Controls on Nutrient Cycling in Estuarine Mangrove Lake Sediments
Abstract
1. Introduction
2. Materials and Methods
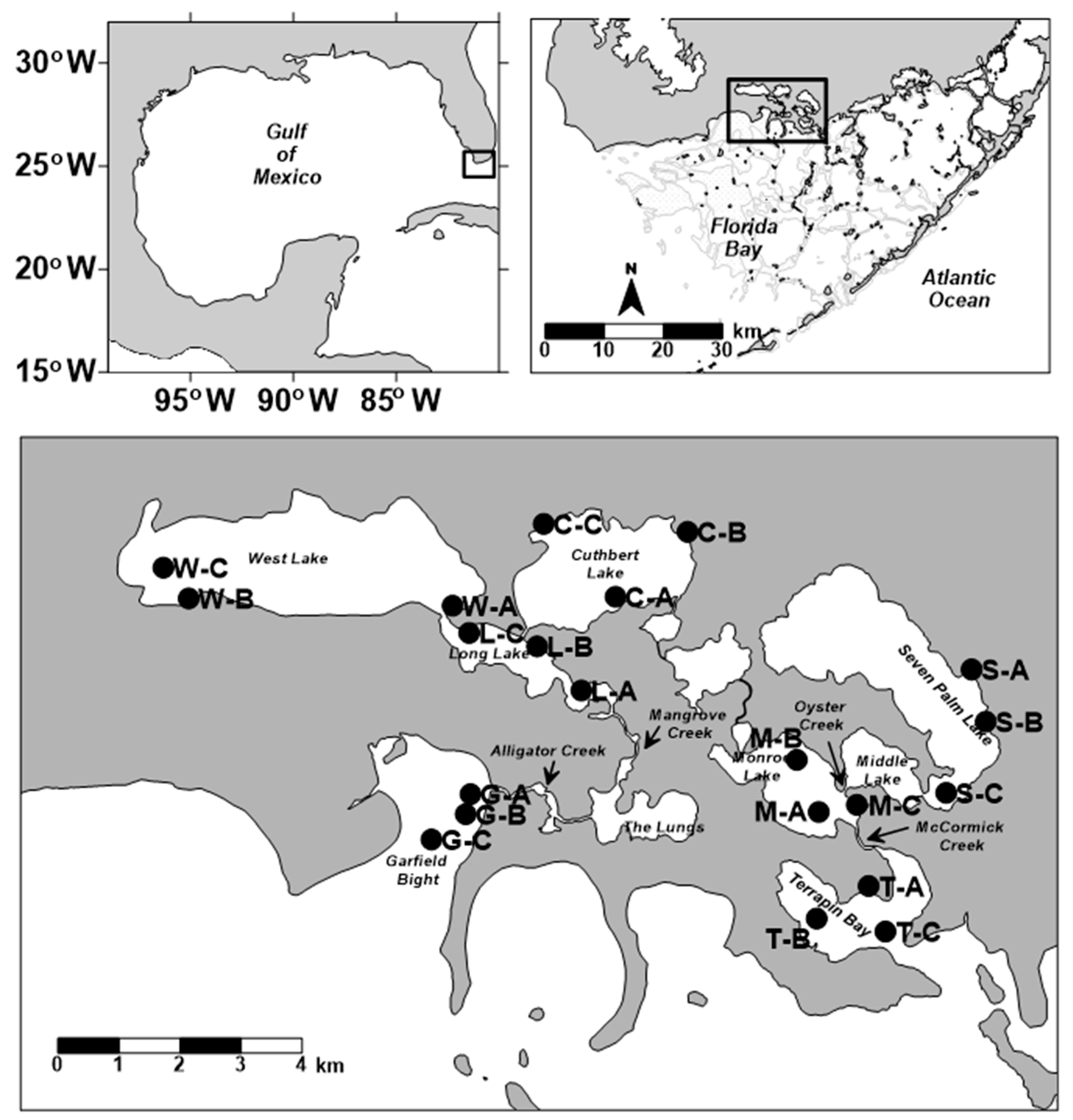
2.1. Sediment Field Collections
2.2. Macrophyte Coverage, Chlorophyll a and Salinity
2.3. Sediment–Water Exchange Measurements
2.4. Pore Water Chemistry
2.5. Solid Phase Chemistry
2.6. Vertical Diagenetic Rate Profiles
2.7. Oxygen Microelectrode Profiles
2.8. Gas and Solute Analysis
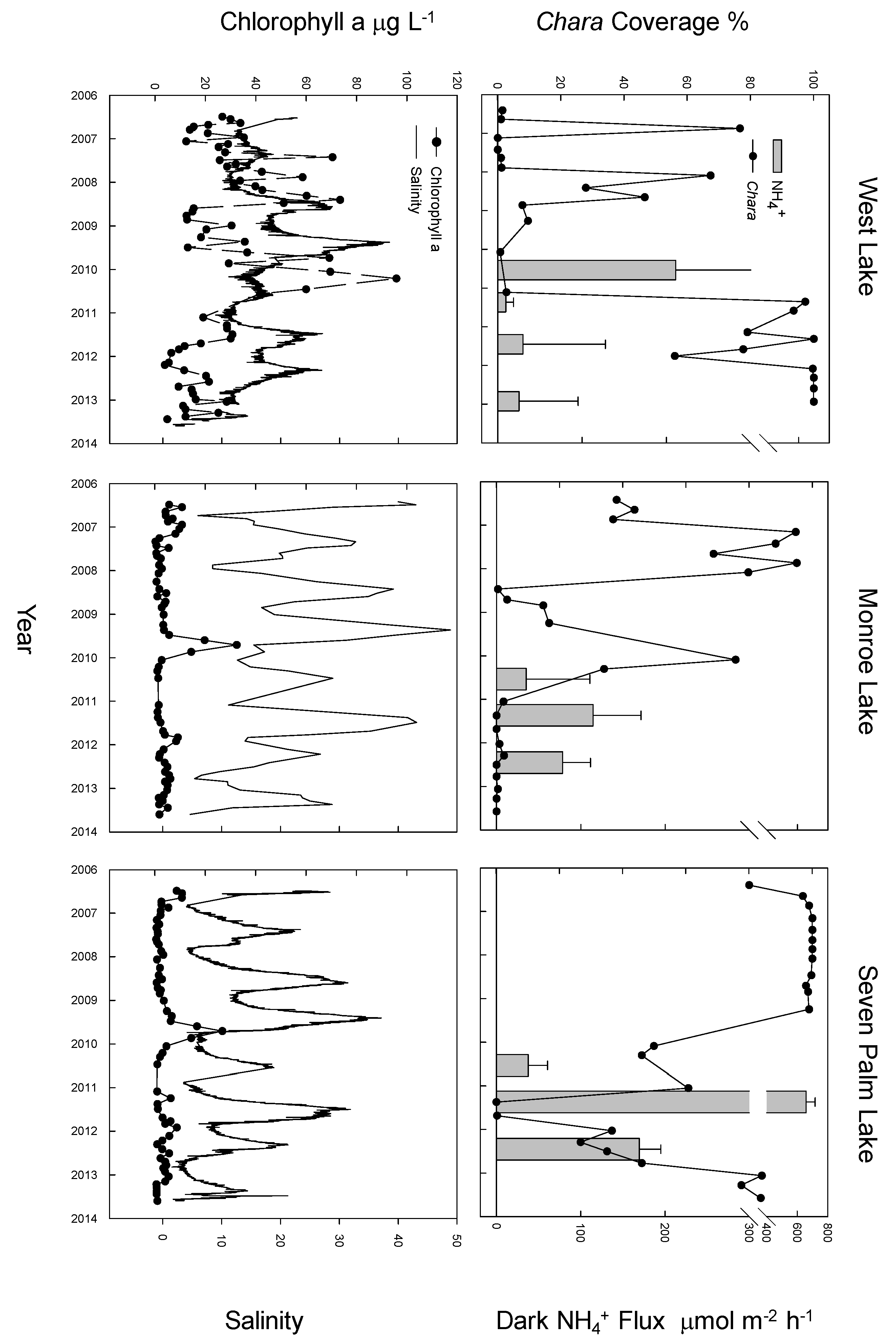
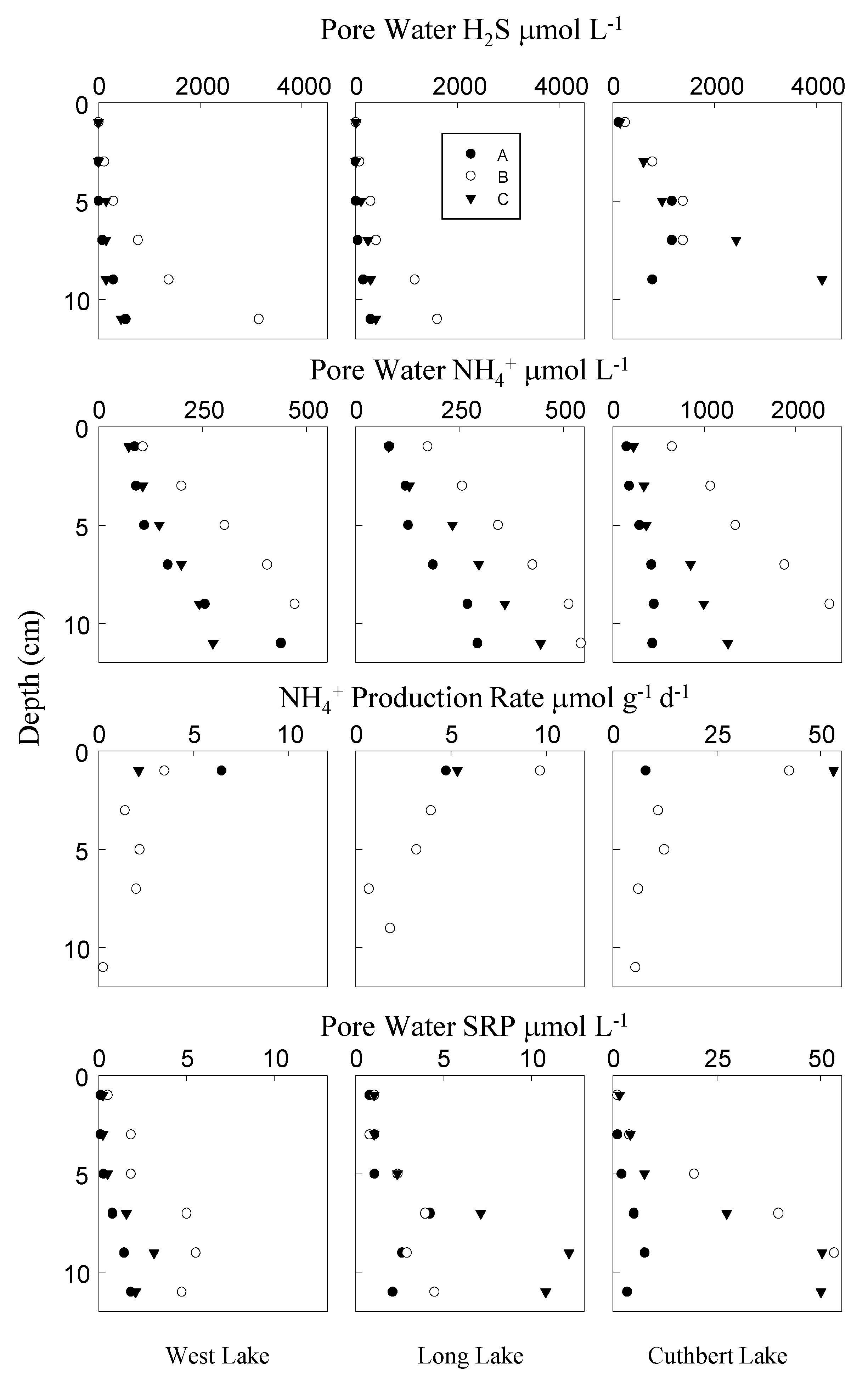
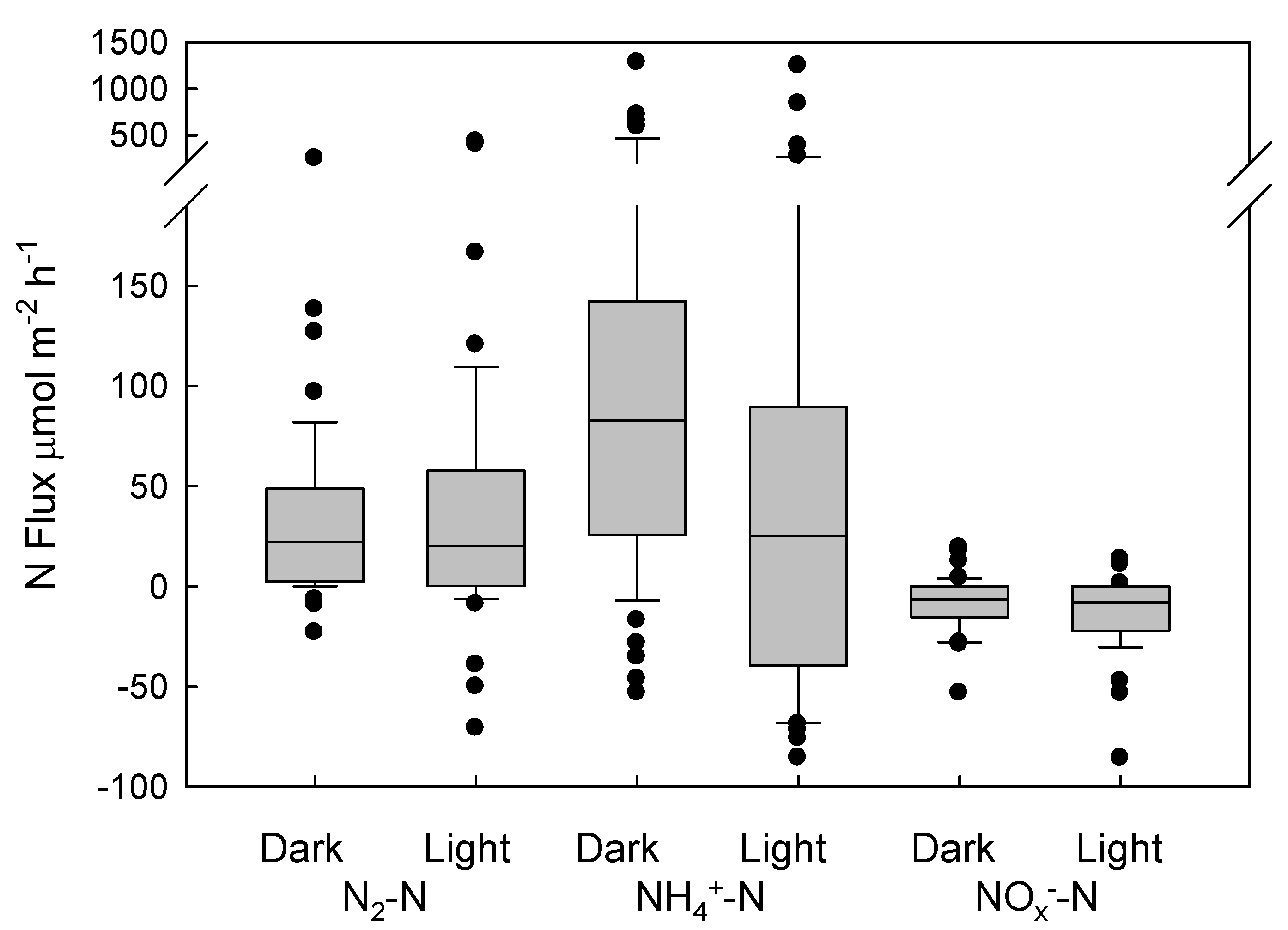
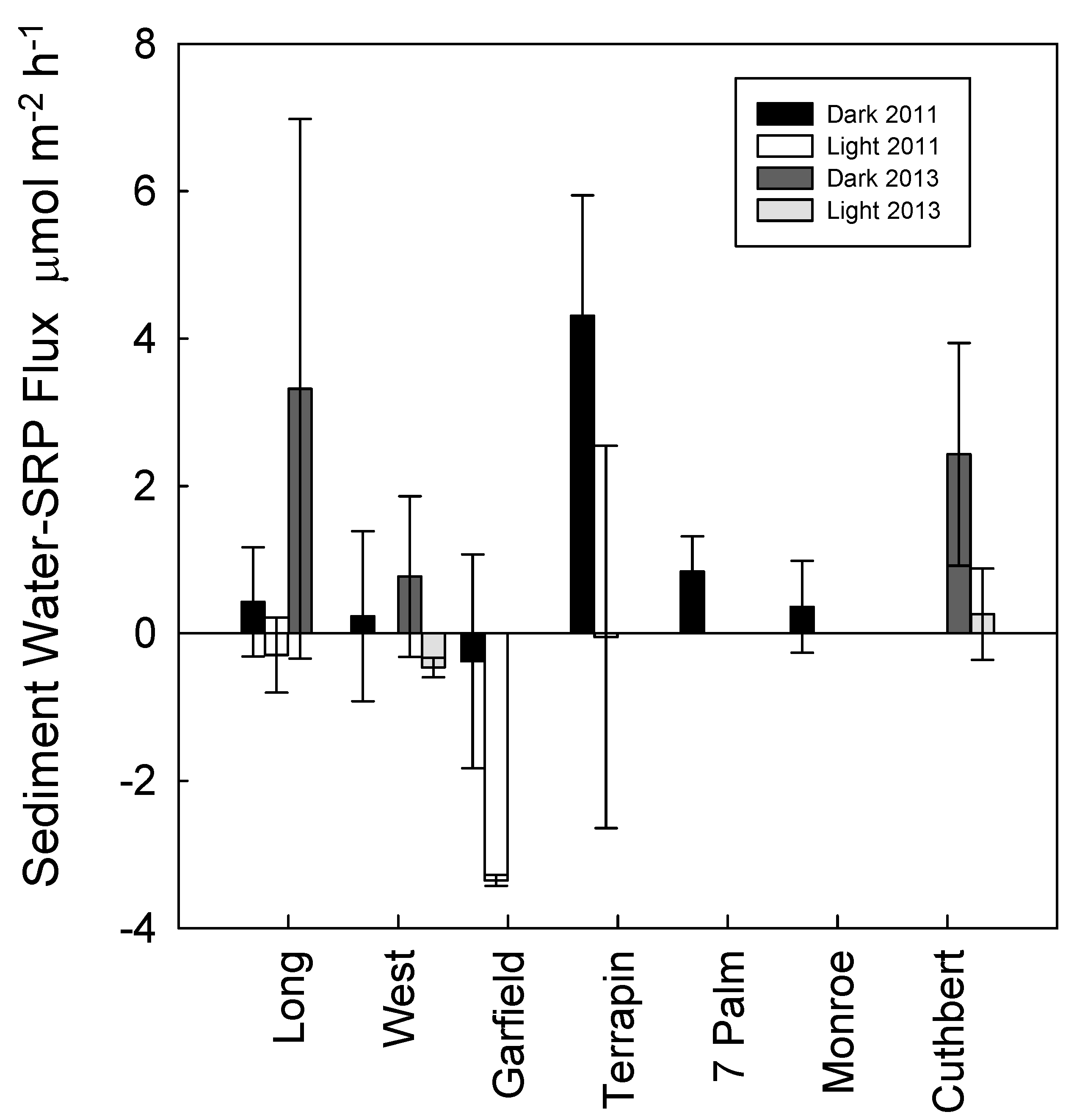
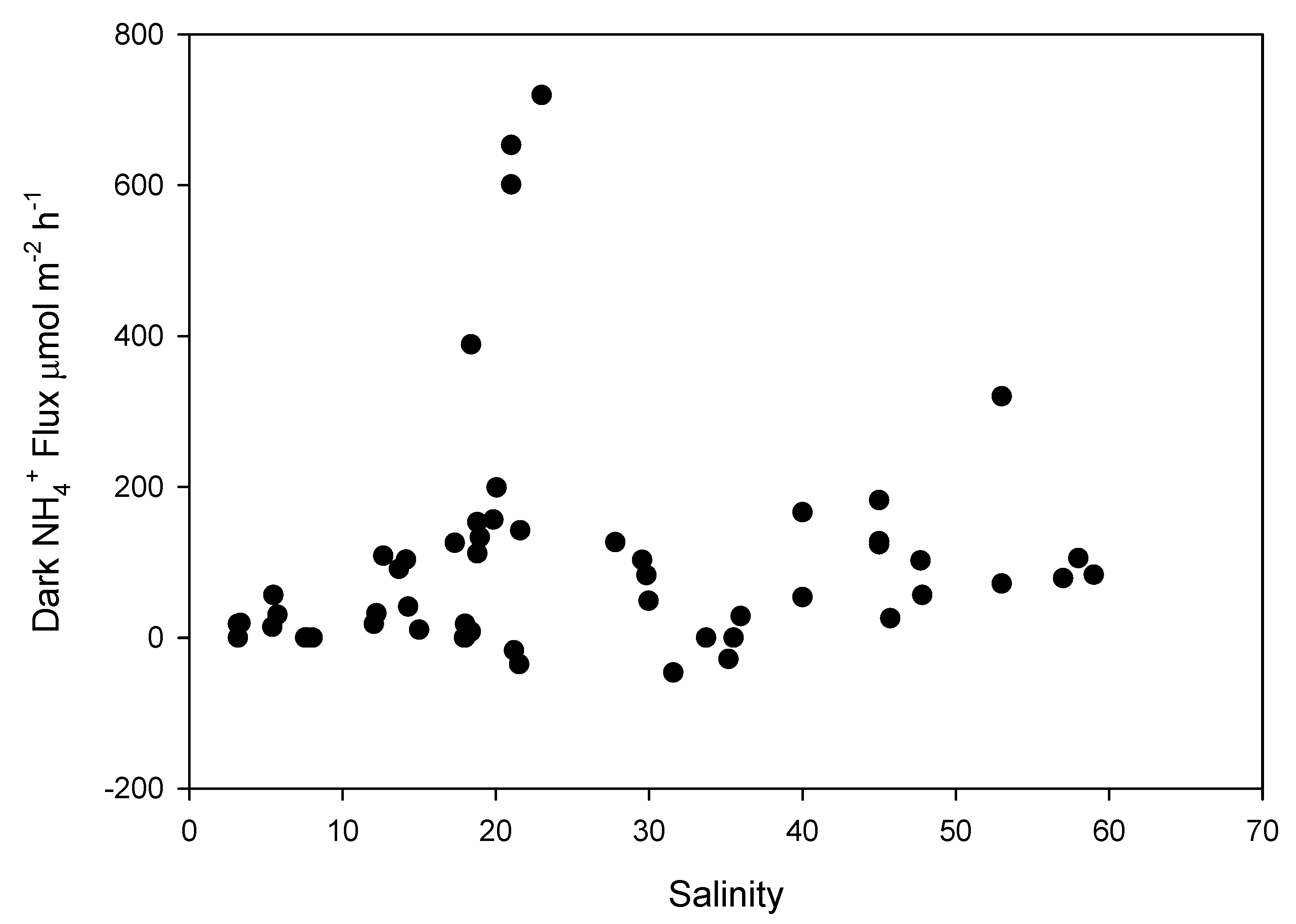
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernhard, A.E.; Landry, Z.C.; Blevins, A.; de la Torre, J.R.; Giblin, A.E.; Stahl, D.A. Abundance of ammonia-oxidizing archaea and bacteria along an estuarine salinity gradient in relation to potential nitrification rates. Appl. Environ. Microbiol. 2010, 76, 1285–1289. [Google Scholar] [CrossRef]
- Kelly, J.R.; Berounsky, V.M.; Nixon, S.W. Benthic-pelagic coupling and nutrient cycling across an experimental eutrophication gradient. Mar. Ecol. Prog. Ser. 1985, 26, 207–219. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Gardner, W.S.; Spratt, A.K. The effect of salinity on ammonium sorption in aquatic sediments: Implications for benthic nutrient recycling. Estuaries 1991, 14, 167–174. [Google Scholar] [CrossRef]
- Weston, N.B.; Giblin, A.E.; Banta, G.T.; Hopkinson, C.S.; Tucker, J. The effects of varying salinity on ammonium exchange in estuarine sediments of the Parker River, Massachusetts. Estuaries Coasts 2010, 33, 985–1003. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Morrison, D.; Fourqurean, J.W. Benthic macrophyte distribution and abundance in estuarine mangrove lakes and estuaries: Relationships to environmental variables. Estuaries Coasts 2011, 34, 20–31. [Google Scholar] [CrossRef]
- Inglett, P.W.; Rivera-Monroy, V.H.; Wozniak, J.R. Biogeochemistry of nitrogen across the everglades landscape. Crit. Rev. Environ. Sci. Technol. 2011, 41, 187–216. [Google Scholar] [CrossRef]
- Koch, G.R.; Childers, D.L.; Staehr, P.A.; Price, R.M.; Davis, S.E.; Gaiser, E.E. Hydrological conditions control P loading and aquatic metabolism in an oligotrophic, subtropical estuary. Estuaries Coasts 2012, 35, 292–307. [Google Scholar] [CrossRef][Green Version]
- Sutula, M.A.; Perez, B.C.; Reyes, E.; Childers, D.L.; Davis, S.; Day, J.W.; Rudnick, D.; Sklar, F. Factors affecting spatial and temporal variability in material exchange between the Southern Everglades wetlands and Florida Bay (USA). Estuar. Coast. Shelf Sci. 2003, 57, 757–781. [Google Scholar] [CrossRef]
- Kotun, K.; Renshaw, A. Taylor slough hydrology. Wetlands 2014, 34, 9–22. [Google Scholar] [CrossRef]
- Armitage, A.R.; Frankovich, T.A.; Fourqurean, J.W. Variable responses within epiphytic and benthic microalgal communities to nutrient enrichment. Hydrobiologia 2006, 569, 423–435. [Google Scholar] [CrossRef]
- Ferdie, M.; Fourqurean, J.W. Responses of seagrass communities to fertilization along a gradient of relative availability of nitrogen and phosphorus in a carbonate environment. Limnol. Oceanogr. 2004, 49, 2082–2094. [Google Scholar] [CrossRef]
- McCarthy, M.J.; Gardner, W.S.; Lavrentyev, P.J. Water column nitrogen cycling and microbial plankton in Florida Bay. Contrib. Mar. Sci. 2009, 38, 49–62. [Google Scholar]
- Gardner, W.S.; McCarthy, M.J. Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: Why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 2009, 95, 185–198. [Google Scholar] [CrossRef]
- Chambers, R.M.; Fourqurean, J.W.; Macko, S.A.; Hoppenot, R. Biogeochemical effects of iron availability on primary producers in a shallow marine carbonate environment. Limnol. Oceanogr. 2001, 46, 1278–1286. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Jones, R.D.; Zieman, J.C. Process influencing water column nutrient characteristics and phosphorus limitation of phytoplankton biomass in Florida Bay, FL, USA: Inferences from spatial distributions. Estuar. Coast. Shelf Sci. 1993, 36, 295–314. [Google Scholar] [CrossRef]
- Miller, C.J.; Davis, S.E.; Roelke, D.L.; Li, H.-P.; Driffill, M.J. Factors influencing algal biomass in intermittently connected, subtropical coastal ponds. Wetlands 2009, 29, 759–771. [Google Scholar] [CrossRef]
- Shangguan, Y.; Glibert, P.M.; Alexander, J. Phytoplankton assemblage response to changing nutrients in Florida Bay: Results of mesocosm studies. J. Exp. Mar. Biol. Ecol. 2017, 494, 38–53. [Google Scholar] [CrossRef]
- Accoroni, S.; Totti, C.; Romagnoli, T.; Giulietti, S.; Glibert, P.M. Distribution and potential toxicity of benthic harmful dinoflagellates in waters of Florida Bay and the Florida Keys. Mar. Environ. Res. 2020, 155, 104891. [Google Scholar] [CrossRef]
- Owens, M.S.; Cornwell, J.C. The benthic exchange of O2, N2 and dissolved nutrients using small core incubations. J. Vis. Exp. 2016. [Google Scholar] [CrossRef]
- Evans, J.L.B. The Effect of Benthic Microalgal Photosynthetic Oxygen Production on Nitrogen Fluxes Across the Sediment-Water Interface in a Shallow, Sub-Tropical Estuary. Master’s Thesis, University of Maryland, College Park, MD, USA, 2005. [Google Scholar]
- Bray, J.T.; Bricker, O.P.; Troup, B.N. Phosphate in interstitial waters of anoxic sediments: Oxidation effects during sampling procedure. Science 1973, 180, 1362–1364. [Google Scholar] [CrossRef]
- Cline, J.D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 1969, 14, 454–458. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Oxford, UK, 1984. [Google Scholar]
- Stainton, M.P. A syringe gas-stripping procedure for gas-chromatographic determination of dissolved inorganic and organic carbon in fresh water and carbonates in sediments. J. Fish. Res. Board Can. 1973, 30, 1441–1445. [Google Scholar] [CrossRef]
- Aspila, K.I.; Agemian, H.; Chau, A.S. A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 1976, 101, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Krom, M.D.; Berner, R.A. The diagenesis of phosphorus in a nearshore marine sediment. Geochim. Cosmochim. Acta 1981, 45, 207–216. [Google Scholar] [CrossRef]
- Burdige, D.J. The effects of sediment slurrying on microbial processes, and the role of amino acids as substrates for sulfate reduction in anoxic marine sediments. Biogeochemistry 1989, 8, 1–23. [Google Scholar] [CrossRef]
- Rosenfeld, J.K. Nitrogen diagenesis in Long Island sound sediments. Am. J. Sci. 1981, 281, 436–462. [Google Scholar] [CrossRef]
- Cornwell, J.C.; Owens, M.S. Quantifying sediment nitrogen releases associated with estuarine dredging. Aquat. Geochem. 2011, 17, 499. [Google Scholar] [CrossRef]
- Revsbech, N.P. An oxygen microsensor with a guard cathode. Limnol. Oceanogr. 1989, 34, 474–478. [Google Scholar] [CrossRef]
- Glud, R.N.; Gundersen, J.K.; Jørgensen, B.B.; Revsbech, N.P.; Schulz, H.D. Diffusive and total oxygen uptake of deep-sea sediments in the eastern South Atlantic Ocean: In situ and laboratory measurements. Deep Sea Res. Part I 1994, 41, 1767–1788. [Google Scholar] [CrossRef]
- Kana, T.M.; Darkangelo, C.; Hunt, M.D.; Oldham, J.B.; Bennett, G.E.; Cornwell, J.C. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Anal. Chem. 1994, 66, 4166–4170. [Google Scholar] [CrossRef]
- Colt, J. Computation of Dissolved Gas Concentrations In Water As Functions Of Temperature, Salinity and Pressure; Special Publication 14; American Fisheries Society: Bethesda, MD, USA, 1984. [Google Scholar]
- Chambers, R.M.; Pederson, K.A. Variation in soil phosphorus, sulfur, and iron pools among south Florida wetlands. Hydrobiologia 2006, 569, 63–70. [Google Scholar] [CrossRef][Green Version]
- Vermeer, C.P.; Escher, M.; Portielje, R.; de Klein, J.J.M. Nitrogen uptake and translocation by Chara. Aquat. Bot. 2003, 76, 245–258. [Google Scholar] [CrossRef]
- Kufel, L.; Kufel, I. Chara beds acting as nutrient sinks in shallow lakes—A review. Aquat. Bot. 2002, 72, 249–260. [Google Scholar] [CrossRef]
- Frankovich, T.A.; Barr, J.G.; Morrison, D.; Fourqurean, J.W. Differing temporal patterns of Chara hornemannii cover correlate to alternate regimes of phytoplankton and submerged aquatic-vegetation dominance. Mar. Freshw. Res. 2012, 63, 1005–1014. [Google Scholar] [CrossRef]
- Steinman, A.D.; Meeker, R.H.; Rodusky, A.J.; Davis, W.P.; Hwang, S.-J. Ecological properties of charophytes in a large subtropical lake. J. N. Am. Benthol. Soc. 1997, 16, 781–793. [Google Scholar] [CrossRef]
- Shangguan, Y.; Glibert, P.M.; Alexander, J.A.; Madden, C.J.; Murasko, S. Nutrients and phytoplankton in semienclosed lagoon systems in Florida Bay and their responses to changes in flow from Everglades restoration: Responses in Florida Bay to everglades restoration. Limnol. Oceanogr. 2017, 62, S327–S347. [Google Scholar] [CrossRef]
- Owens, M.S.; Cornwell, J.C. Temporal enhancement of denitrification in bioirrigated estuarine sediments. Aquat. Sci. 2020, 82, 1–12. [Google Scholar] [CrossRef]
- Grenz, C.; Cloern, J.E.; Hager, S.W.; Cole, B.E. Dynamics of nutrient cycling and related benthic nutrient and oxygen fluxes during a spring phytoplankton bloom in South San Francisco Bay (USA). Mar. Ecol. Prog. Ser. 2000, 197, 67–80. [Google Scholar] [CrossRef]
- Caraco, N.; Cole, J.; Likens, G.E. A comparison of phosphorus immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 1990, 9, 277–290. [Google Scholar] [CrossRef]
- Froelich, P.N. Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism. Limnol. Oceanogr. 1988, 33, 649–668. [Google Scholar] [CrossRef]
- Hochard, S.; Pinazo, C.; Grenz, C.; Evans, J.L.B.; Pringault, O. Impact of microphytobenthos on the sediment biogeochemical cycles: A modeling approach. Ecol. Modell. 2010, 221, 1687–1701. [Google Scholar] [CrossRef]
- Nils, R.-P. Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: On the influence of benthic microalgae. Limnol. Oceanogr. 2003, 48, 93–105. [Google Scholar] [CrossRef]
- Giblin, A.E.; Tobias, C.R.; Song, B.; Weston, N.; Banta, G.T.; Rivera-Monroy, V.H. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 2013, 26, 124–131. [Google Scholar] [CrossRef]
- Joye, S.B.; Hollibaugh, J.T. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 1995, 270, 623–625. [Google Scholar] [CrossRef]
- Castañeda-Moya, E.; Twilley, R.R.; Rivera-Monroy, V.H.; Zhang, K.; Davis, S.E.; Ross, M. Sediment and nutrient deposition associated with Hurricane Wilma in mangroves of the Florida Coastal Everglades. Estuaries Coasts 2010, 33, 45–58. [Google Scholar] [CrossRef]
- Koch, M.S.; Benz, R.E.; Rudnick, D.T. Solid-phase phosphorus pools in highly organic carbonate sediments of northeastern Florida Bay. Estuar. Coast. Shelf Sci. 2001, 52, 279–291. [Google Scholar] [CrossRef]
- Lund, E.H. Phosphate contents of sediments near bird rookeries in South Florida. Econ. Geol. 1957, 52, 582–583. [Google Scholar] [CrossRef]
- Neely, M.B. Benthic Microalgae and Nutrient Flux in Florida Bay, USA. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2008. [Google Scholar]
- Jensen, H.S.; Nielsen, O.I.; Koch, M.S.; de Vicentea, I. Phosphorus release with carbonate dissolution coupled to sulfide oxidation in Florida Bay seagrass sediments. Limnol. Oceanogr. 2009, 54, 1753–1764. [Google Scholar] [CrossRef]
- Fourqurean, J.W.; Zieman, J.C.; Powell, G.V.N. Relationships between porewater nutrients and seagrasses in a subtropical carbonate environment. Mar. Biol. 1992, 114, 57–65. [Google Scholar]
- Yarbro, L.A.; Carlson, P.R. Community oxygen and nutrient fluxes in seagrass beds of Florida Bay, USA. Estuaries Coasts 2008, 31, 877–897. [Google Scholar] [CrossRef]
- Liu, K.J.; Li, H.P.; Davis, S.E. Benthic exchange of C, N, and P along the estuarine ecotone of lower Taylor Slough, Florida (USA): Effect of seasonal flows and phosphorus availability. Wetlands 2014, 34, 113–122. [Google Scholar] [CrossRef]
- Boyer, J.N.; Dailey, S.K.; Gibson, P.J.; Rogers, M.T. The role of dissolved organic matter bioavailability in promoting phytoplankton blooms in Florida Bay. Hydrobiologia 2006, 569, 71–85. [Google Scholar] [CrossRef]
- Childers, D.L.; Boyer, J.N.; Davis, S.E.; Madden, C.J.; Rudnick, D.T.; Sklar, F.H. Relating precipitation and water management to nutrient concentrations in the oligotrophic “upside-down” estuaries of the Florida Everglades. Limnol. Oceanogr. 2006, 51, 602–616. [Google Scholar] [CrossRef]
- Rudnick, D.T.; Chen, Z.; Childers, D.L.; Fontaine, T.D. Phosphorus and nitrogen inputs to Florida Bay: The importance of the Everglades watershed. Estuaries 1999, 22, 398–416. [Google Scholar] [CrossRef]
- Sfriso, A.; Pavoni, B.; Marcomini, A.; Raccanelli, S.; Orio, A.A. Particulate matter deposition and nutrient fluxes onto the sediments of the venice lagoon. Environ. Technol. 1992, 13, 473–483. [Google Scholar] [CrossRef]
- Castel, J.; Caumette, P.; Herbert, R. Eutrophication gradients in coastal lagoons as exemplified by the Bassin d’Arcachon and the Etang du Prévost. Hydrobiologia 1996, 329, 9–28. [Google Scholar] [CrossRef]

| Site | Date | Sediment Flux | Porewater | Diagenetic NH4+ | O2 Profiles | Chara |
|---|---|---|---|---|---|---|
| West | July 2010 | ✕ | ✕ | |||
| May 2011 | ✕ | ✕ | ||||
| June 2012 | ✕ | ✕ | ||||
| December 2013 | ✕ | ✕ | ✕ | ✕ | ✕ | |
| Long | July 2010 | ✕ | ||||
| May 2011 | ✕ | |||||
| December 2013 | ✕ | ✕ | ✕ | ✕ | ||
| Cuthbert | June 2012 | ✕ | ||||
| December 2013 | ✕ | ✕ | ✕ | ✕ | ||
| Seven Palm | July 2010 | ✕ | ✕ | |||
| May 2011 | ✕ | ✕ | ||||
| June 2012 | ✕ | ✕ | ||||
| Monroe | July 2010 | ✕ | ✕ | |||
| May 2011 | ✕ | ✕ | ||||
| June 2012 | ✕ | ✕ | ||||
| Terrapin | July 2010 | ✕ | ||||
| May 2011 | ✕ | |||||
| Garfield | July 2010 | ✕ | ||||
| May 2011 | ✕ |
| Site | Date | S | T | Chl a | n | Org C | N | IP | OP | CaCO3-C |
|---|---|---|---|---|---|---|---|---|---|---|
| Psu | °C | mg m−2 | % | % | mg g−1 | mg g−1 | % | |||
| West | July 2010 | 19 | 30 | 10 ± 10 | ||||||
| May 2011 | 17 | 31 | 21 ± 8 | |||||||
| June 2012 | 21 | 31 | 22 ± 12 | |||||||
| December 2013 | 8 | 25 | 22 ± 12 | 12 | 4 ± 2 | 0.6 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 9 ± 1 | |
| Long | July 2010 | 30 | 32 | 26 ± 12 | ||||||
| May 2011 | 54 | 31 | 60 ± 24 | |||||||
| December 2013 | 6 | 25 | 24 ± 3 | 9 | 6 ± 1 | 0.7 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 6 ± 1 | |
| Cuthbert | June 2012 | 18 | 30 | 35 ± 7 | ||||||
| December 2013 | 3 | 24 | 40 ± 15 | 6 | 13 ± 4 | 1.3 ± 0.8 | 0.9 ± 0.4 | 0.5 ± 0.2 | 4 ± 2 | |
| Seven Palm | July 2010 | 20 | 34 | 27 ± 5 | ||||||
| May 2011 | 22 | 30 | 55 ± 13 | |||||||
| June 2012 | 12 | 31 | 25 ± 7 | 9 | 6 ± 1 | 0.8 ± 0.8 | 0.2 ± 0.0 | 0.2 ± 0.1 | 7 ± 1 | |
| Monroe | July 2010 | 30 | 34 | 23 ± 15 | ||||||
| May 2011 | 42 | 30 | 19 ± 4 | |||||||
| June 2012 | 14 | 31 | 21 ± 4 | 9 | 4 ± 1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 8 ± 2 | |
| Terrapin | July 2010 | 36 | 33 | 31 ± 9 | ||||||
| May 2011 | 45 | 31 | 32 ± 5 | 6 | 4 ± 1 | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 8 ± 1 | |
| Garfield | July 2010 | 47 | 32 | 45 ± 17 | ||||||
| May 2011 | 58 | 33 | 16 ± 1 | 6 | 5 ± 1 | 0.6 ± 0.2 | 0.3 ± 0.1 | 0.2 ± 0.1 | 7 ± 1 |
| Site | Date | NH4+ | SRP | |
|---|---|---|---|---|
| West | July 2010 | Chara | −0.71 | |
| May 2011 | ||||
| June 2012 | ||||
| December 2013 | ||||
| Seven Palm | July 2010 | Chara | −0.91 | −0.85 |
| May 2011 | ||||
| December 2012 | ||||
| Monroe | July 2010 | Chara | −0.52 | −0.28 |
| May 2011 | ||||
| December 2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owens, M.S.; Kelly, S.P.; Frankovich, T.A.; Rudnick, D.T.; Fourqurean, J.W.; Cornwell, J.C. Controls on Nutrient Cycling in Estuarine Mangrove Lake Sediments. J. Mar. Sci. Eng. 2021, 9, 626. https://doi.org/10.3390/jmse9060626
Owens MS, Kelly SP, Frankovich TA, Rudnick DT, Fourqurean JW, Cornwell JC. Controls on Nutrient Cycling in Estuarine Mangrove Lake Sediments. Journal of Marine Science and Engineering. 2021; 9(6):626. https://doi.org/10.3390/jmse9060626
Chicago/Turabian StyleOwens, Michael S., Stephen P. Kelly, Thomas A. Frankovich, David T. Rudnick, James W. Fourqurean, and Jeffrey C. Cornwell. 2021. "Controls on Nutrient Cycling in Estuarine Mangrove Lake Sediments" Journal of Marine Science and Engineering 9, no. 6: 626. https://doi.org/10.3390/jmse9060626
APA StyleOwens, M. S., Kelly, S. P., Frankovich, T. A., Rudnick, D. T., Fourqurean, J. W., & Cornwell, J. C. (2021). Controls on Nutrient Cycling in Estuarine Mangrove Lake Sediments. Journal of Marine Science and Engineering, 9(6), 626. https://doi.org/10.3390/jmse9060626







