Upwelling Irradiance below Sea Ice—PAR Intensities and Spectral Distributions
Abstract
1. Introduction
2. Materials and Methods
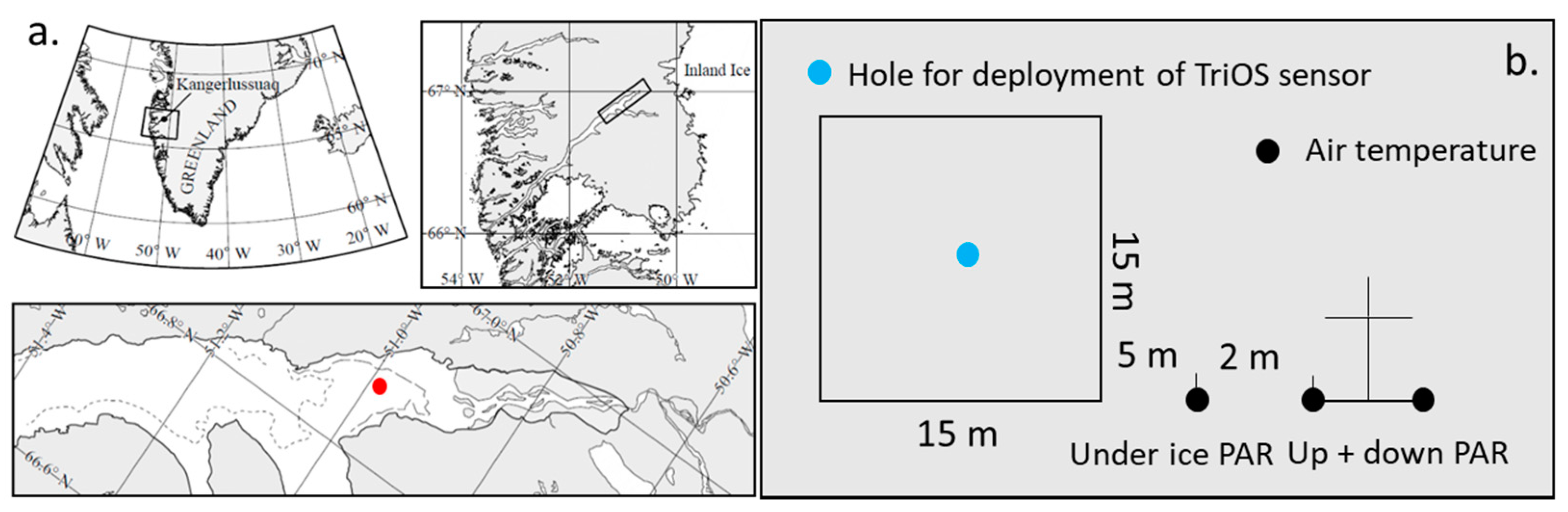
2.1. Study Area and Sampling
2.2. Optical Measurements
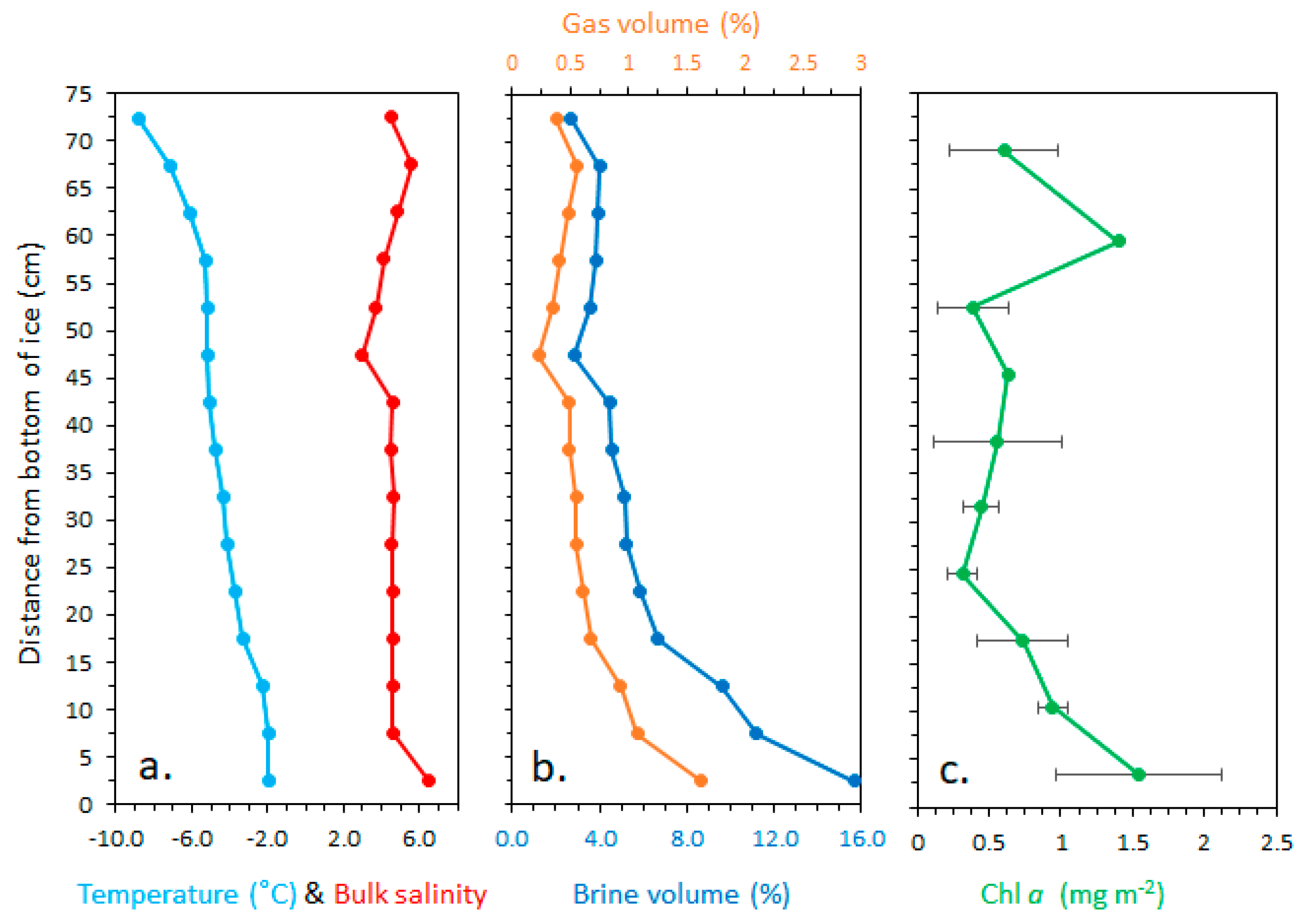
2.3. Ice Cores, Bulk Salinity, and Temperatures
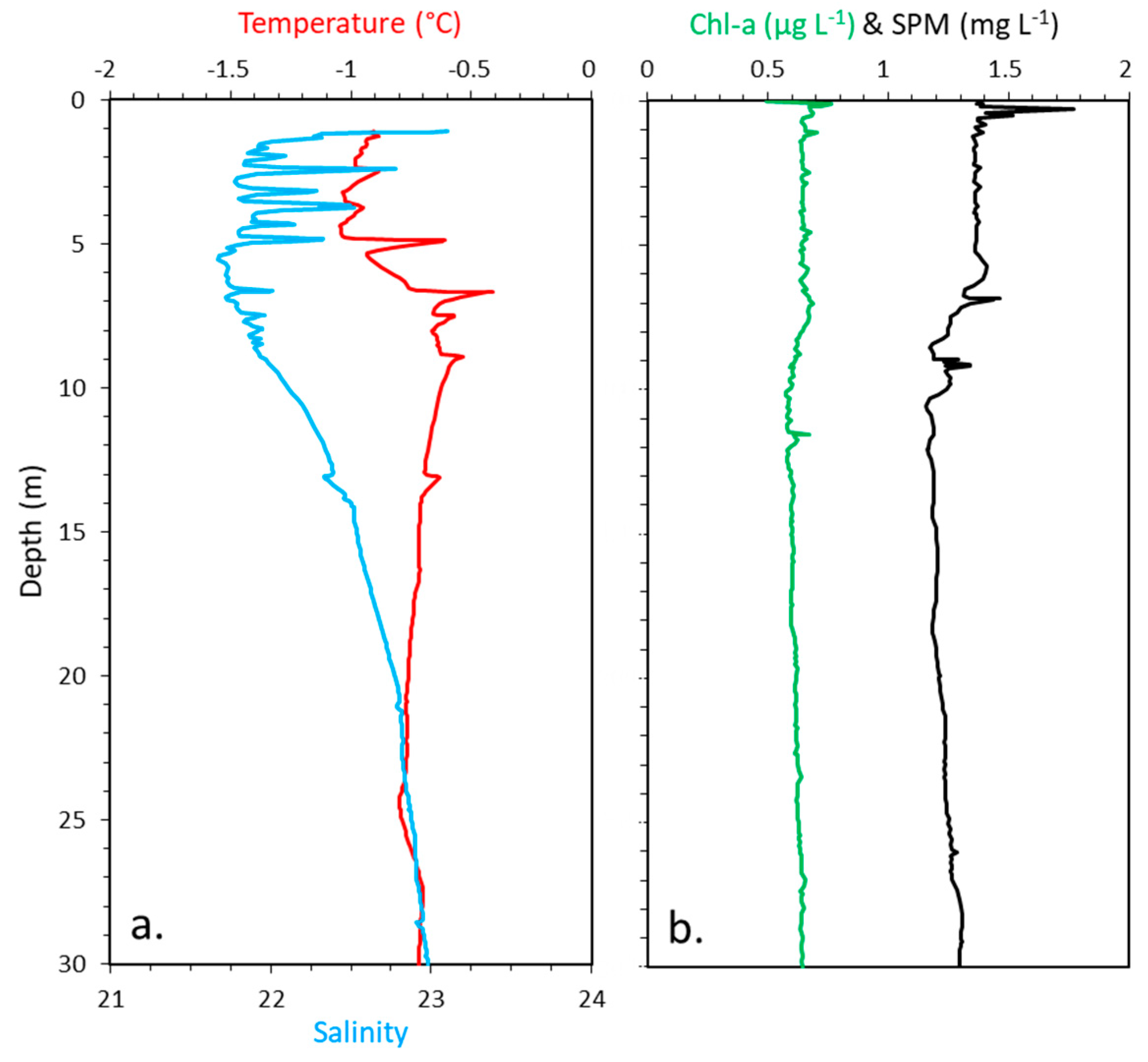
2.4. CTD, Chlorophyll a, Suspended Particulate Matter
2.5. Data Treatment
2.6. PAR Partitioning
3. Results
3.1. Physical and Optical Properties of the Snow, Ice, and Water Column
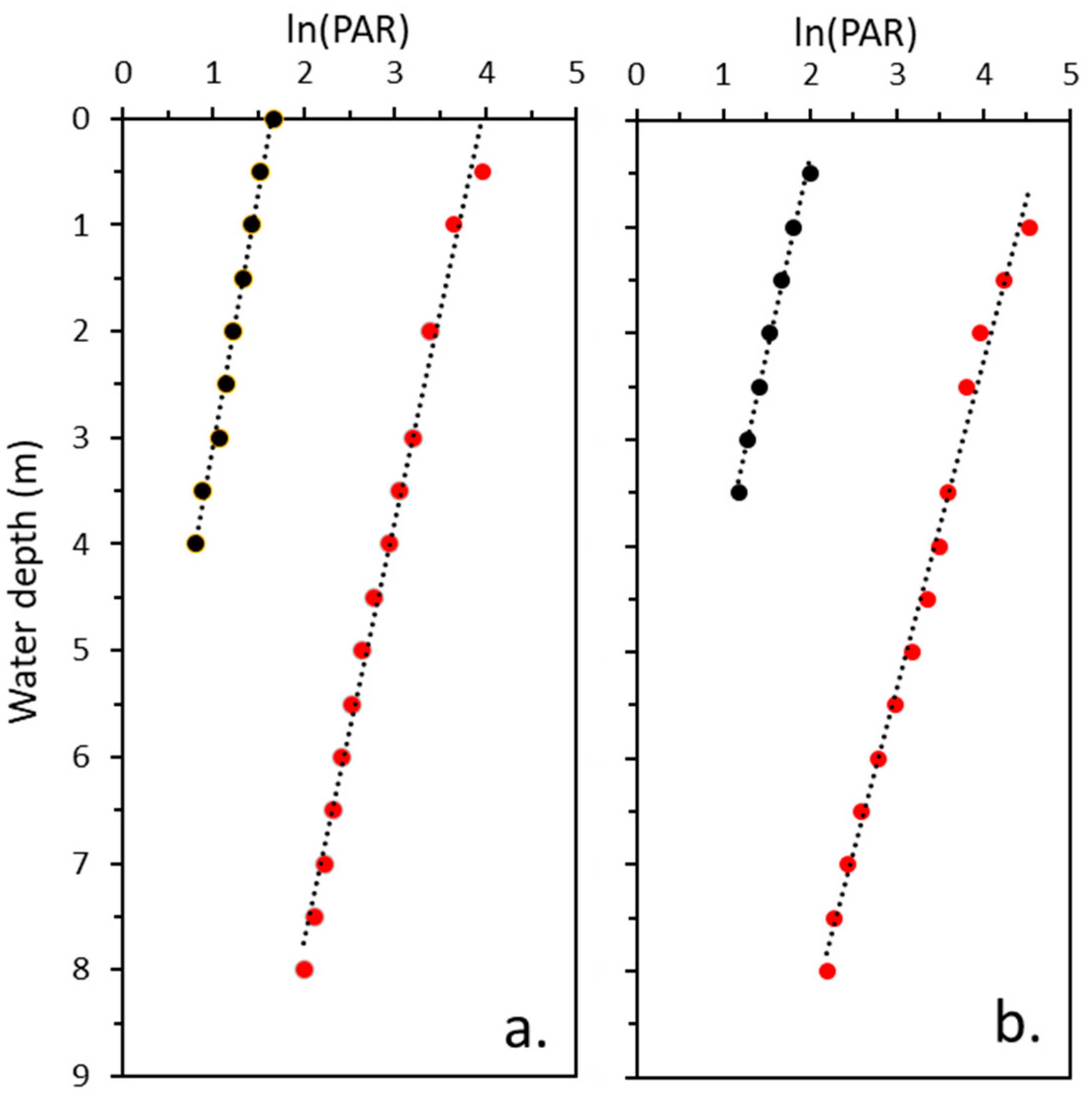
3.2. Upwelling and Downwelling PAR
3.3. Partitioning
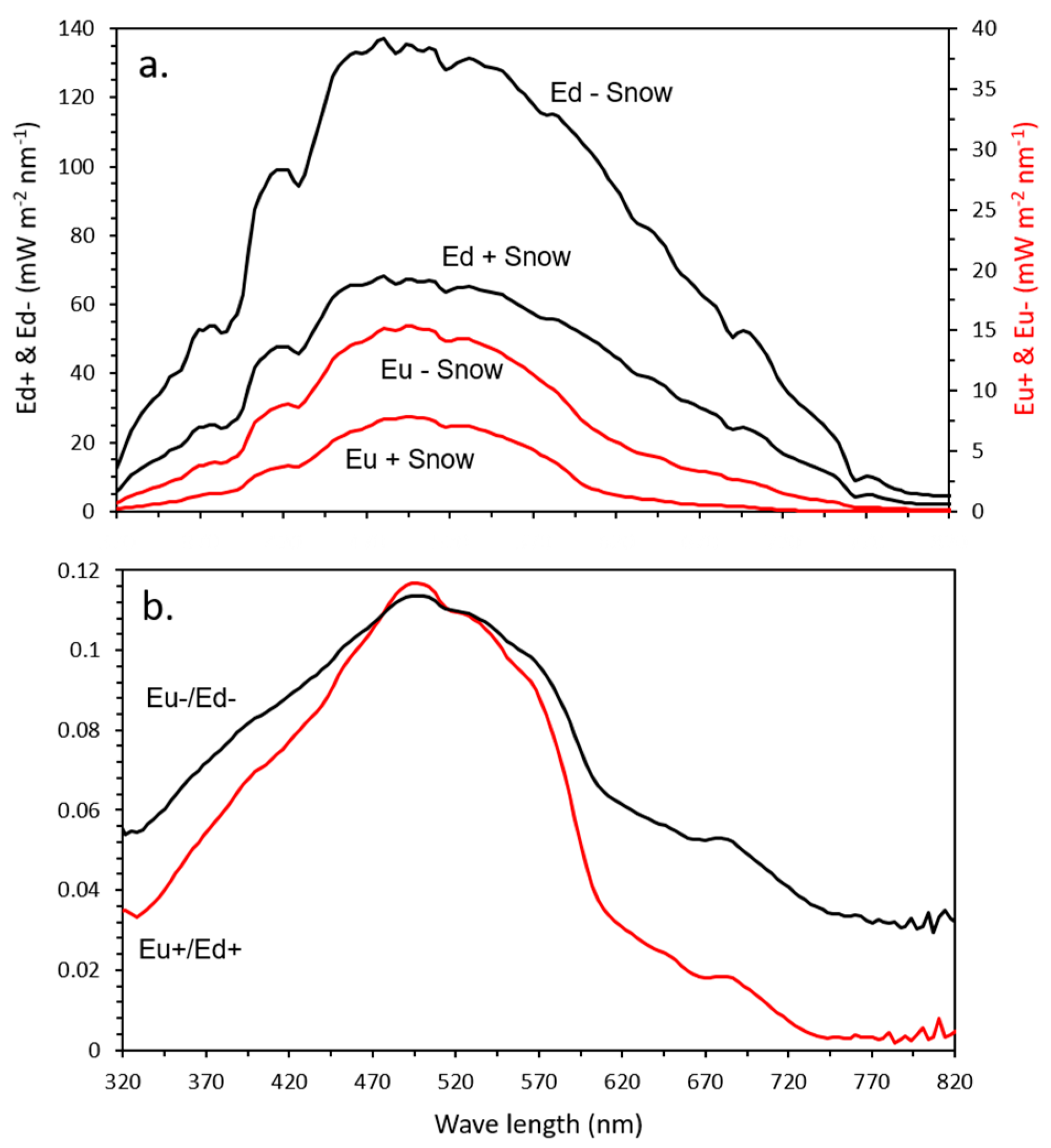
3.4. Spectral Distribution
3.5. Time-Series of Downwelling, Under-Ice, and Upwelling PAR
4. Discussion
4.1. Spectral Distribution and Kd(PAR)
4.2. Under-Ice Light Field and Reflectances
4.3. Low Light Adaptation
4.4. Acclimation to Low Light, Snow Melt, and Removal
4.5. Light Saturation Point Ek
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parkinson, C.L. Spatially mapped reductions in the length of the Arctic sea ice season. Geophys. Res. Lett. 2014, 41, 4316–4322. [Google Scholar] [CrossRef]
- Parkinson, C.L. A 40-y record reveals gradual Antarctic sea ice increases followed by decreases at rates far exceeding the rates see in the Arctic. Proc. Natl. Acad. Sci. USA 2019, 116, 14414–14423. [Google Scholar] [CrossRef]
- Thackeray, C.W.; Hall, A. An emergent constraint on future Arctic sea-ice albedo feedback. Nat. Clim. Chang. 2019, 9, 972–978. [Google Scholar] [CrossRef]
- Lund-Hansen, L.C.; Søgaard, D.H.; Sorrell, B.K.; Gradinger, R.; Meiners, K.M. Arctic Sea Ice Ecology–Seasonal Dynamics in Algal and Bacterial Productivity; Springer: Berlin, Germany, 2020; 178p, Available online: https://www.springer.com/gp/book/9783030374716 (accessed on 30 July 2021).
- Kolbach, D.; Schaafsma, F.L.; Graeve, M.; Lebreton, B.; Lange, B.A.; David, C.; Vortkamp, M.; Flores, H. Strong linkage of polar cod (Boreogadus saida) to sea ice algae-produced carbon: Evidence from stomach content, fatty acid and stable isotope analyses. Prog. Oceanogr. 2017, 152, 62–74. [Google Scholar] [CrossRef]
- Arrigo, K. Sea ice as a habitat for primary producers. In Sea Ice, 3rd ed.; Thomas, D.N., Ed.; Wiley Blackwell: Oxford, UK, 2017; 652p. [Google Scholar]
- Ehn, J.; Mundy, C.J. Assessment of light absorption within highly scattering bottom sea ice from under-ice light measurements: Implications for Arctic ice algae primary production. Limnol. Oceanogr. 2013, 58, 893–902. [Google Scholar] [CrossRef]
- Lund-Hansen, L.C.; Hawes, I.; Sorrell, B.K.; Nielsen, M.H. Removal of snow cover inhibits spring growth of Arctic ice algae through physiological and behavioral effects. Polar Biol. 2013, 37, 471–481. [Google Scholar] [CrossRef]
- Campbell, K.; Mundy, C.J.; Belzile, C.; Delaforge, A.; Rysgaard, S. Seasonal dynamics of algal and bacterial communities in Arctic sea ice under variable snow cover. Polar Biol. 2018, 41, 1869–1896. [Google Scholar] [CrossRef]
- Hancke, K.; Lund-Hansen, L.C.; Pedersen, S.; King, M.d.; Andersen, P.; Sorrell, B.K. Extreme low light requirement for algae growth underneath sea ice: A case study from Station Nord, NE Greenland. J. Geophys. Res. Oceans 2018, 123, 985–1000. [Google Scholar] [CrossRef]
- Terrado, R.; Lovejoy, C.; Massana, R.; Vincent, W. Microbial food web responses to light and nutrients beneath the coastal Arctic Ocean sea ice during the winter-spring transition. J. Mar. Syst. 2008, 74, 964–977. [Google Scholar] [CrossRef]
- Light, B.; Grenfell, T.; Perovich, D. Transmission and absorption of solar radiation by Arctic sea ice during the melt season. J. Geophys. Res. Oceans 2008, 113, C03023. [Google Scholar] [CrossRef]
- Hamre, B.; Winther, J.; Gerland, S.; Stamnes, J.; Stamnes, K. Modeled and measured optical transmittance of snow-covered first-year sea ice in Kongsfjorden, Svalbard. J. Geophys. Res. Oceans 2004, 109, C10006. [Google Scholar] [CrossRef]
- Perovich, D. Sea ice and sunlight. In Sea Ice, 3rd ed.; Thomas, D.N., Ed.; Wiley Blackwell: Oxford, UK, 2017; 652p. [Google Scholar]
- Matsuoka, A.; Huot, Y.; Shimada, K.; Saitoh, S.; Babin, M. Bio-optical characteristics of the western Arctic Ocean: Implications for ocean color algorithms. Can. J. Remote Sens. 2007, 33, 503–518. [Google Scholar] [CrossRef]
- Doxaran, D.; Ehn, J.; Bélanger, S.; Matsuoka, A.; Hooker, S.; Babin, M. Optical characterisation of suspended particles in the Mackenzie River plume (Canadian Arctic Ocean) and implications for ocean colour remote sensing. Biogeosciences 2012, 9, 3213–3229. [Google Scholar] [CrossRef]
- Bélanger, S.; Babin, M. Impact of sea ice on the retrieval of water-leaving reflectance, chlorophyll a concentration and inherent optical properties from satellite ocean color data. Remote Sens. Environ. 2007, 111, 51–68. [Google Scholar] [CrossRef]
- Tang, S.; Larouche, P.; Niemi, A.; Michel, C. Regional algorithms for remote-sensing estimates of total suspended matter in the Beaufort Sea. Int. J. Remote Sens. 2013, 34, 6562–6576. [Google Scholar] [CrossRef]
- Kirk, J.T.O. Light and Photosynthesis in the aquatic ecosystems; Cambridge University Press: Cambridge, UK, 1994; 509p. [Google Scholar]
- Ralph, P.J.; Gademann, R. Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 2005, 82, 222–237. [Google Scholar] [CrossRef]
- Mernild, S.; Hasholt, B. Observed runoff, jökulhlaups and suspended sediment load from the Greenland ice sheet at Kangerlussuaq, West Greenland, 2007 and 2008. J. Glaciol. 2009, 55, 855–858. [Google Scholar] [CrossRef]
- Nielsen, M.H.; Erbs-Hansen, D.; Knudsen, K.L. Water masses in Kangerlussuaq, a large fjord in West Greenland: The processes of formation and the associated foraminiferal fauna. Polar Res. 2010, 29, 159–175. [Google Scholar] [CrossRef]
- Lund-Hansen, L.C. Diffuse attenuation coefficients Kd(PAR) at the estuarine Nort Sea-Baltic Ses transition: Time-series, partitioning, absorption, and scattering. Estuar. Coast. Shelf Sci. 2004, 61, 251–259. [Google Scholar] [CrossRef]
- Lund-Hansen., L.C.; Hawes, I.; Nielsen, M.H.; Dahllöf, I.; Sorrell, B. Summer meltwater and spring sea ice primary production, light climate and nutrients in an Arctic estuary, Kangerlussuaq, west Greenland. Arc. Ant. Alp. Res. 2018, 50, e1414468. [Google Scholar] [CrossRef]
- Sorrell, B.K.; Hawes, I.; Stratmann, T.; Lund-Hansen, L.C. Photobiological effects on ice algae of a rapid whole-fjord loss of snow cover during spring growth in Kangerlussuaq, a West Greenland Fjord. J. Mar. Sci. Eng. 2021, 9, 814. [Google Scholar] [CrossRef]
- Cox, G.F.N.; Weeks, W.F. Equations for determining the gas and brine volumes in sea-ice samples. J. Glaciol. 1983, 102, 306–316. [Google Scholar] [CrossRef]
- Smith, R.; Baker, K. Optical properties of the clearest natural waters (200–800 nm). App. Opt. 1981, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lund-Hansen, L.C.; Andersen, T.J.; Nielsen, M.H.; Pejrup, M. Suspended matter, Chl-a, CDOM, grain sizes, and optical properties in the Arctic fjord-type estuary, Kangerlussuaq, West Greenland durung summer. Estuaries Coasts 2010, 33, 1442–1451. [Google Scholar] [CrossRef]
- Sirevaag, A. Turbulent exchange coefficients for the ice/ocean interface in case of rapid melting. Geophys. Res. Let. 2009, 36, GL036587(1–5). [Google Scholar] [CrossRef]
- Legendre, L.; Gosselin, M. In situ spectroradiometric estimation of microalgal biomass in first-year sea ice. Polar Biol. 1991, 4, 113–115. [Google Scholar] [CrossRef]
- Forrest, A.; Lund-Hansen, L.C.; Sorrell, B.K.; Bowden-Flyod, I.; Lucieer, V.; Cossu, R.; Lange, B.; Hawes, I. Exploring spatial heterogeneity of antarctic sea ice algae using an autonomous underwater vehicle mounted irradiance sensor. Front. Earth Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Søgaard, D.; Sorrell, B.K.; Sejr, M.; Andersen, P.; Rysgaard, S.; Hansen, P.J.; Skyttä, A.; Lemcke, S.; Lund-Hansen, L.C. An under-ice bloom of mixotrophic haptophytes in low nutrient and freshwater-influenced Arctic waters. Sci. Rep. 2021, 11, 2915. [Google Scholar] [CrossRef] [PubMed]
- Lund-Hansen, L.C.; Markager, S.; Hancke, K.; Stratmann, T.; Rysgaard, S.; Ramløv, H.; Sorrell, B. Effects of sea-ice light attenuation and CDOM absorption in the water below the Eurasian sector of central Arctic Ocean (>88° N). Polar Res. 2015, 34, 1–12. [Google Scholar] [CrossRef]
- Laney, S.; Krishfield, R.; Toole, J.; Hammar, T.; Ashjian, C. Assessing algal biomass and bio-optical distributions in perennially ice-covered polar ocean ecosystems. Polar Sci. 2014, 8, 73–85. [Google Scholar] [CrossRef]
- Matthes, L.; Ehn, J.; Girard, S.; Pogorzelec, N.; Babin, N.; Mundy, C.J. Average cosine coefficient and spectral distribution of the light field under sea ice: Implications for primary production. Elementa 2019, 7, 25. [Google Scholar] [CrossRef]
- Light, B. Theoretical and observational techniques for estimating light scattering in first-year Arctic sea ice. In Light Scattering Reviews 5; Springer Praxis Books; Kokhanovsky, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Jassby, I.A.; Platt, T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef]
- Lund-Hansen, L.C.; Hawes, I.; Hancke, K.; Salmansen, N.; Nielsen, J.R.; Balslev, L.; Sorrell, B.K. Effects of increased irradiance and biomass, photobiology, nutritional quality, and pigment composition of Arctic sea ice algae. Mar. Ecol. Prog. Ser. 2020, 648, 95–110. [Google Scholar] [CrossRef]
- Mundy, C.J.; Barber, D.; Michel, C. Variability of snow and ice thermal, physical and optical properties pertinent to sea ice algae biomass during spring. J. Mar. Sys. 2005, 58, 107–120. [Google Scholar] [CrossRef]
- Matthew, S.; Massom, R.A. Snow in the sea ice system: Friend or foe? In Sea Ice, 3rd ed.; Thomas, D.N., Ed.; Wiley Blackwell: Oxford, UK, 2017; 652p. [Google Scholar]

| +Snow | −Snow | |
|---|---|---|
| Kd(PAR) m−1 | 0.25 | 0.32 |
| Ku(PAR) m−1 | 0.21 | 0.27 |
| I0d (Down) µmol photons m−2 s−1 | 46.8 | 105.9 |
| I0u (Up) µmol photons m−2 s−1 | 5.1 | 8.2 |
| Irradiance reflectance (%) | 11 | 8 |
| Water | CDOM | Phyto | SPM | |
|---|---|---|---|---|
| Kd(PAR) (%) | 10 | 7 | 11 | 72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lund-Hansen, L.C.; Bjerg-Nielsen, M.; Stratmann, T.; Hawes, I.; Sorrell, B.K. Upwelling Irradiance below Sea Ice—PAR Intensities and Spectral Distributions. J. Mar. Sci. Eng. 2021, 9, 830. https://doi.org/10.3390/jmse9080830
Lund-Hansen LC, Bjerg-Nielsen M, Stratmann T, Hawes I, Sorrell BK. Upwelling Irradiance below Sea Ice—PAR Intensities and Spectral Distributions. Journal of Marine Science and Engineering. 2021; 9(8):830. https://doi.org/10.3390/jmse9080830
Chicago/Turabian StyleLund-Hansen, Lars Chresten, Michael Bjerg-Nielsen, Tanja Stratmann, Ian Hawes, and Brian K. Sorrell. 2021. "Upwelling Irradiance below Sea Ice—PAR Intensities and Spectral Distributions" Journal of Marine Science and Engineering 9, no. 8: 830. https://doi.org/10.3390/jmse9080830
APA StyleLund-Hansen, L. C., Bjerg-Nielsen, M., Stratmann, T., Hawes, I., & Sorrell, B. K. (2021). Upwelling Irradiance below Sea Ice—PAR Intensities and Spectral Distributions. Journal of Marine Science and Engineering, 9(8), 830. https://doi.org/10.3390/jmse9080830







