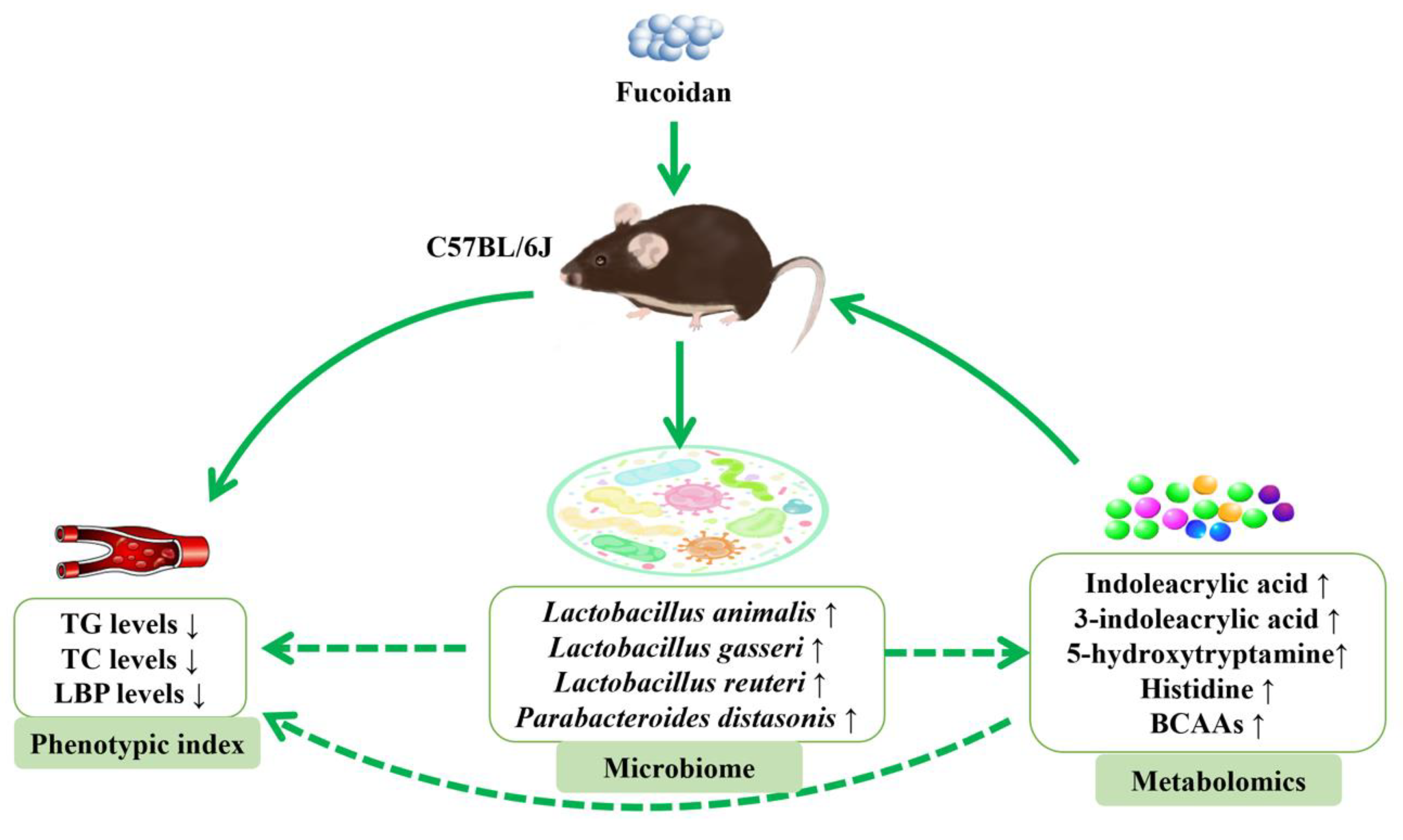
Microbiome-Metabolomics Reveals Prebiotic Benefits of Fucoidan Supplementation in Mice
Abstract
1. Introduction
2. Materials
2.1. Animals and Diets
2.2. Biochemical Analyses
2.3. Microbiota Analysis
2.4. Metabolome Analysis
2.5. Statistical Analysis
3. Results
3.1. Effects of Fucoidan on Body Weight and Plasma Lipid Profile
3.2. Effects of Fucoidan on Microbial Communities
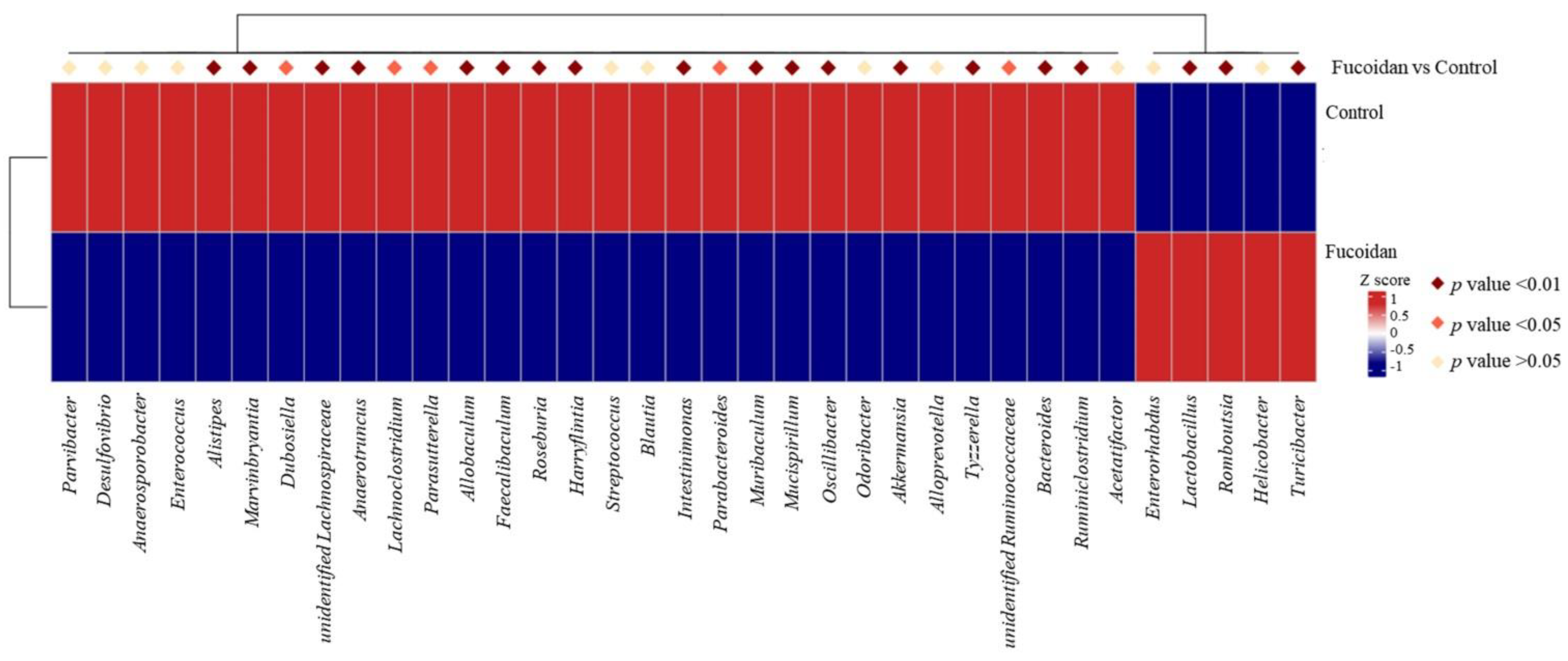
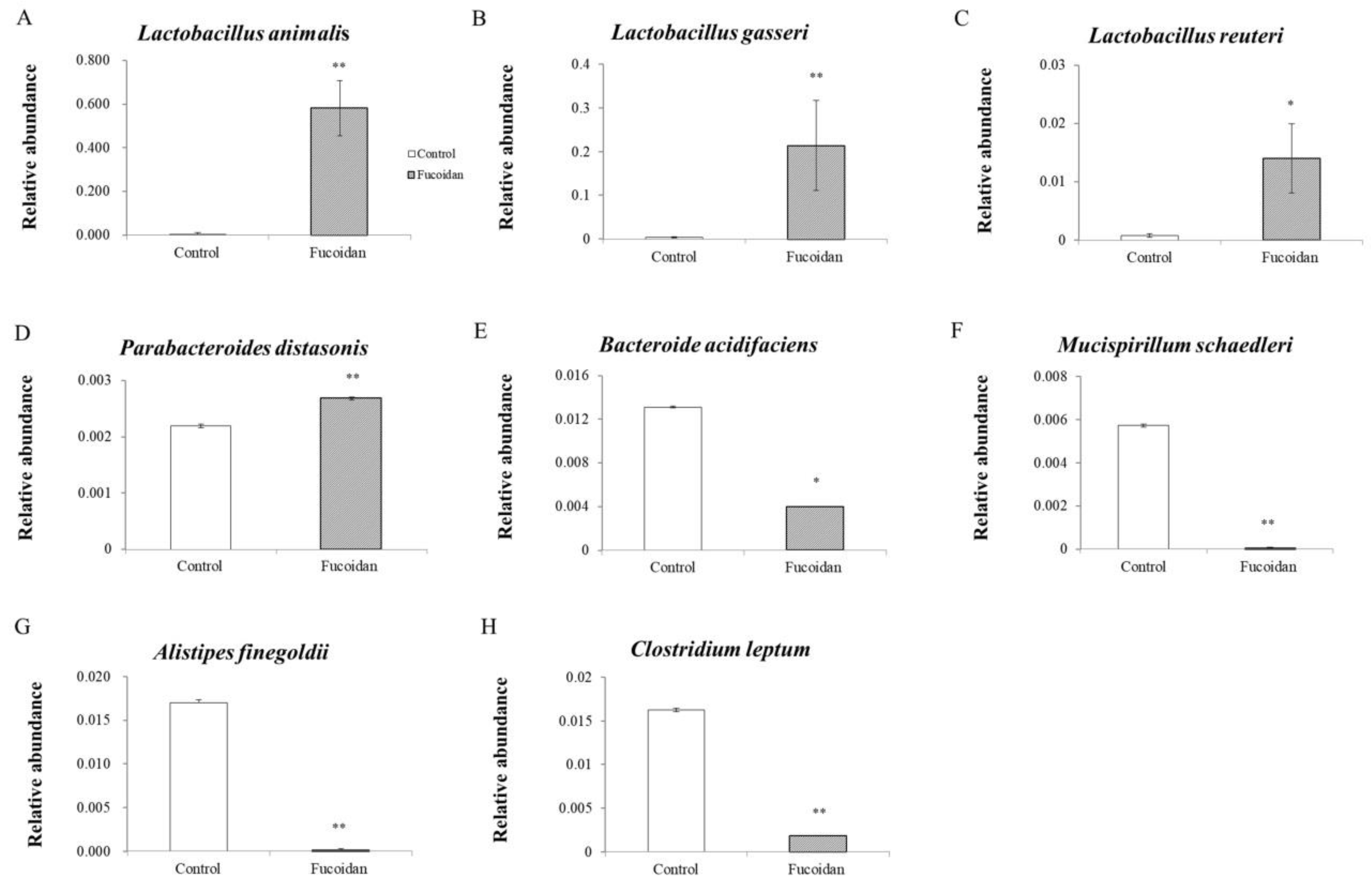
3.3. Effects of Fucoidan on Bacterial Genera and Species
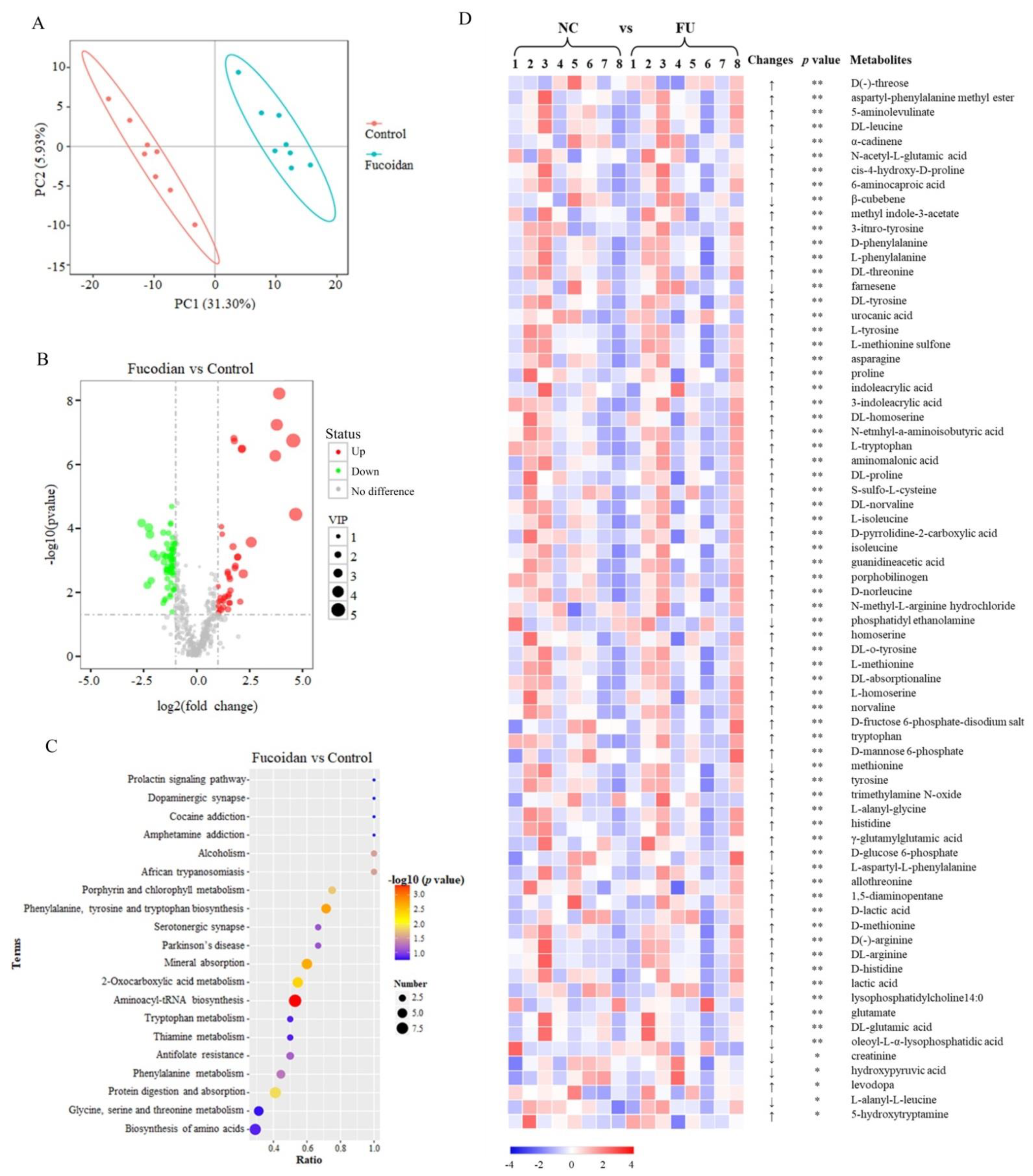
3.4. Effects of Fucoidan on the Metabolomics
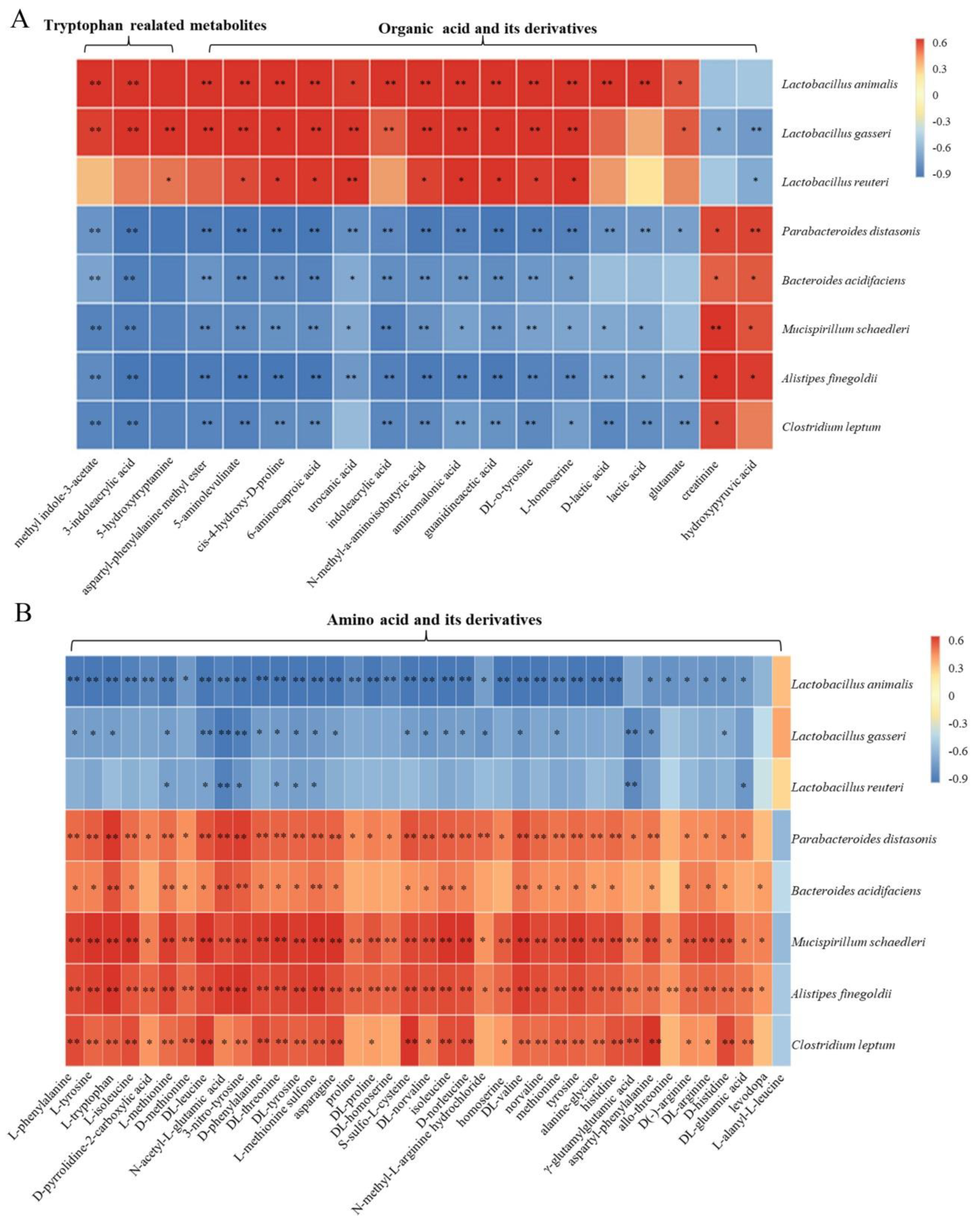
3.5. Spearman Correlation Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A



References
- Hendrikx, T.; Schnabl, B. Indoles: Metabolites produced by intestinal bacteria capable of controlling liver disease manifestation. J. Intern. Med. 2019, 286, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. USA 2020, 117, 19376–19387. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Medina, D.A.; Ortuzar, V.; Gotteland, M.; Garrido, D. Anti-inflammatory effect of microbial consortia during the utilization of dietary polysaccharides. Food Res. Int. 2018, 109, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: A randomised cross-over trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2018, 68, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40 e27. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 2018, 23, 41–53 e44. [Google Scholar] [CrossRef] [PubMed]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, S.; Miao, J.; Li, Y.; Wang, Z.; Wang, M.; Yu, Q. Lactobacillus reuteri maintains intestinal epithelial regeneration and repairs damaged intestinal mucosa. Gut Microbes 2020, 11, 997–1014. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, Y.T.; Zhu, L.M.; Liu, Z.Y.; Ye, C.Q.; Qin, S. Inulin with different degrees of polymerization protects against diet-induced endotoxemia and inflammation in association with gut microbiota regulation in mice. Sci. Rep. 2020, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Piko, P.; Fiatal, S.; Werissa, N.A.; Bekele, B.B.; Racz, G.; Kosa, Z.; Sandor, J.; Adany, R. The effect of haplotypes in the cetp and lipc genes on the triglycerides to HDL-c ratio and its components in the roma and hungarian general populations. Genes 2020, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Roubille, F.; Sultan, A.; Huet, F.; Leclercq, F.; Macia, J.C.; Gervasoni, R.; Delseny, D.; Akodad, M.; Roubille, C. Is hypertriglyceridemia atherogenic? Presse Med. 2018, 47, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yun, J.M.; Kim, M.K.; Kwon, O.; Cho, B. Lactobacillus gasseri BNR17 supplementation reduces the visceral fat accumulation and waist circumference in obese adults: A randomized, double-blind, placebo-controlled Trial. J. Med. Food 2018, 21, 454–461. [Google Scholar] [CrossRef]
- Yonejima, Y.; Ushida, K.; Mori, Y. Lactobacillus gasseri NT decreased visceral fat through enhancement of lipid excretion in feces of KK-A(y) mice. Biosci. Biotechnol. Biochem. 2013, 77, 2312–2315. [Google Scholar] [CrossRef]
- Citronberg, J.S.; Curtis, K.R.; White, E.; Newcomb, P.A.; Newton, K.; Atkinson, C.; Song, X.; Lampe, J.W.; Hullar, M.A. Association of gut microbial communities with plasma lipopolysaccharide-binding protein (LBP) in premenopausal women. ISME J. 2018, 12, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Pastor Rojo, O.; Lopez San Roman, A.; Albeniz Arbizu, E.; de la Hera Martinez, A.; Ripoll Sevillano, E.; Albillos Martinez, A. Serum lipopolysaccharide-binding protein in endotoxemic patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2007, 13, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Li, Y.; Han, D.; Hong, J.; Yang, N.; He, J.; Peng, R.; Mi, X.; Kuang, C.; et al. Baicalin ameliorates cognitive impairment and protects microglia from LPS-induced neuroinflammation via the SIRT1/HMGB1 pathway. Oxidative Med. Cell. Longev. 2020, 2020, 4751349. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wei, Y.; Lu, Q.; Qin, D.; Zhang, M.; Du, X.; Xu, W.; Yu, X.; He, C.; Li, N.; et al. Melatonin alleviates LPS-induced endoplasmic reticulum stress and inflammation in spermatogonial stem cells. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Gebremariam, H.G.; Qazi, K.R.; Somiah, T.; Pathak, S.K.; Sjolinder, H.; Sverremark Ekstrom, E.; Jonsson, A.B. Lactobacillus gasseri suppresses the production of proinflammatory cytokines in -infected macrophages by inhibiting the expression of ADAM17. Front. Immunol. 2019, 10, 2326. [Google Scholar] [CrossRef]
- Juarez, G.E.; Villena, J.; Salva, S.; de Valdez, G.F.; Rodriguez, A.V. Lactobacillus reuteri CRL1101 beneficially modulate lipopolysaccharide-mediated inflammatory response in a mouse model of endotoxic shock. J. Funct. Foods 2013, 5, 1761–1773. [Google Scholar] [CrossRef]
- Wlodarska, M.; Luo, C.; Kolde, R.; d’Hennezel, E.; Annand, J.W.; Heim, C.E.; Krastel, P.; Schmitt, E.K.; Omar, A.S.; Creasey, E.A.; et al. Indoleacrylic Acid Produced by Commensal Peptostreptococcus Species Suppresses Inflammation. Cell Host Microbe 2017, 22, 25–37 e26. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Chimienti, G.; Orlando, A.; D’Attoma, B.; Clemente, C.; Russo, F. Effects of long-term administration of Lactobacillus reuteri DSM-17938 on circulating levels of 5-HT and BDNF in adults with functional constipation. Benef. Microbes 2019, 10, 137–147. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of gut microbiota composition by serotonin signaling influences intestinal immune response and susceptibility to colitis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Vistoli, G.; Katunga, L.A.; Funai, K.; Regazzoni, L.; Monroe, T.B.; Gilardoni, E.; Cannizzaro, L.; Colzani, M.; De Maddis, D.; et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018, 128, 5280–5293. [Google Scholar] [CrossRef] [PubMed]
- Christian, F. Histidine metabolism boosts cancer therapy. Nature 2019, 559, 484–485. [Google Scholar]
- Kanarek, N.; Petrova, B.; Sabatini, D.M. Dietary modifications for enhanced cancer therapy. Nature 2020, 579, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Ten Have, G.A.M.; Jansen, L.; Schooneman, M.G.; Engelen, M.; Deutz, N.E.P. Metabolic flux analysis of Branched-chain amino and keto acids (BCAA, BCKA) and beta-Hydroxy beta-methylbutyric acid (HMB) across multiple organs in the pig. Am. J. Physiol. Endocrinol. Metab. 2021. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Cerezo, A.; Cerrillo, I.; Ortega, A.; FernAndez-PachOn, M.S. Intake of branched chain amino acids favors post-exercise muscle recovery and may improve muscle function: Optimal dosage regimens and consumption conditions. J. Sports Med. Phys. Fitness 2021. [Google Scholar] [CrossRef]
- Wyant, G.A.; Abu-Remaileh, M.; Wolfson, R.L.; Chen, W.W.; Freinkman, E.; Danai, L.V.; Vander Heiden, M.G.; Sabatini, D.M. mTORC1 activator SLC38A9 is required to efflux essential amino acids from lysosomes and use protein as a nutrient. Cell 2017, 171, 642–654.e612. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Qin, S.; Li, W.; Zhang, Y.; Wang, Y.; Chang, X.; Li, L. Microbiome-Metabolomics Reveals Prebiotic Benefits of Fucoidan Supplementation in Mice. J. Mar. Sci. Eng. 2021, 9, 505. https://doi.org/10.3390/jmse9050505
Yuan J, Qin S, Li W, Zhang Y, Wang Y, Chang X, Li L. Microbiome-Metabolomics Reveals Prebiotic Benefits of Fucoidan Supplementation in Mice. Journal of Marine Science and Engineering. 2021; 9(5):505. https://doi.org/10.3390/jmse9050505
Chicago/Turabian StyleYuan, Jingyi, Song Qin, Wenjun Li, Yubing Zhang, Yuting Wang, Xiulian Chang, and Lili Li. 2021. "Microbiome-Metabolomics Reveals Prebiotic Benefits of Fucoidan Supplementation in Mice" Journal of Marine Science and Engineering 9, no. 5: 505. https://doi.org/10.3390/jmse9050505
APA StyleYuan, J., Qin, S., Li, W., Zhang, Y., Wang, Y., Chang, X., & Li, L. (2021). Microbiome-Metabolomics Reveals Prebiotic Benefits of Fucoidan Supplementation in Mice. Journal of Marine Science and Engineering, 9(5), 505. https://doi.org/10.3390/jmse9050505








