Cockle as Second Intermediate Host of Trematode Parasites: Consequences for Sediment Bioturbation and Nutrient Fluxes across the Benthic Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Experimental Infection
2.3. Experimental Procedure
2.4. Sediment Characteristics
2.5. Benthic Flux Measurement
2.6. Quantification of Bioturbation Rates
2.6.1. Bioirrigation
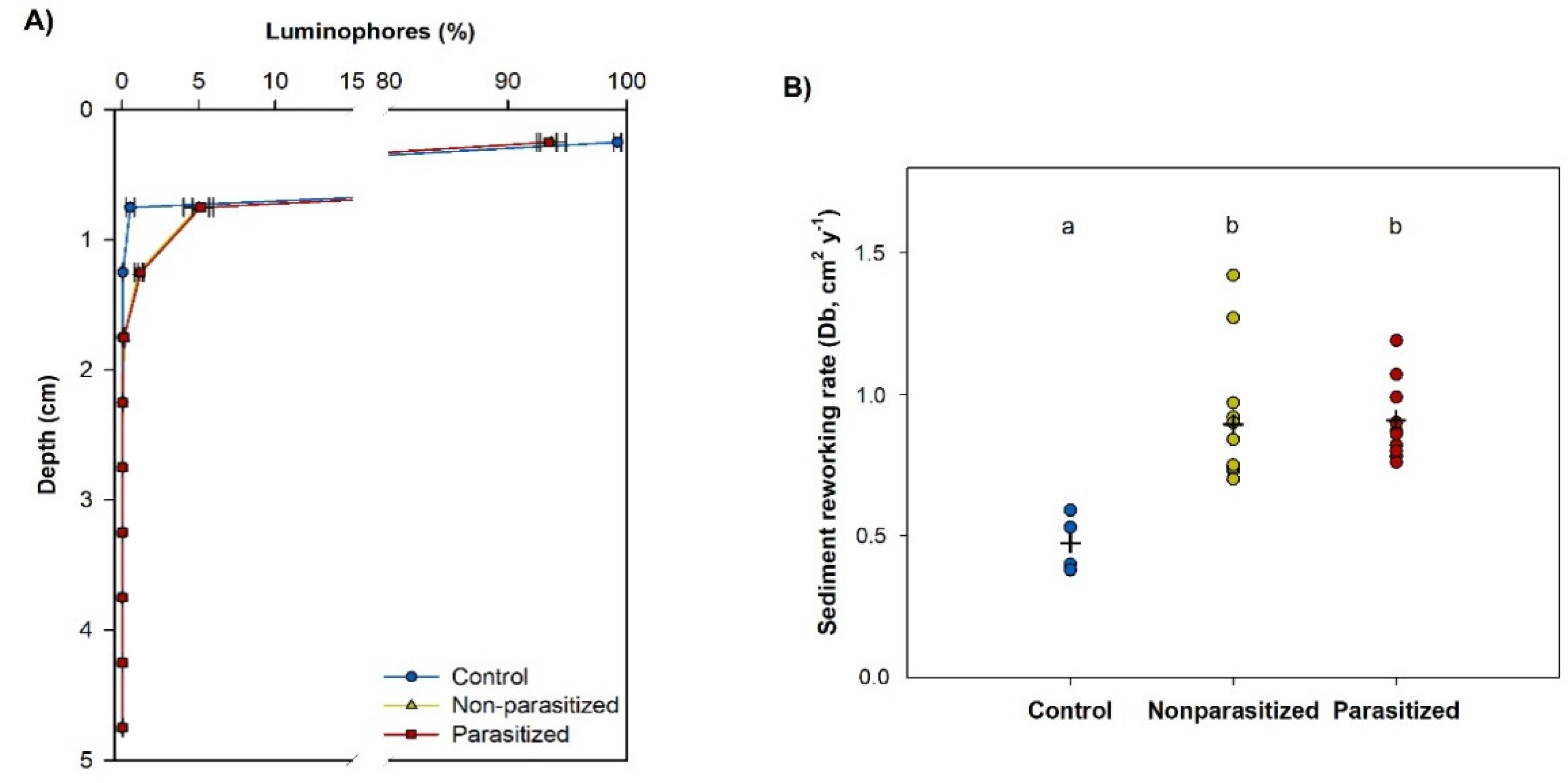
2.6.2. Sediment Reworking
2.7. Parasite Diagnosis
2.8. Data Analysis
3. Results
3.1. Parasite Diagnosis
3.2. Sediment Characteristics
3.3. Bioturbation
3.3.1. Bioirrigation
3.3.2. Sediment Reworking
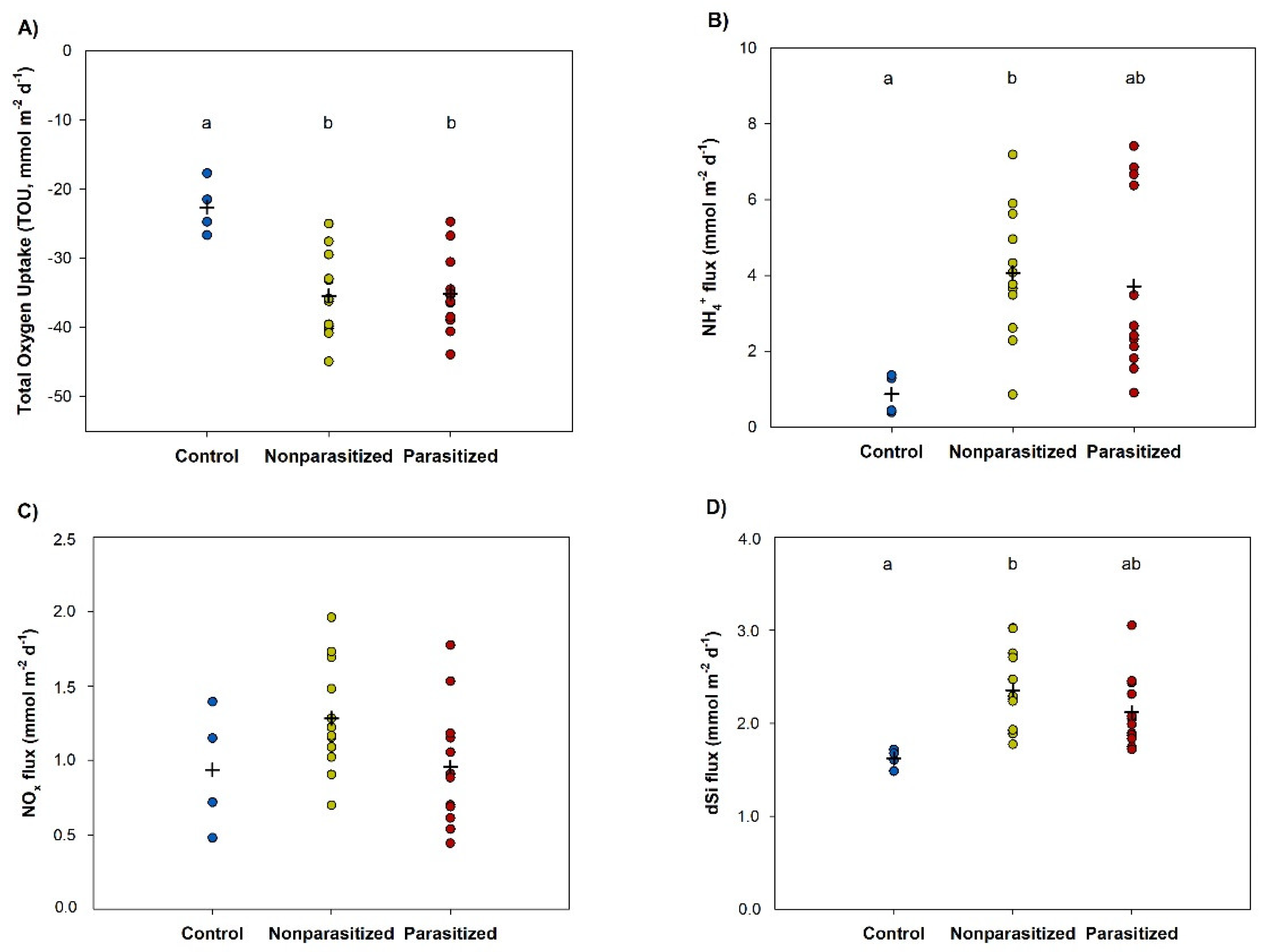
3.4. Benthic Fluxes Measurements
3.5. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dobson, A.; Lafferty, K.D.; Kuris, A.M.; Hechinger, R.F.; Jetz, W. Homage to Linnaeus: How many parasites? How many hosts? Proc. Natl. Acad. Sci. USA 2008, 105, 11482–11489. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Ostfeld, R.S.; Keesing, F. Frontiers in research on biodiversity and disease. Ecol. Lett. 2015, 18, 1119–1133. [Google Scholar] [CrossRef]
- Preston, D.L.; Mischler, J.A.; Townsend, A.R.; Johnson, P.T.J. Disease Ecology Meets Ecosystem Science. Ecosystems 2016, 19, 737–748. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Poulin, R. Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 2002, 124, 101–117. [Google Scholar] [CrossRef]
- Sato, T.; Egusa, T.; Fukushima, K.; Oda, T.; Ohte, N.; Tokuchi, N.; Watanabe, K.; Kanaiwa, M.; Murakami, I.; Lafferty, K.D. Nematomorph parasites indirectly alter the food web and ecosystem function of streams through behavioural manipulation of their cricket hosts. Ecol. Lett. 2012, 15, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.A.; Donohue, I.; Picard, J.; O’Keeffe, F.; Holland, C.V. Infection with behaviour-manipulating parasites enhances bioturbation by key aquatic detritivores. Parasitology 2019, 146, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Pascal, L.; Grémare, A.; Montaudouin, X.; Deflandre, B.; Romero-Ramirez, A.; Maire, O. Parasitism in ecosystem engineer species: A key factor controlling marine ecosystem functioning. J. Anim. Ecol. 2020, 89, 2192–2205. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G.; Lawton, J.H.; Moshe, S. Organisms as Ecosystem Engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 2002, 296, 2158–2162. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Tech. L’oie 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Malham, S.K.; Hutchinson, T.H.; Longshaw, M. A review of the biology of European cockles (Cerastoderma spp.). J. Mar. Biol. Assoc. U. K. 2012, 92, 1563–1577. [Google Scholar] [CrossRef]
- Tyler-Walters, H. Cerastoderma edule Common Cockle. In Marine Life Information Network: Biology and Sensitivity Key Information Reviews; Hiscock, K., Tyler-Walters, H., Eds.; Marine Biological Association of the United Kingdom: Plymouth, UK, 2007. [Google Scholar]
- Swanberg, I.L. The influence of the filter-feeding bivalve Cerastoderma edule L. on microphytobenthos: A laboratory study. J. Exp. Mar. Biol. Ecol. 1991, 151, 93–111. [Google Scholar] [CrossRef]
- Ciutat, A.; Widdows, J.; Readman, J. Influence of cockle Cerastoderma edule bioturbation and tidal-current cycles on resuspension of sediment and polycyclic aromatic hydrocarbons. Mar. Ecol. Prog. Ser. 2006, 328, 51–64. [Google Scholar] [CrossRef]
- Rakotomalala, C.; Grangeré, K.; Ubertini, M.; Forêt, M.; Orvain, F. Modelling the effect of Cerastoderma edule bioturbation on microphytobenthos resuspension towards the planktonic food web of estuarine ecosystem. Ecol. Model. 2015, 316, 155–167. [Google Scholar] [CrossRef]
- Dairain, A.; Maire, O.; Meynard, G.; Orvain, F. Does parasitism influence sediment stability? Evaluation of trait-mediated effects of the trematode Bucephalus minimus on the key role of cockles Cerastoderma edule in sediment erosion dynamics. Sci. Total Environ. 2020, 733, 139307. [Google Scholar] [CrossRef] [PubMed]
- Carss, D.N.; Brito, A.C.; Chainho, P.; Ciutat, A.; de Montaudouin, X.; Otero, R.M.F.; Filgueira, M.I.; Garbutt, A.; Goedknegt, M.A.; Lynch, S.A.; et al. Ecosystem services provided by a non-cultured shellfish species: The common cockle Cerastoderma edule. Mar. Environ. Res. 2020, 158, 104931. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.; Banta, G. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Krantzberg, G. The influence of bioturbation on physical, chemical and biological parameters in aquatic environments: A review. Environ. Pollut. Ser. A Ecol. Biol. 1985, 39, 99–122. [Google Scholar] [CrossRef]
- Volkenborn, N.; Polerecky, L.; Wethey, D.; DeWitt, T.; Woodin, S. Hydraulic activities by ghost shrimp Neotrypaea californiensis induce oxic−anoxic oscillations in sediments. Mar. Ecol. Prog. Ser. 2012, 455, 141–156. [Google Scholar] [CrossRef]
- Aller, R.C. The Effects of Macrobenthos on Chemical Properties of Marine Sediment and Overlying Water. In Animal-Sediment Relations; McCall, P., Tevesz, M.J.S., Eds.; Springer US: Boston, MA, USA, 1982; pp. 53–102. [Google Scholar] [CrossRef]
- Aller, R.C. Sedimentary Diagenesis, Depositional Environments, and Benthic Fluxes. Treatise Geochem. 2014, 8, 293–334. [Google Scholar] [CrossRef]
- De Montaudouin, X.; Kisielewski, I.; Bachelet, G.; Desclaux, C. A census of macroparasites in an intertidal bivalve community, Arcachon Bay, France. Oceanol. Acta 2000, 23, 453–468. [Google Scholar] [CrossRef]
- Thieltges, D.W.; Krakau, M.; Andresen, H.; Fottner, S.; Reise, K. Macroparasite community in molluscs of a tidal basin in the Wadden Sea. Helgol. Mar. Res. 2006, 60, 307–316. [Google Scholar] [CrossRef]
- Lauckner, G. Diseases of Mollusca: Bivalvia. In Diseases of Marine Animals; Kinne, O., Ed.; Biologische Anstalt Helgoland: Hamburg, Germany, 1983; Volume 2. [Google Scholar]
- De Montaudouin, X.; Thieltges, D.W.; Gam, M.; Krakau, M.; Pina, S.; Bazairi, H.; Dabouineau, L.; Russell-Pinto, F.; Jensen, K.T. Digenean trematode species in the cockle Cerastoderma edule: Identification key and distribution along the north-eastern Atlantic shoreline. J. Mar. Biol. Assoc. U. K. 2009, 89, 543–556. [Google Scholar] [CrossRef]
- Esch, G.W.; Barger, M.A.; Fellis, K.J. The Transmission of Digenetic Trematodes: Style, Elegance, Complexity. Integr. Comp. Biol. 2002, 42, 304–312. [Google Scholar] [CrossRef]
- Poulin, R.; Combes, C. Interactions Durables: Ecologie et Evolution du Parasitisme. J. Parasitol. 1997, 83, 177. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Magalhães, A.R.; Barracco, M.A. Effects of Bucephalus sp. (Trematoda: Bucephalidae) on Perna perna mussels from a culture station in Ratones Grande Island, Brazil. J. Invertebr. Pathol. 2002, 79, 154–162. [Google Scholar] [CrossRef]
- Thieltges, D.W. Parasite Induced Summer Mortality in the Cockle Cerastoderma edule by the Trematode Gymnophallus choledochus. Hydrobiologia 2006, 559, 455–461. [Google Scholar] [CrossRef]
- Dubois, S.; Savoye, N.; Sauriau, P.; Billy, I.; Martinez, P.; De Montaudouin, X. Digenean trematodes–marine mollusc relationships: A stable isotope study. Dis. Aquat. Org. 2009, 84, 65–77. [Google Scholar] [CrossRef]
- Magalhães, L.; Freitas, R.; De Montaudouin, X. Review: Bucephalus minimus, a deleterious trematode parasite of cockles Cerastoderma spp. Parasitol. Res. 2015, 114, 1263–1278. [Google Scholar] [CrossRef]
- Magalhães, L.; Daffe, G.; Freitas, R.; De Montaudouin, X. Monorchis parvus and Gymnophallus choledochus: Two trematode species infecting cockles as first and second intermediate host. Parasitology 2020, 147, 643–658. [Google Scholar] [CrossRef]
- De Montaudouin, X.; Binias, C.; Lassalle, G. Assessing parasite community structure in cockles Cerastoderma edule at various spatio-temporal scales. Estuar. Coast. Shelf Sci. 2012, 110, 54–60. [Google Scholar] [CrossRef]
- Bakhmet, I.; Nikolaev, K.; Levakin, I. Effect of infection with Metacercariae of Himasthla elongata (Trematoda: Echinostomatidae) on cardiac activity and growth rate in blue mussels (Mytilus edulis) In Situ. J. Sea Res. 2017, 123, 51–54. [Google Scholar] [CrossRef]
- Robaldo, R.; Monserrat, J.; Cousin, J.; Bianchini, A. Effects of metacercariae (Digenea: Microphallidae) on the hepatopancreas of Chasmagnathus granulata (Decapoda: Grapsidae). Dis. Aquat. Org. 1999, 37, 153–157. [Google Scholar] [CrossRef]
- Desclaux, C.; De Montaudouin, X.; Bachelet, G. Cockle Cerastoderma edule population mortality: Role of the digenean parasite Himasthla quissetensis. Mar. Ecol. Prog. Ser. 2004, 279, 141–150. [Google Scholar] [CrossRef]
- Gam, M.; De Montaudouin, X.; Bazairi, H. Do trematode parasites affect cockle (Cerastoderma edule) secondary production and elimination? J. Mar. Biol. Assoc. U. K. 2009, 89, 1395–1402. [Google Scholar] [CrossRef]
- Magalhães, L.; de Montaudouin, X.; Figueira, E.; Freitas, R. Interactive effects of contamination and trematode infection in cockles biochemical performance. Environ. Pollut. 2018, 243, 1469–1478. [Google Scholar] [CrossRef]
- Magalhães, L.; Freitas, R.; de Montaudouin, X. How costly are metacercarial infections in a bivalve host? Effects of two trematode species on biochemical performance of cockles. J. Invertebr. Pathol. 2020, 177, 107479. [Google Scholar] [CrossRef]
- Wegeberg, A.M.; Jensen, K.T. In Situ growth of juvenile cockles, Cerastoderma edule, experimentally infected with larval trematodes (Himasthla interrupta). J. Sea Res. 2003, 50, 37–43. [Google Scholar] [CrossRef]
- De Montaudouin, X.; Bazairi, H.; Culloty, S. Effect of trematode parasites on cockle Cerastoderma edule growth and condition index: A transplant experiment. Mar. Ecol. Prog. Ser. 2012, 471, 111–121. [Google Scholar] [CrossRef][Green Version]
- Paul-Pont, I.; Gonzalez, P.; Baudrimont, M.; Jude, F.; Raymond, N.; Bourrasseau, L.; Le Goïc, N.; Haynes, F.; Legeay, A.; Paillard, C.; et al. Interactive effects of metal contamination and pathogenic organisms on the marine bivalve Cerastoderma edule. Mar. Pollut. Bull. 2010, 60, 515–525. [Google Scholar] [CrossRef]
- Helluy, S.; Thomas, F. Effects of Microphallus papillorobustus (Platyhelminthes: Trematoda) on serotonergic immunoreactivity and neuronal architecture in the brain of Gammarus insensibilis (Crustacea: Amphipoda). Proc. R. Soc. B Boil. Sci. 2003, 270, 563–568. [Google Scholar] [CrossRef]
- Seppälä, O.; Karvonen, A.; Valtonen, E.T. Parasite-induced change in host behaviour and susceptibility to predation in an eye fluke–fish interaction. Anim. Behav. 2004, 68, 257–263. [Google Scholar] [CrossRef]
- Seppälä, O.; Karvonen, A.; Valanko, E.T. Host manipulation by parasites and risk of non-host predation: Is manipulation costly in an eye fluke-fish interaction? Evol. Ecol. Res. 2006, 8, 871–879. [Google Scholar]
- Lefevre, T.; Lebarbenchon, C.; Gauthier-Clerc, M.; Missé, D.; Poulin, R.; Thomas, F. The ecological significance of manipulative parasites. Trends Ecol. Evol. 2008, 24, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Vannatta, J.T.; Minchella, D.J. Parasites and their impact on ecosystem nutrient cycling. Trends Parasitol. 2018, 34, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Dairain, A.; Legeay, A.; De Montaudouin, X. Influence of parasitism on bioturbation: From host to ecosystem functioning. Mar. Ecol. Prog. Ser. 2019, 619, 201–214. [Google Scholar] [CrossRef]
- Thomas, F.; Poulin, R. Manipulation of a mollusc by a trophically transmitted parasite: Convergent evolution or phylogenetic inheritance? Parasitology 1998, 116, 431–436. [Google Scholar] [CrossRef]
- Jensen, K.T.; Castro, N.F.; Bachelet, G. Infectivity of Himasthla spp. (Trematoda) in cockle (Cerastoderma edule) spat. J. Mar. Biol. Assoc. U. K. 1999, 79, 265–271. [Google Scholar] [CrossRef]
- Mouritsen, K.N.; Poulin, R. Parasites boosts biodiversity and changes animal community structure by trait-mediated indirect effects. Oikos 2005, 108, 344–350. [Google Scholar] [CrossRef]
- De Montaudouin, X.; Blanchet, H.; Desclaux-Marchand, C.; Lavesque, N.; Bachelet, G. Cockle infection by Himasthla quissetensis—I. From cercariae emergence to metacercariae infection. J. Sea Res. 2016, 113, 99–107. [Google Scholar] [CrossRef]
- Seppälä, O.; Karvonen, A.; Valtonen, E.T. Phenotypic Variation in Infectivity of Diplostomum spathaceum cercariae within a population. J. Parasitol. 2007, 93, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Hapman, J.R.; Nakagawa, S.; Coltman, D.; Slate, J.; Heldon, B.C. A quantitative review of heterozygosity-fitness correlations in animal populations. Mol. Ecol. 2009, 18, 2746–2765. [Google Scholar] [CrossRef] [PubMed]
- De Montaudouin, X. Factors involved in growth plasticity of cockles Cerastoderma edule (L.), identified by field survey and transplant experiments. J. Sea Res. 1996, 36, 251–265. [Google Scholar] [CrossRef]
- Dabouineau, L.; Ponsero, A. Synthèse Sur la Biologie des Coques Cerastoderma edule, 2nd ed.; Réserve Naturelle Baie de St-Brieuc; CCSD: Villeurbanne, France, 2009; pp. 1–24. [Google Scholar]
- Magalhães, L.; Correia, S.; de Montaudouin, X.; Freitas, R. Spatio-temporal variation of trematode parasites community in Cerastoderma edule cockles from Ria de Aveiro (Portugal). Environ. Res. 2018, 164, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.; Kennedy, H.; Papadimitriou, S. The effect of acidification on the determination of organic carbon, total nitrogen and their stable isotopic composition in algae and marine sediment. Rapid Commun. Mass Spectrom. 2005, 19, 1063–1068. [Google Scholar] [CrossRef]
- Burdige, D.J. Geochemistry of Marine Sediments; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Hannides, A.K.; Dunn, S.M.; Aller, R.C. Diffusion of organic and inorganic solutes through macrofaunal mucus secretions and tube linings in marine sediments. J. Mar. Res. 2005, 63, 957–981. [Google Scholar] [CrossRef]
- Lepore, B.J.; Barak, P. A Colorimetric Microwell Method for Determining Bromide Concentrations. Soil Sci. Soc. Am. J. 2009, 73, 1130–1136. [Google Scholar] [CrossRef]
- Andersson, J.H.; Middelburg, J.J.; Soetaert, K. Identifiability and uncertainty analysis of bio-irrigation rates. J. Mar. Res. 2006, 64, 407–429. [Google Scholar] [CrossRef]
- Romero-Ramirez, A.; Grémare, A.; Bernard, G.; Pascal, L.; Maire, O.; Duchene, J. Development and validation of a video analysis software for marine benthic applications. J. Mar. Syst. 2016, 162, 4–17. [Google Scholar] [CrossRef][Green Version]
- Maire, O.; Lecroart, P.; Meysman, F.; Rosenberg, R.; Duchêne, J.; Grémare, A. Quantification of sediment reworking rates in bioturbation research: A review. Aquat. Biol. 2008, 2, 219–238. [Google Scholar] [CrossRef]
- Wegeberg, A.M.; de Montaudouin, X.; Jensen, K. Effect of intermediate host size (Cerastoderma edule) on infectivity of cercariae of three Himasthla species (Echinostomatidae, Trematoda). J. Exp. Mar. Biol. Ecol. 1999, 238, 259–269. [Google Scholar] [CrossRef]
- Meysman, F.J.R.; Galaktionov, O.S.; Gribsholt, B.; Middelburg, J.J. Bio-irrigation in permeable sediments: An assessment of model complexity. J. Mar. Res. 2006, 64, 589–627. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Rosenberg, R.; Carcaillet, F.; Norling, K.; Mauclaire, L. Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat. Microb. Ecol. 2004, 36, 271–284. [Google Scholar] [CrossRef]
- François, F.; Dalègre, K.; Gilbert, F.; Stora, G. Variabilité spécifique à l’intérieur des groupes fonctionnels. Étude du remaniement sédimentaire de deux bivalves Veneridae, Ruditapes decussatus et Venerupis aurea. C. R. Acad. Sci. Ser. III Sci. Vie 1999, 322, 339–345. [Google Scholar] [CrossRef]
- Woodin, S.A.; Volkenborn, N.; Pilditch, C.A.; Lohrer, A.M.; Wethey, D.; Hewitt, J.E.; Thrush, S.F. Same pattern, different mechanism: Locking onto the role of key species in seafloor ecosystem process. Sci. Rep. 2016, 6, 26678. [Google Scholar] [CrossRef]
- Glud, R.N. Oxygen dynamics of marine sediments. Mar. Biol. Res. 2008, 4, 243–289. [Google Scholar] [CrossRef]
- Newell, R.I.E.; Bayne, B.L. Seasonal changes in the physiology, reproductive condition and carbohydrate content of the cockle Cardium (=Cerastoderma) edule (Bivalvia: Cardiidae). Mar. Biol. 1980, 56, 11–19. [Google Scholar] [CrossRef]
- Sandwell, D.R.; Pilditch, C.A.; Lohrer, A.M. Density dependent effects of an infaunal suspension-feeding bivalve (Austrovenus stutchburyi) on sandflat nutrient fluxes and microphytobenthic productivity. J. Exp. Mar. Biol. Ecol. 2009, 373, 16–25. [Google Scholar] [CrossRef]
- Henriksen, K.; Rasmussen, M.B.; Jensen, A. Effect of bioturbation on microbial nitrogen transformations in the sediment and fluxes of ammonium and nitrate to the overlaying water. Ecol. Bull. 1983, 35, 193–205. [Google Scholar]
- Magni, P.; Montani, S.; Takada, C.; Tsutsumi, H. Temporal scaling and relevance of bivalve nutrient excretion on a tidal flat of the Seto Inland Sea, Japan. Mar. Ecol. Prog. Ser. 2000, 198, 139–155. [Google Scholar] [CrossRef]
- Camillini, N.; Larsen, M.; Glud, R. Behavioural patterns of the soft-shell clam Mya arenaria: Implications for benthic oxygen and nitrogen dynamics. Mar. Ecol. Prog. Ser. 2019, 622, 103–119. [Google Scholar] [CrossRef]
- Vernberg, W.B.; Vernberg, F.J. Respiratory Metabolism of a Trematode Metacercaria and Its Host. Aspeas of the Biology of Symbiosis; Cheng, T.C., Ed.; University Park Press: Baltimore, MD, USA, 1971; pp. 91–102. [Google Scholar]
- Magalhães, L.; De Montaudouin, X.; Figueira, E.; Freitas, R. Trematode infection modulates cockles biochemical response to climate change. Sci. Total. Environ. 2018, 637–638, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Desclaux, C.; De Montaudouin, X.; Bachelet, G. Cockle emergence at the sediment surface: “Favourization” mechanism by digenean parasites? Dis. Aquat. Org. 2002, 52, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, K.N. The parasite-induced surfacing behaviour in the cockle Austrovenus stutchburyi: A test of an alternative hypothesis and identification of potential mechanisms. Parasitology 2002, 124, 521–528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandnes, J.; Forbes, T.; Hansen, R.; Sandnes, B.; Rygg, B. Bioturbation and irrigation in natural sediments, described by animal-community parameters. Mar. Ecol. Prog. Ser. 2000, 197, 169–179. [Google Scholar] [CrossRef]
- Thrush, S.F.; Hewitt, J.E.; Gibbs, M.; Lundquist, C.; Norkko, A. Functional Role of large organisms in intertidal communities: Community effects and ecosystem function. Ecosystems 2006, 9, 1029–1040. [Google Scholar] [CrossRef]
- Zwarts, L.; Wanink, J. Siphon size and burying depth in deposit- and suspension-feeding benthic bivalves. Mar. Biol. 1989, 100, 227–240. [Google Scholar] [CrossRef]
- Bakhmet, I.; Nikolaev, K.; Levakin, I.; Ekimov, D. Influence of Himasthla elongata (Trematoda: Echinostomatidae) metacercariae on heart rate in blue mussels (Mytilus edulis). J. Invertebr. Pathol. 2019, 166, 107220. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richard, A.; de Montaudouin, X.; Rubiello, A.; Maire, O. Cockle as Second Intermediate Host of Trematode Parasites: Consequences for Sediment Bioturbation and Nutrient Fluxes across the Benthic Interface. J. Mar. Sci. Eng. 2021, 9, 749. https://doi.org/10.3390/jmse9070749
Richard A, de Montaudouin X, Rubiello A, Maire O. Cockle as Second Intermediate Host of Trematode Parasites: Consequences for Sediment Bioturbation and Nutrient Fluxes across the Benthic Interface. Journal of Marine Science and Engineering. 2021; 9(7):749. https://doi.org/10.3390/jmse9070749
Chicago/Turabian StyleRichard, Anaïs, Xavier de Montaudouin, Auriane Rubiello, and Olivier Maire. 2021. "Cockle as Second Intermediate Host of Trematode Parasites: Consequences for Sediment Bioturbation and Nutrient Fluxes across the Benthic Interface" Journal of Marine Science and Engineering 9, no. 7: 749. https://doi.org/10.3390/jmse9070749
APA StyleRichard, A., de Montaudouin, X., Rubiello, A., & Maire, O. (2021). Cockle as Second Intermediate Host of Trematode Parasites: Consequences for Sediment Bioturbation and Nutrient Fluxes across the Benthic Interface. Journal of Marine Science and Engineering, 9(7), 749. https://doi.org/10.3390/jmse9070749




