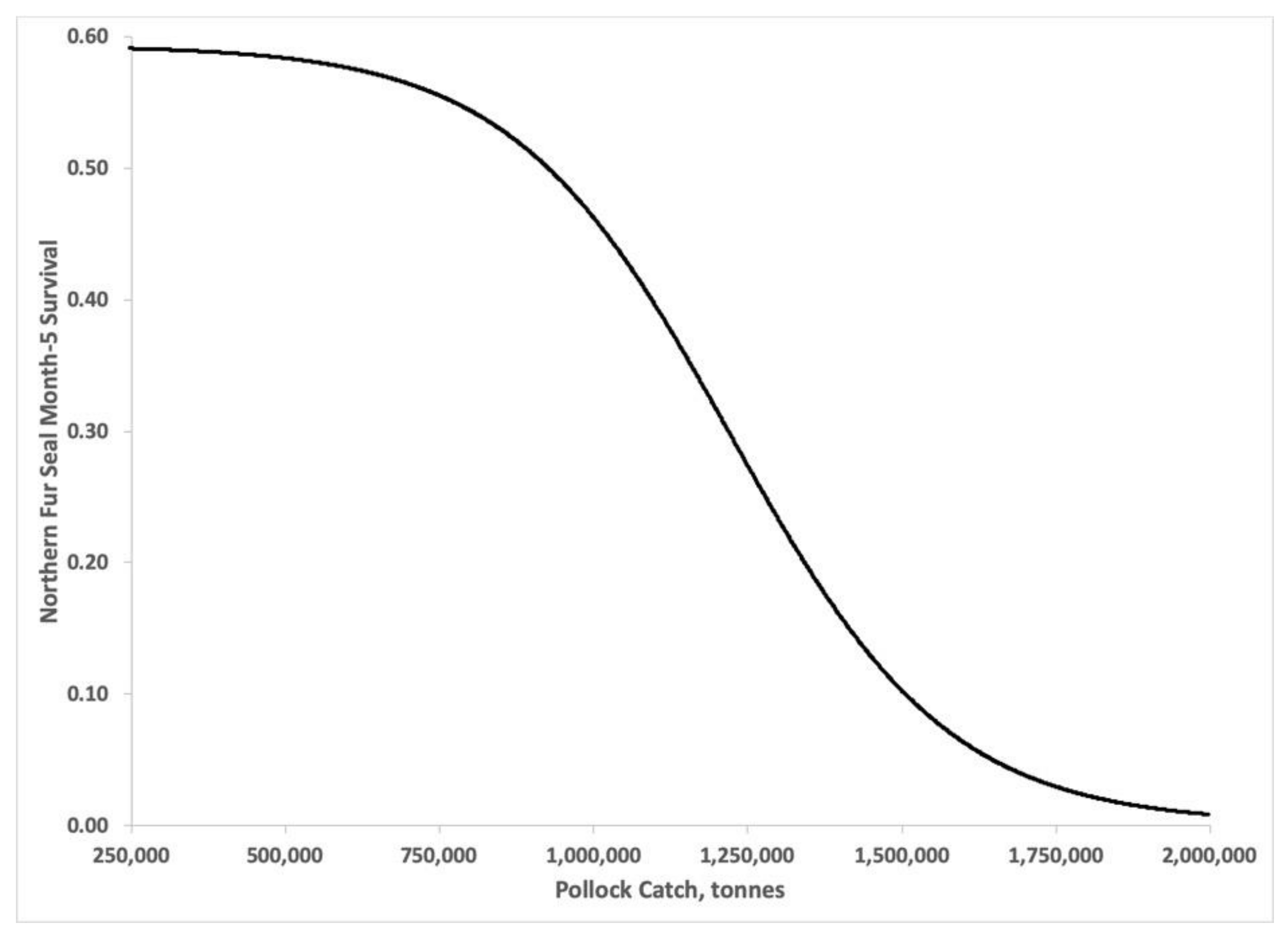
3.3. Bayesian Estimates of St. Paul Fur Seal Survival as a Function of Pollock Catch
4. Discussion
4.1. Comparison of Pollock Catch and Biomass Models
4.2. Pollock Catch and Regime Shift Effects on Fur Seal Births
4.3. Fur Seal Size Selectivity of Consumed Pollock
4.4. Fur Seal Population Response to Competition
4.5. Restorative Capacity of the Pribilof Northern Fur Seal Herd
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kenyon, K.W.; Scheffer, V.B.; Chapman, D.G. A Population Study of the Alaska Fur-Seal Herd; Special Scientific Report—Wildlife 12; U.S. Fish and Wildlife Service: Washington, DC, USA, 1954; p. 77. [Google Scholar]
- U.S. Department of Commerce. Conservation Plan for the Eastern Pacific Stock of Northern Fur Seal (Callorhinus ursinus); National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Alaska Regional Office—Protected Resources Division: Juneau, AK, USA, 2007; p. 99. [Google Scholar]
- Towell, R.G.; Ream, R.R.; Sterling, J.T.; Bengtson, J.L.; Williams, M.; Alaska Fisheries Science Center; National Marine Fisheries Service; National Oceanic and Atmospheric Administration. 2018 Northern Fur Seal Pup Production and Adult Male Counts on the Pribilof Islands, Alaska; Memorandum to the Record; Alaska Fisheries Science Center: Seattle, WA, USA, 2019. [Google Scholar]
- Muto, M.M.; Helker, V.T.; Delean, J.; Angliss, R.P.; Boveng, P.L.; Breiwick, J.M.; Brost, M.M.; Cameron, M.F.; Clapham, P.J.; Dahle, S.P.; et al. Alaska Marine Mammal Stock Assessments, 2019; NOAA Tech. Memo. NMFS–AFSC–404; National Technical Information Service: Springfield, VA, USA, 2020; pp. 30–40. [Google Scholar]
- Lander, R.H. A life table and biomass estimate for Alaskan fur seals. Fish. Res. 1981, 1, 55–70. [Google Scholar] [CrossRef]
- York, A.E.; Hartley, J.R. Pup production following harvest of female northern fur seals. Can. J. Fish. Aquat. Sci. 1981, 38, 84–90. [Google Scholar] [CrossRef]
- York, A.E.; Kozloff, P.A. On the estimation of numbers of northern fur seal, Callorhinus ursinus, pups born on St. Paul Island, 1980−6. Fish. Bull. 1987, 85, 367–375. [Google Scholar]
- Trites, A.; Larkin, P.A. The decline and fall of the Pribilof fur seal (Callorhinus ursinus): A simulation study. Can. J. Fish Aquat. Sci. 1989, 46, 1437–1445. [Google Scholar] [CrossRef]
- Trites, A.W. Northern fur seals: Why have they declined? Aquat. Mamm. 1992, 18, 3–18. [Google Scholar]
- Towell, R.G.; Ream, R.R.; York, A.E. Decline in northern fur seal (Callorhinus ursinus) pup production on the Pribilof Islands. Mar. Mamm. Sci. 2006, 22, 486–491. [Google Scholar] [CrossRef]
- Scheffer, V.B. The Year of the Seal; Charles Scribner’s Sons: New York, NY, USA, 1970; p. 205. [Google Scholar]
- Perez, M.A.; McAlister, W.B. Estimates of Food Consumption by Marine Mammals in the Eastern Bering Sea; NOAA Tech. Memo NMFS–AFSC–14; National Technical Information Service: Springfield, VA, USA, 1993; p. 36. [Google Scholar]
- Elliot, H.W. A monograph of the Seal-Islands of Alaska; Government Printing Office: Washington, DC, USA, 1882; p. 176.
- Jordan, D.S.; Clark, G.A. The history, condition, and needs of the herd of fur seals resorting to the Pribilof Islands. In The Fur Seals and Fur-Seal Islands of the North Pacific Ocean, Part 1; Jordan, D.S., Ed.; Government Printing Office: Washington, DC, USA, 1898; p. 249. [Google Scholar]
- Lucas, F.A. The breeding habits of Pribilof fur seal. In The Fur Seals and Fur-Seal Islands of the North Pacific Ocean, Part 3; Jordan, D.S., Ed.; Government Printing Office: Washington, DC, USA, 1899; pp. 43–57. [Google Scholar]
- Bartholomew, G.A., Jr.; Hoel, P.G. Reproductive behavior of the Alaska fur seal, Callorhinus ursinus. J. Mammal. 1953, 34, 417–436. [Google Scholar] [CrossRef]
- Roppel, A.Y.; Davey, S.P. Evolution of fur seal management on the Pribilof Islands. J. Wildl. Manag. 1965, 29, 448–463. [Google Scholar] [CrossRef]
- Siddon, E.C.; Zador, S.G.; Hunt, G.L., Jr. Ecological responses to climate perturbations and minimal sea ice in the northern Bering Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020, 181–182, 104914. [Google Scholar] [CrossRef]
- Ichihara, T. Possible effect of surface wind force on the sex-specific mortality of young fur seals in the eastern Pacific. Bull. Far Seas. Fish. Res. Lab. 1974, 11, 1–8. [Google Scholar]
- Lea, M.A.; Johnson, D.; Ream, R.; Sterling, J.; Melin, S.; Gelatt, T. Extreme weather events influence dispersal of naive northern fur seals. Biol. Lett. 2009, 5, 252–257. [Google Scholar] [CrossRef]
- McHuron, E.A.; Luxa, K.; Pelland, N.A.; Holsman, K.; Ream, R.; Zeppelin, T.; Sterling, J.T. Practical application of a bioenergetic model to inform management of a declining fur seal population and their commercially important prey. Front. Mar. Sci. 2020, 7, 1027. [Google Scholar] [CrossRef]
- Spraker, T.R.; Lander, M.E. Causes of mortality in Northern fur seals (Callorhinus ursinus), St. Paul island, Pribilof islands, Alaska, 1986–2006. J. Wildl. Dis. 2010, 46, 450–473. [Google Scholar] [CrossRef] [PubMed]
- Ianelli, J.; Fissel, B.; Holman, K.; Honkalehto, T.; Kotwicki, S.; Monnahan, C.; Siddon, E.; Stienessen, S.; Thorson, J. Assessment of the walleye pollock stock in the eastern Bering Sea. In North Pacific Fishery Management Council Bering Sea and Aleutian Islands Stock Assessment and Fishery Evaluation; North Pacific Fishery Management Council: Anchorage, AK, USA, 2019; p. 169. [Google Scholar]
- Zeppelin, T.; Pelland, N.; Sterling, J.; Brost, B.; Melin, S.; Johnson, D.; Lea, M.A.; Ream, R. Migratory strategies of juvenile nortern fur seals (Callorhinus ursinus): Bridging the gap between pups and adults. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef]
- Townsend, C.H. Pelagic sealing. In The Fur Seals and Fur-Seal Islands of the North Pacific Ocean, Part 3; Jordan, D.S., Ed.; Washington, Government Printing Office: Washington, DC, USA, 1899; pp. 223–274. [Google Scholar]
- Springer, A.M.; Ream, R.R.; Iverson, S.J. Seasonal Foraging Strategies and Consequences for Northern Fur Seals at Colonies with Opposite Population Trends—Year 2; North Pacific Research Board Final Report Project 514; North Pacific Research Board: Anchorage, AK, USA, 2008; p. 79. [Google Scholar]
- Robson, B.W.; Goebel, M.E.; Baker, J.D.; Ream, R.R.; Loughlin, T.R.; Francis, R.C.; Antonelis, G.A.; Costa, D.P. Separation of foraging habitat among breeding sites of a colonial marine predator, the northern fur seal (Callorhinus ursinus). Can. J. Zool. 2004, 82, 20–29. [Google Scholar] [CrossRef]
- Benoit-Bird, K.J.; Battaile, B.C.; Nordstrom, C.A.; Trites, A.W. Foraging behavior of northern fur seals closely matches the hierarchical patch scales of prey. Mar. Ecol. Prog. Ser. 2013, 479, 283–302. [Google Scholar] [CrossRef][Green Version]
- Kenyon, K.W.; Wilke, F. Migration of the northern fur seal, Callorhinus ursinus. J. Mammol. 1953, 34, 86–98. [Google Scholar] [CrossRef]
- Ragen, T.J.; Antonelis, G.A.; Kiyota, M. Early migration of northern fur seal pups from St. Paul Island, Alaska. J. Mammol. 1995, 76, 1137–1148. [Google Scholar] [CrossRef]
- Baker, J.D. Post-weaning migration of northern fur seal Callorhinus ursinus pups from the Pribilof Islands, Alaska. Mar. Ecol. Prog. Ser. 2007, 341, 243–255. [Google Scholar] [CrossRef]
- Baker, J.D.; Donohue, M.J. Ontogeny of swimming and diving in northern fur seal (Callorhinus ursinus) pups. Can. J. Zool. 2000, 78, 100–109. [Google Scholar] [CrossRef]
- Abegglen, C.E.; Roppel, A.Y. Fertility in the northern fur seal, 1956–1957. J. Wildl. Mgmt. 1959, 23, 75–81. [Google Scholar] [CrossRef]
- Bancroft, H.H. The works of Hubert Howe Bancroft. In History of Alaska, 1730–1885; A.L. Bancroft & Company: San Francisco, CA, USA, 1886; Volume 33, p. 775. [Google Scholar]
- Trites, A.W. Estimating the juvenile survival rate of male northern fur seals (Callorhinus ursinus). Can. J. Fish. Aquat. Sci. 1989, 46, 1428–1436. [Google Scholar] [CrossRef]
- Kozloff, P. Fur Seal Investigations, 1982; NOAA Tech. Memo. NMFS F/NWC–71; National Technical Information Service: Springfield, VA, USA, 1985; p. 127. [Google Scholar]
- Kajimura, H. Opportunistic feeding of northern fur seals in the eastern North Pacific Ocean. In Fur Seal Investigations, 1982; NOAA Tech. Memo. NMFS F/NWC–71; Kozloff, P., Ed.; U.S. National Technical Information Service: Springfield, VA, USA, 1985; pp. 61–70. [Google Scholar]
- Sterling, J.T.; Ream, R.R. At-sea behavior of juvenile male northern fur seals (Callorhinus ursinus). Can. J. Zool. 2004, 82, 1621–1637. [Google Scholar] [CrossRef]
- Scheffer, V.B. The food of the Alaska fur seal, Wildlife Leaflet 329; U.S. Department Interior, Fish and Wildlife Service: Washington, DC, USA, 1950; p. 16. [Google Scholar]
- Wilke, F. Alaska Fur Seal Investigations, Pribilof Islands, Alaska; U.S. Fish and Wildlife Service, Bureau of Commercial Fisheries, Section of Marine Mammal Research: Seattle, WA, USA, 1955; p. 46. [Google Scholar]
- Wilke, F.; Kenyon, K.W. The food of fur seals in the eastern Bering Sea. J. Wildl. Manag. 1957, 21, 237–238. [Google Scholar] [CrossRef]
- Fiscus, C.H.; Niggol, K.; Wilke, F. Pelagic Fur Seal Investigations, California to British Columbia; U.S. Fish and Wildlife Service, Bureau of Commercial Fisheries, Marine Mammal Research: Seattle, WA, USA, 1961; p. 87. [Google Scholar]
- Perez, M.A.; Bigg, M.A. Diet of northern fur seals, Callorhinus ursinus, off Western North America. Fish. Bull. 1986, 84, 957–971. [Google Scholar]
- Sinclair, E.T.; Loughlin, T.R.; Pearcy, W. Prey selection by northern fur seals (Callorhinus ursinus) in the eastern Bering Sea. Fish. Bull. 1994, 92, 144–156. [Google Scholar]
- Hanna, G.D. The Alaska Fur Seal Islands; NOAA Tech. Memo. NOS–ORR–16; Lindsay, J.A., Ed.; U.S. National Technical Information Service: Springfield, VA, USA, 2008; p. 295. [Google Scholar]
- Lucas, F.A. Food of the northern fur seals. In The Fur Seals and Fur-Seal Islands of the North Pacific Ocean, Part 3; Jordan, D.S., Ed.; Washington, Government Printing Office: Washington, DC, USA, 1899; pp. 59–67. [Google Scholar]
- Sinclair, E.H. Fur Seal Investigations, 1994; NOAA Tech. Memo. NMFS–AFSC–69; U.S. National Technical Information Service: Springfield, VA, USA, 1996; p. 144. [Google Scholar]
- Goebel, M. Northern Fur Seal Lactation, Attendance and Reproductive Success in Two Years of Contrasting Oceanography. Ph.D. Thesis, University of California at Santa Cruz, Santa Cruz, CA, USA, December 2002. [Google Scholar]
- Gudmundson, C.J.; Zeppelin, T.K.; Ream, R.R. Application of two methods for determining diet of northern fur seals (Callorhinus ursinus). Fish. Bull. 2006, 104, 445–455. [Google Scholar]
- Zeppelin, T.K.; Ream, R.R. Foraging habitats based on the diet of female northern fur seals (Callorhinus ursinus) on the Pribilof Islands, Alaska. J. Zool. 2006, 270, 565–576. [Google Scholar] [CrossRef]
- Sterling, J.T. Northern fur seal foraging behaviors, food webs, and interactions with oceanographic features in the eastern Bering Sea. Ph.D. Dissertation, University of Washington, Seattle, WA, USA, 2009. [Google Scholar]
- Gentry, R.L.; Goebel, M.E.; Roberts, W.E. Behavior and biology, Pribilof Islands, Alaska. In Fur Seal Investigations, 1984; NOAA Tech. Memo. NMFS F/NWC–97; Kozloff, P., Ed.; U.S. National Technical Information Service: Springfield, VA, USA, 1986; pp. 29–40. [Google Scholar]
- Gentry, R.L.; Kooyman, G.E.; Goebel, M.E. Feeding and diving behavior of northern fur seals. In Fur Seals: Maternal Strategies on Land and at Sea; Gentry, R., Kooyman, G., Eds.; Princeton University Press: Princeton, NJ, USA, 1986; pp. 61–78. [Google Scholar] [CrossRef]
- Sinclair, E.H.; Antonelis, G.A.; Robson, B.W.; Ream, R.R.; Loughlin, T.R. Northern fur seal, Callorhinus ursinus, predation on juvenile walleye pollock, Theragra chalcogramma. In The Importance of Prerecruit Walleye Pollock to the Bering Sea and North Pacific Ecosystems; NOAA Technical Report 126; Brodeur, R., Livingston, P., Loughlin, T., Hollowed, A., Eds.; Alaska Fisheries Science Center, National Marine Fisheries Service: Seattle, WA, USA, 1996; pp. 167–178. [Google Scholar]
- Kuhn, C.E.; Ream, R.R.; Sterling, J.T.; Thomason, J.R.; Towell, R.G. Spatial segregation and the influence of habitat on the foraging behavior of northern fur seals (Callorhinus ursinus). Can. J. Zool. 2014, 92, 861–873. [Google Scholar] [CrossRef]
- Kuhn, C.E.; Baker, J.D.; Towell, R.G.; Ream, R.R. Evidence of localized resource depletion following a natural colonization event by a large marine predator. J. Anim. Ecol. 2014, 83, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Testa, J.W. Fur Seal Investigations, 2013–2014; NOAA Tech. Memo. NMFS–AFSC–316; National Technical Information Service: Springfield, VA, USA, 2016; p. 124. [Google Scholar]
- Testa, J.W. Fur Seal Investigations, 2015–2016; NOAA Tech. Memo. NMFS–AFSC–375; National Technical Information Service: Springfield, VA, USA, 2018; p. 107. [Google Scholar]
- Strong, J.W.; Criddle, K.R. Fishing for Pollock in a Sea of Change: A Historical Analysis of the Bering Sea Pollock Fishery; Alaska Sea Grant, University of Alaska Press: Fairbanks, AK, USA, 2013; p. 177. [Google Scholar] [CrossRef]
- Bakkala, R.G. Structure and Historical Changes in the Groundfish Complex of the Eastern Bering Sea; NOAA Tech. Rept. NMFS–114; U.S. National Technical Information Service: Springfield, VA, USA, 1993; p. 100. [Google Scholar]
- Murai, S.; Gangmark, H.A.; French, R.R. All-Nation Removals of Groundfish, Herring, and Shrimp from the Eastern Bering Sea and Northeast Pacific Ocean, 1964–1980; NOAA Tech. Memo. NMFS F/NWC–14; U.S. National Technical Information Service: Springfield, VA, USA, 1981; p. 40. [Google Scholar]
- Fisheries Monitoring and Analysis Division . Alaska Fisheries Science Center: Seattle, WA, USA, Unpublished work on observed groundfish fisheries data, foreign, joint-venture, and domestic, 1973–2019. 2021.
- Seber, G.A.F. The Estimation of Animal Abundance and Related Parameters, 2nd ed.; The Blackburn Press: Caldwell, NJ, USA, 2002; p. 654. [Google Scholar]
- Pyper, B.J.; Peterman, R.M. Comparison of methods to account for autocorrelation in correlation analyses of fish data. Can. J. Fish. Aquat. Sci. 1998, 55, 2127–2140. [Google Scholar] [CrossRef]
- Swartzman, G.L.; Haar, R.T. Interactions between fur seal populations and fisheries in the Bering Sea. Fish. Bull. 1983, 81, 121–132. [Google Scholar]
- Holling, C.S. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Wilke, F.; Niggol, K.; Fiscus, C.H. Pelagic Fur Seal Investigations, California to British Columbia; U.S. Fish and Wildlife Service, Bureau of Commercial Fisheries, Section of Marine Mammal Research: Seattle, WA, USA, 1958; p. 96. [Google Scholar]
- Spiegelhalter, D.J.; Best, N.G.; Carlin, B.P.; van der Linde, A. The deviance information criterion: 12 years on. J. R. Stat. Soc. Ser. B Stat. Methodol. 2014, 76, 485–493. [Google Scholar] [CrossRef]
- Bakkala, R.G.; Wespestad, V.G.; Low, L.L. Historical trends in abundance and current condition of walleye pollock in the eastern Bering Sea. Fish. Res. 1987, 5, 199–215. [Google Scholar] [CrossRef]
- Rose, G.A.; Kulka, D.W. Hyperaggregation of fish and fisheries: How catch-per-unit-effort increased as the northern cod (Gadus morhua) declined. Can. J. Fish. Aq. Sci. 1999, 56, 118–127. [Google Scholar] [CrossRef]
- Smith, G.B. The biology of walleye pollock. In Fisheries Oceanoagraphy—Eastern Bering Sea Shelf; NWAFC Processed Report 79-20; U.S. Department of Commerce: Springfield, VA, USA, 1979; pp. 213–279. [Google Scholar]
- Wespestad, V.G.; Terry, J.M. Biological and economic yields for eastern Bering Sea walleye pollock under differing fishing regimes. North Am. J. Fish. Manag. 1984, 4, 204–215. [Google Scholar] [CrossRef]
- Hunt, G.L., Jr.; Stabeno, P.; Walters, G.; Sinclair, E.; Brodeur, R.D.; Napp, J.M.; Bond, N.A. Climate change and control of the southeastern Bering Sea pelagic ecosystem. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5821–5823. [Google Scholar] [CrossRef]
- Trites, A.W.; Donnelly, C.P. The decline of Steller sea lions in Alaska: A review of the nutritional stress hypothesis. Mamm. Rev. 2003, 33, 3–28. [Google Scholar] [CrossRef]
- Sinclair, E.H.; Vlietstra, L.S.; Johnson, D.S.; Zeppelin, T.K.; Byrd, G.V.; Springer, A.M.; Ream, R.R.; Hunt, G.L., Jr. Patterns in prey use among fur seals and seabirds in the Pribilof Islands. Deep Sea Res. Part II Top. Stud. Oceanogr. 2008, 55, 1897–1919. [Google Scholar] [CrossRef]
- Fritz, L.W.; Hinckley, S. A critical review of the regime shift-“junk food”-nutritional stress hypothesis for the decline of the western stock of Steller sea lion. Mar. Mamm. Sci. 2005, 21, 476–518. [Google Scholar] [CrossRef]
- Mueter, F.J.; Ladd, C.; Palmer, M.C.; Norcross, B.L. Bottom-up and top-down controls of walleye pollock (Theragra chalcogramma) on the eastern Bering Sea shelf. Prog. Oceanogr. 2006, 68, 152–183. [Google Scholar] [CrossRef]
- Barbeaux, S.J.; Dorn, M.; Ianelli, J.; Horne, J. Visualizing Alaska Pollock (Theragra chalcogramma) Aggregation Dynamics; Document CM 2005/ U: 01; International Council for the Exploration of the Seas: Copenhagen, Denmark, 2005; p. 12. [Google Scholar]
- McHuron, E.A.; Sterling, J.T.; Costa, D.P.; Goebel, M.E. Factors affecting energy expenditure in a declining fur seal population. Conserv. Physiol. 2019, 7, coz103. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.P. A conceptual model of the variation in parental attendance in response to environmental fluctuation: Foraging energetics of lactating sea lions and fur seals. Aquat. Conserv. Mar. Freshw. Ecosyst. 2007, 17, S44–S52. [Google Scholar] [CrossRef]
- Baker, J.D.; Fowler, C.W. Pup weight and survival of northern fur seals Callorhinus ursinus. J. Zool. 1992, 227, 231–238. [Google Scholar] [CrossRef]
- North Pacific Fishery Management Council. Fishery Management Plan for Groundfish in the Bering Sea and Aleutian Islands Management Area; North Pacific Fishery Management Council: Anchorage, AK, USA, 2020; pp. 1–175. [Google Scholar]
- Lowry, L.F. Documentation and assessment of marine mammal-fishery interactions in the Bering Sea. In Proceedings of the Transactions of the 47th North American Wildlife and Natural Resources Conference, Portland, OR, USA, 26–31 March 1982; Sobel, K., Ed.; Wildlife Management Institute: Washington, DC, USA, 1982; pp. 300–311. [Google Scholar]
- Laevastu, T.; Favorite, F. Fishing and Stock Fluctuations; Fishing News Books: Totnes, UK, 1988; p. 232. [Google Scholar]
- Fréon, P.; Gerlotto, F.; Soria, M. Changes in school structure according to external stimuli: Description and influence on acoustic assessment. Fish. Res. 1992, 15, 45–66. [Google Scholar] [CrossRef]
- Gende, S.M.; Sigler, M.F. Persistence of forage fish ‘hot spots’ and its association with foraging Steller sea lions (Eumetopias jubatus) in southeast Alaska. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 432–441. [Google Scholar] [CrossRef]
- Sigler, M.F.; Womble, J.N.; Vollenweider, J.J. Availability to Steller sea lions (Eumetopias jubatus) of a seasonal prey resource: A pre-spawning aggregation of eulachon (Thaleichthys pacificus). Can. J. Fish. Aquat. Sci. 2004, 61, 1475–1484. [Google Scholar] [CrossRef]
- Sigler, M.F.; Tollit, D.J.; Vollenweider, J.J.; Thedinga, J.F.; Csepp, D.J.; Womble, J.N.; Wong, M.A.; Rehberg, M.J.; Trites, A.W. Steller sea lion foraging response to seasonal changes in prey availability. Mar. Ecol. Prog. Ser. 2009, 388, 243–261. [Google Scholar] [CrossRef]
- Sigler, M.F.; Gende, S.M.; Csepp, D.J. Association of foraging Steller sea lions with persistent prey hot spots in southeast Alaska. Mar. Ecol. Prog. Ser. 2017, 571, 233–243. [Google Scholar] [CrossRef]
- Womble, J.N.; Willson, M.F.; Sigler, M.F.; Kelly, B.P.; VanBlaricom, G.R. Distribution of Steller sea lions (Eumetopias jubatus) in relation to spring spawning fish in SE Alaska. Mar. Ecol. Prog. Ser. 2005, 294, 271–282. [Google Scholar] [CrossRef]
- Womble, J.N.; Sigler, M.F.; Willson, M.F. Linking seasonal distribution patterns with prey availability in a central-place forager, the Steller sea lion. J. Biogeogr. 2009, 36, 439–451. [Google Scholar] [CrossRef]
- Womble, J.N.; Sigler, M. Seasonal availability of abundant, energy-rich prey influences the abundance and diet of a marine predator, the Steller sea lion Eumetopias jubatus. Mar. Ecol. Prog. Ser. 2006, 325, 281–293. [Google Scholar] [CrossRef]
- Malavaer, M.Y.G. Modeling the Energetics of Steller Sea Lions (Eumetopias jubatus) along the Oregon Coast. Master’s Thesis, Oregon State University, Corvallis, OR, USA, 2002. [Google Scholar]
- Trenberth, K.E.; Hurrell, J.W. Decadal atmosphere-ocean variations in the Pacific. Clim. Dyn. 1994, 9, 303–319. [Google Scholar] [CrossRef]
- Hare, S.R.; Mantua, N.J. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog. Oceanogr. 2000, 47, 103–145. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Ware, D.M. Long-term variability in zooplankton biomass in the subarctic Pacific Ocean. Fish. Oceanogr. 1992, 1, 32–38. [Google Scholar] [CrossRef]
- Gargett, A.E. The optimal stability window: A mechanism underlying decadal fluctuations in North Pacific salmon stocks? Fish. Oceanogr. 1997, 6, 109–117. [Google Scholar] [CrossRef]
- Francis, R.C.; Hare, S.R.; Hollowed, A.B.; Wooster, W.S. Effects of interdecadal climate variability on the oceanic ecosystems of the NE Pacific. Fish. Oceanogr. 1998, 7, 1–21. [Google Scholar] [CrossRef]
- Anderson, P.J.; Piatt, J.F. Community reorganization in the Gulf of Alaska following ocean climate regime shift. Mar. Ecol. Prog. Ser. 1999, 189, 117–123. [Google Scholar] [CrossRef]
- Hare, S.R.; Mantua, N.J.; Francis, R.C. Inverse production regimes: Alaska and west coast Pacific salmon. Fisheries 1999, 24, 6–14. [Google Scholar] [CrossRef]
- Benson, A.J.; Trites, A.W. Ecological effects of regime shifts in the Bering Sea and eastern North Pacific Ocean. Fish Fish. 2002, 3, 95–113. [Google Scholar] [CrossRef]
- Conners, M.E.; Hollowed, A.B.; Brown, E. Retrospective analysis of Bering Sea bottom trawl surveys: Regime shift and ecosystem reorganization. Prog. Oceanogr. 2002, 55, 209–222. [Google Scholar] [CrossRef]
- Kajimura, H.; Sanger, G.A. Fur seal feeding on walleye pollock T. chalcogramma in the eastern Bering Sea. In Fur Seal Investigations, 1975; U.S. National Technical Information Service, U.S. Department of Commerce: Springfield, VA, USA, 1976; pp. 69–71. [Google Scholar]
- Lloyd, D.S.; McRoy, C.P.; Day, R.H. Discovery of northern fur seals (Callorhinus ursinus) breeding on Bogoslof Island, southeastern Bering. Sea. Arctic 1981, 34, 318–320. [Google Scholar] [CrossRef]
- Sinclair, E.H. Fur Seal Investigations, 1995; NOAA Tech. Memo. NMFS–AFSC–69; U.S. Department of Commerce: Springfield, VA, USA, 1997; p. 188. [Google Scholar]
- Merrick, R. Census of northern fur seals on Bogoslof Island. In Fur Seal Investigations, 1989; NOAA Tech. Memo. NMFS F/NWC–190; Kajimura, H., Ed.; U.S. National Technical Information Service: Springfield, VA, USA, 1990; p. 40. [Google Scholar]
- Ream, R.R.; Baker, J.D.; Towell, R.G. Bogoslof Island studies, 1997. In Fur Seal Investigations, 1997; NOAA Tech. Memo. NMFS–AFSC–106; Sinclair, E., Robson, B.W., Eds.; U.S. National Technical Information Service: Springfield, VA, USA, 1999; pp. 81–92. [Google Scholar]
- O’Corry-Crowe, G.; Gelatt, T.; Rea, L.; Bonin, C.; Rehberg, M. Crossing to safety: Dispersal, colonization, and mate choice in evolutionary distinct populations of Steller sea lions. Eumetopias jubatus. Molec. Ecol. 2014, 23, 5415–5435. [Google Scholar] [CrossRef] [PubMed]
| Age | Age-Specific Survival | Cumulative Survival | Fecundity a | Proportion of Population Fecundity b | Cumulative Proportion of Population Fecundity to Age |
|---|---|---|---|---|---|
| 0 | 1.000 | 0 | 0 | 0 | |
| 1 | 0.500 | 0.500 | 0 | 0 | 0 |
| 2 | 0.800 | 0.400 | 0 | 0 | 0 |
| 3 | 0.840 | 0.336 | 0 | 0 | 0 |
| 4 | 0.920 | 0.309 | 0.0205 | 0.0063 | 0.0063 |
| 5 | 0.940 | 0.290 | 0.1855 | 0.0538 | 0.0601 |
| 6 | 0.940 | 0.273 | 0.3500 | 0.0956 | 0.156 |
| 7 | 0.945 | 0.258 | 0.4000 | 0.1032 | 0.259 |
| 8 | 0.950 | 0.245 | 0.4250 | 0.1041 | 0.363 |
| 9 | 0.950 | 0.233 | 0.4350 | 0.1014 | 0.464 |
| 10 | 0.938 | 0.219 | 0.4400 | 0.0964 | 0.561 |
| 11 | 0.924 | 0.202 | 0.4400 | 0.0889 | 0.650 |
| 12 | 0.906 | 0.183 | 0.4400 | 0.0805 | 0.730 |
| 13 | 0.884 | 0.162 | 0.4350 | 0.0705 | 0.801 |
| 14 | 0.858 | 0.139 | 0.4200 | 0.0584 | 0.859 |
| 15 | 0.876 | 0.122 | 0.4050 | 0.0494 | 0.909 |
| 16 | 0.789 | 0.096 | 0.3850 | 0.0370 | 0.946 |
| 17 | 0.743 | 0.071 | 0.3550 | 0.0252 | 0.971 |
| 18 | 0.692 | 0.044 | 0.3150 | 0.0139 | 0.985 |
| 19 | 0.630 | 0.031 | 0.2800 | 0.0087 | 0.993 |
| 20 | 0.564 | 0.017 | 0.2350 | 0.0040 | 0.997 |
| 21 | 0.490 | 0.008 | 0.1850 | 0.0015 | 0.999 |
| 22 | 0.411 | 0.003 | 0.1300 | 0.0004 | 0.999 |
| 23 | 0.330 | 0.001 | 0.0505 | 0.0001 | 1 |
| 24+ | <0.330 | <0.001 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Short, J.W.; Geiger, H.J.; Fritz, L.W.; Warrenchuk, J.J. First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea. J. Mar. Sci. Eng. 2021, 9, 975. https://doi.org/10.3390/jmse9090975
Short JW, Geiger HJ, Fritz LW, Warrenchuk JJ. First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea. Journal of Marine Science and Engineering. 2021; 9(9):975. https://doi.org/10.3390/jmse9090975
Chicago/Turabian StyleShort, Jeffrey W., Harold J. Geiger, Lowell W. Fritz, and Jonathan J. Warrenchuk. 2021. "First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea" Journal of Marine Science and Engineering 9, no. 9: 975. https://doi.org/10.3390/jmse9090975
APA StyleShort, J. W., Geiger, H. J., Fritz, L. W., & Warrenchuk, J. J. (2021). First-Year Survival of Northern Fur Seals (Callorhinus ursinus) Can Be Explained by Pollock (Gadus chalcogrammus) Catches in the Eastern Bering Sea. Journal of Marine Science and Engineering, 9(9), 975. https://doi.org/10.3390/jmse9090975







