Analysis of Manganese Bioaccumulated in Mediterranean Blue Mussel (Mytilus galloprovincialis) from the Bay of Mali Ston (Adriatic Sea, Croatia) during Diarrhetic Shellfish Poisoning Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mussel Sampling
2.3. Reagents and Standard Solutions
2.4. Extraction of Manganese in Tissue Samples
2.5. Atomic Absorption Spectroscopy (AAS) Analysis
2.6. Data Analysis
3. Results
3.1. Spatial Distribution of Manganese during DSP Toxicity
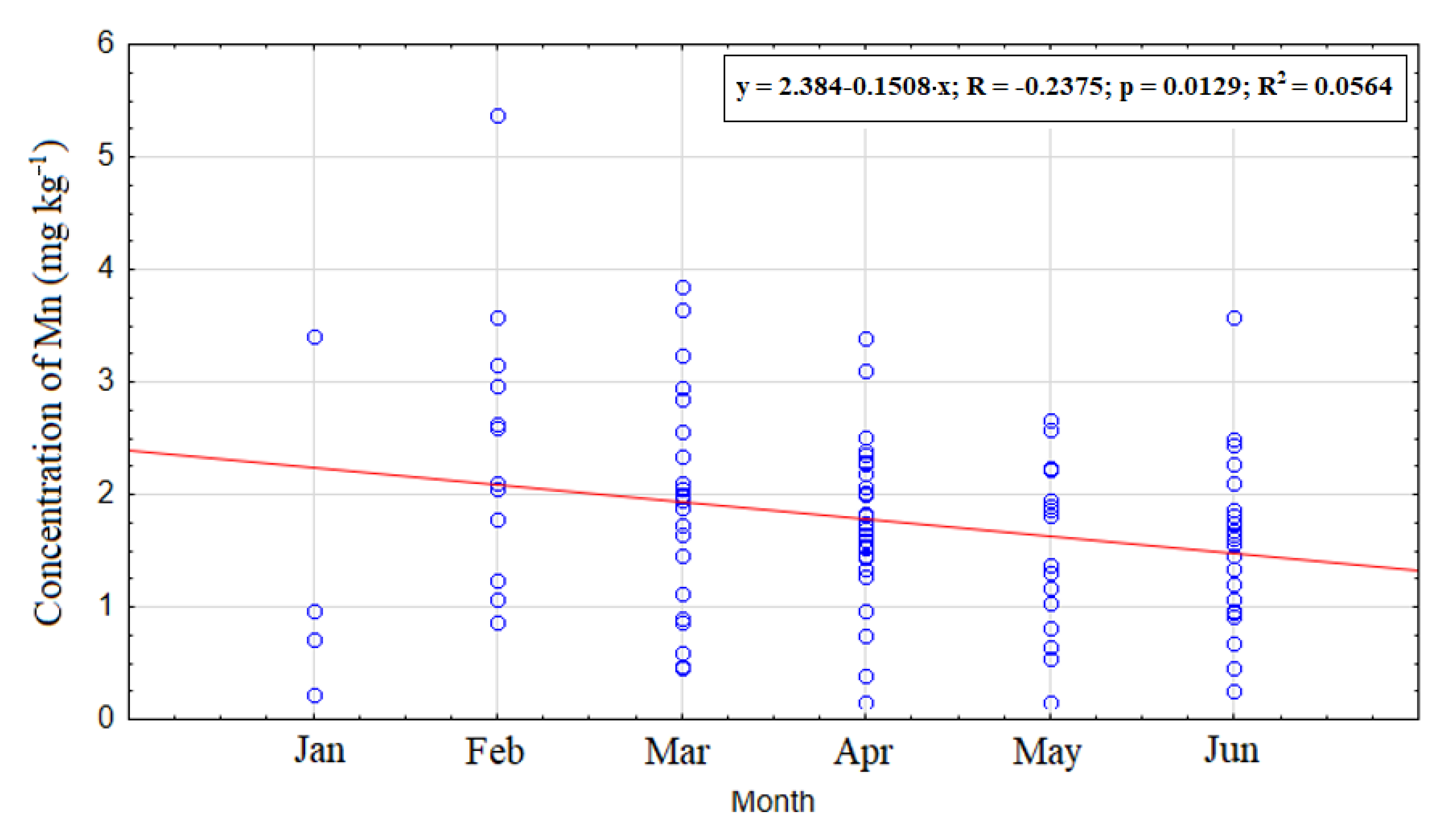
3.2. Temporal Distribution of Manganese during the Period of DSP Shellfish Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinčić, D.; Kwokal, Ž.; Peharec, Ž.; Marguš, D.; Branica, M. Distribution of Zn, Pb, Cd and Cu between seawater and transplanted mussels (Mytilus galloprovincialis). Sci. Total Environ. 1992, 119, 211–230. [Google Scholar] [CrossRef]
- McCulloch, A.W.; Body, R.K.; de Freitas, A.S.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; Quilliam, M.A.; Wright, J.L.; Boyko, V.J.; McLaren, J.W. Zinc from oyster tissue as causative factor in mouse deaths in official bioassay for paralytic shellfish poison. J. Assoc. Off. Anal Chem. 1989, 72, 384–386. [Google Scholar] [CrossRef]
- Park, D.L.; Adams, W.N.; Graham, S.L.; Jackson, R.C. Variability of mouse bioassay for determination of paralytic shellfish poisoning. J. Assoc. Off. Anal. Chem. 1986, 69, 547–550. [Google Scholar] [CrossRef]
- Rhodes, L.; Selwood, A.; McNabb, P.; Briggs, L.; Adamson, J.; van Ginkel, R.; Laczka, O.F. Trace metal effects on the production of biotoxins by microalgae. Afr. J. Mar. Sci. 2006, 28, 393–397. [Google Scholar] [CrossRef]
- Libes, S.M. An Introduction to Marine Biogeochemistry; John Willey and Sons Inc.: New York, NY, USA, 1992. [Google Scholar]
- El-Moselhy, K.M.; Gabal, M.N. Trace metals in water, sediments and marine organisms from the northern part of Gulf of Suez, Red Sea. J. Mar. Syst. 2004, 46, 39–46. [Google Scholar] [CrossRef]
- Wedepohl, K. The composition of the continental crust. Geochim. Cosmochi. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Van Hulten, M.; Dutay, J.-C.; Middag, R.; de Baar, H.; Roy-Barman, M.; Gehlen, M.; Tagliabue, A.; Sterl, A. Manganese in the world ocean: A first global model. Biogeosci. Discuss. 2016, 1–38. [Google Scholar] [CrossRef]
- Raven, J.A. Predictions of Mn and Fe use efficiencies of phototropic growth as a function of light availability for growth and C assimilation pathway. N. Phytol. 1990, 116, 1–18. [Google Scholar] [CrossRef]
- Sarmiento, J.; Gruber, N. Ocean Biogeochemical Dynamics; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Baker, A.; Landing, W.; Bucciarelli, E.; Fietz, S.; Hayes, C.; Kadko, D.; Morton, P.; Rogan, N.; Sarthou, G.; Shelley, R.; et al. Trace element and isotope deposition across the air-sea interface: Progress and research needs. Philos. Trans. R. Soc. A 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Homoky, W.B.; Weber, T.; Berelson, W.M.; Conway, T.M.; Henderson, G.M.; Vun Hulten, M.M.P.; Jeandel, C.; Severmann, S.; Tagliabue, A. Quantifying trace element and isotope fluxes at the ocean-sediment boundary. Philos. Trans. R. Soc. A 2016, 374. [Google Scholar] [CrossRef]
- Middag, R.; de Baar, H.; Laan, P.; Huhn, O. The effects of continental margins and water mass circulation on the distribution of dissolved aluminium and manganese in Drake Passage. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef]
- Pakhomova, S.; Hall, P.; Kononets, M.; Rozanov, A.; Tengberg, A.; Verenshinin, A. Fluxes of iron and manganese across the sediment-water interface under various redox conditions. Mar. Chem. 2007, 107, 319–331. [Google Scholar] [CrossRef]
- Aguilar-Islas, A.; Burland, K. Dissolved manganese and silicic acid in the Columbia River plume. Mar. Chem. 2006, 101, 233–247. [Google Scholar] [CrossRef]
- Jeandel, C. Overview of the mechanisms that could explain the “Boundary Exchange” at the land-ocean contact. Philos. Trans. R. Soc. A 2016, 374. [Google Scholar] [CrossRef] [PubMed]
- Middag, R.; de Baar, H.; Laan, P.; Klunder, M. Fluvial and hydrothermal input of manganese into the Arctic Ocean. Geochim. Cosmochim. Acta 2011, 75, 2393–2408. [Google Scholar] [CrossRef]
- Bogdanović, T.; Ujević, I.; Sedak, M.; Listeš, E.; Šimat, V.; Petričević, S.; Poljak, V. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Kljaković-Gašpić, Z.; Odžak, N.; Ujević, I.; Zvonarić, T.; Barić, A. Biomonitoring of trace metals (Cu, Cd, Cr, Hg, Pb, Zn) in eastern Adriatic using the Mediterranean blue mussel (2001–2005). Fresenius Environ. Bull. 2006, 15, 1041–1048. [Google Scholar]
- Cullaj, A.; Lazo, P.; Duka, S. Heavy metals and metallonine levels in mussel samples from the Albanian sea coast. MAP/MED Pol. In Biological Effects Monitoring Programme—Achievements and Future Orientations, Proceedings of the Workshop, Alessandria, Italy, 20–21 December 2006; MAP Technical Reports Serial No. 166; UNEP/MAP: Athens, Greece, 2007; pp. 141–151. [Google Scholar]
- Cardellicchio, N.; Buccolieri, A.; di Leo, A.; Giandomenico, S.; Spada, L. Levels of metals in reared mussels from Taranto Gulf (Ionian Sea, Southern Italy). Food Chem. 2008, 107, 890–896. [Google Scholar] [CrossRef]
- Ščančar, J.; Zuliani, T.; Turk, T.; Milačić, R. Organotin compounds and selected metals in the marine environment of Northern Adriatic Sea. Environ. Monit. Assess. 2007, 127, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Stanković, S.; Jović, M.; Milanov, R.; Joksimović, D. Trace elements concentrations (Zn, Cu, Pb, Cd, As and Hg) in the Mediterranean mussel (Mytilus galloprovincialis) and evaluation of mussel quality and possible human health risk from cultivated and wild sites of the southeastern Adriatic Sea, Montenegro. J. Serb. Chem. Soc. 2011, 76, 1725–1737. [Google Scholar] [CrossRef]
- Marković, J.; Joksimović, D.; Stanković, S. Trace element concentrations in wild mussels from the coastal area of the southeastern Adriatic, Montenegro. Arch Biol. Sci. 2012, 64, 265–275. [Google Scholar] [CrossRef]
- Ramšak, A.; Ščančar, J.; Horvat, M. Evaluation of metallothioneins in blue mussels (Mytilus galloprovincialis) as a biomarker of mercury and cadmium exposure in the Slovenian waters (Gulf of Trieste): A long-term field study. Acta Adriat. 2012, 53, 71–86. [Google Scholar]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity and bioaccumulation. J. Chem. 2019, 6730305. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hickey, B.M.; Bates, S.S. Toxic Diatoms; Elsevier Science Publishers: New York, NY, USA, 2008; pp. 219–237. [Google Scholar]
- Yasumoto, T.; Murata, M. Marine toxins. Chem. Rev. 1993, 5, 1897–1901. [Google Scholar] [CrossRef]
- Rainbow, P.S. Heavy metal levels in the marine invertebrates. In Heavy Metal Levels in the Marine Environment; Furness, R.W., Rainbow, P.S., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 67–79. [Google Scholar]
- Ujević, I.; Nazlić, N.; Ninčević-Gladan, Ž.; Marasović, I. Gymnodimine and spirolide in shellfish during DSP toxicity in central and southern Adriatic Sea. In Proceedings of the 16th International Conference on Harmful Algae, Abstract Book Wellington, Welington, New Zeland, 27–31 October 2014. [Google Scholar]
- Suzuki, T.; Yoshizawa, R.; Kawamura, T.; Yamasaki, M. Interference of free fatty acids from the hepatopancreas of mussels with the mouse bioassay for shellfish toxins. Lipids 1996, 31, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Hayashi, K.; Itabashi, Y. Toxic effect of free unsaturated fatty acids in the mouse bioassay of diarrhetic shellfish toxin by intraperitoneal injection. Bull. Jpn. Soc. Sci. Fish 1984, 50, 1413–1418. [Google Scholar] [CrossRef]
- Stabell, O.B.; Yndestad, M.; Heidenreich, B. Paralytic shellfish toxins seem absent in extracts of diarrhetic shellfish toxins. Environ. Toxicol. Chem. 1991, 10, 331–334. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Chadha, R.K.; Ratnayabe, W.M.; Truelove, J.F. An incident of elevated levels of unsaturated free fatty acids in mussels from Nova Scotia and their toxic effect in mice after intraperitoneal injection. Nat. Toxins 1994, 2, 318–321. [Google Scholar] [CrossRef]
- Vilarino, N.; Fonfria, E.S.; Molgo, J.; Romulo, A.; Botana, L.M. Detection of gymnodimine-A and 13-desmethyl spirolide phycotoxins by fluorescence polarization. Anal. Chem. 2009, 81, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Ninčević Gladan, Ž.; Ujević, I.; Milandri, A.; Marasović, I.; Ceredi, A.; Pigozzi, S.; Arapov, J.; Skejić, S. Lipophilic toxin profile in Mytilus galloprovincialis during episodes of diarrhetic shellfish poisoning (DSP) in the NE Adriatic Sea in 2006. Molecules 2011, 16, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Espina, B.; Otero, P.; Louzao, M.C.; Alfonso, A.; Botana, L.M. 13-Desmethyl spirolide-c and 13,19-didesmethyl spirolide-c trans-epithelial permeabilities: Human intestinal permeability modelling. Toxycology 2011, 287, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Maanan, M. Heavy metals concentrations in marine molluscs from the Moroccan coastal region. Environ. Pollut. 2007, 153, 176–183. [Google Scholar] [CrossRef]
- Maanan, M.; Zourarah, B.; Carruesco, C.; Aajjane, A.; Naud, J. Distribution of heavy metals in Sidi Moussa lagoon sediments (Atlantic Moroccan Coast). J. Afr. Earth Sci. 2004, 39, 473–483. [Google Scholar] [CrossRef]
- Joksimović, D.; Tomić, I.; Stanković, A.R.; Jović, M.; Stanković, S. Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussel quality and possible risks of high human consumption. Food Chem. 2011, 127, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Perošević, A.; Pezo, L.; Joksimović, D.; Đurović, D.; Milašević, I.; Radomirović, M.; Stanković, S. The impacts of seawater physicochemical parameters and sedimental metal contents on trace metal concentrations in mussels—A chemometric approach. Environ. Sci. Pollut. Res. 2018, 25, 28248–28263. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, D.; Notti, A.; Di Mento, R.; Cicero, A.M. Seasonal, spatial and inter-annual variations of trace metals in mussels from the Adriatic Sea: Aregional gradient of arsenic, and implications for monitoring the impact of off-shore activities. Chemosphere 2008, 72, 1524–1533. [Google Scholar] [CrossRef]
- Romeo, M.; Frasila, C.; Gnassia-Berelli, M.; Damiens, G.; Micu, D.; Mustata, G. Biomonitoring of trace metals in the Black Sea (Romania) using mussels Mytilus galloprovincialis. Water Res. 2005, 39, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, S.; Kiribsoglu, C.; Gungor, N. Heavy metals in organisms and sediments from Turkish coast of the Black Sea, 1997–1998. Environ. Int. 2002, 27, 521–526. [Google Scholar] [CrossRef]
- Catsiki, V.A.; Florou, H. Study on the behavior of the heavy metals Cu, Cr, Ni, Zn, Fe, Mn and 137Cs in an estuarine ecosystem using Mytilus galloprovincialis as a bioindicator species: The case of Thermaikos gulf, Greece. J. Environ. Radioact. 2005, 86, 31–44. [Google Scholar] [CrossRef]
- Paerl, H.W. Nuisance phytoplankton blooms in coastal, estaurine, and inland waters. Limnol. Oceanogr. 1988, 33, 823–843. [Google Scholar] [CrossRef]
- Lewetus, A.J.; Willis, B.M.; Hayes, K.C.; Burkholder, J.M.; Glasgow, H.B.; Gilbert, P.M.; Burke, M.K. Mixotrophy and nitrogen uptake by Pfiesteria piscicida (Dinophyceae). J. Phycol. 1999, 35, 1430–1437. [Google Scholar] [CrossRef]
- Gilbert, P.M.; Magnien, R.; Lomas, M.W.; Alexander, J.; Tan, C.; Haramoto, E.; Trice, M.; Kana, T.M. Harmful algal blooms in the Chesapeake and coastal bays of Maryland, USA: Comparison of 1997, 1998 and 1999 events. Estuaries 2001, 24, 875–883. [Google Scholar] [CrossRef]
- Mountfort, D.; Beuzenberg, V.; MacKenzie, L.; Rhodes, L. Enhancement of growth and gymnodimine production by the marine dinoflagellatae, Karenia selliformis. Harmful Algae 2006, 5, 658–664. [Google Scholar] [CrossRef]
- Ujević, I.; Vuletić, N.; Lušić, J.; Nazlić, N.; Kušpilić, G. Bioaccumulation of trace metals in mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP toxicity episodes. Molecules 2015, 20, 13031–13040. [Google Scholar] [CrossRef]
- Regoli, F.; Orlando, E. Seasonal variation of trace metal concentration in the digestive gland of the Mediterranean mussel Mytilus galloprovincialis: Comparison between polluted and non-polluted site. Arch. Environ. Contam. Toxicol. 1994, 27, 36–43. [Google Scholar] [CrossRef]
- Borchardt, T.; Burchert, S.; Krabe, L.; Zeitner, R. Enhanced heavy metal concentrations in Mytilus edulis from the central North Sea. Sci. Mar. 1989, 53, 725–728. [Google Scholar]
- Cossa, D. A review of the use of Mytilus sas quantitative indicators of cadmium and mercury concentration in coastal waters. Oceanol. Acta 1989, 12, 417–432. [Google Scholar]
- Odžak, N.; Zvonarić, T.; Kljaković-Gašpić, Z.; Barić, A. Biomonitoring of copper, cadmium, lead, zinc and chromium in the Kaštela Bay using transplanted mussels. Fresenius Environ. Bull. 2001, 10, 37–41. [Google Scholar]
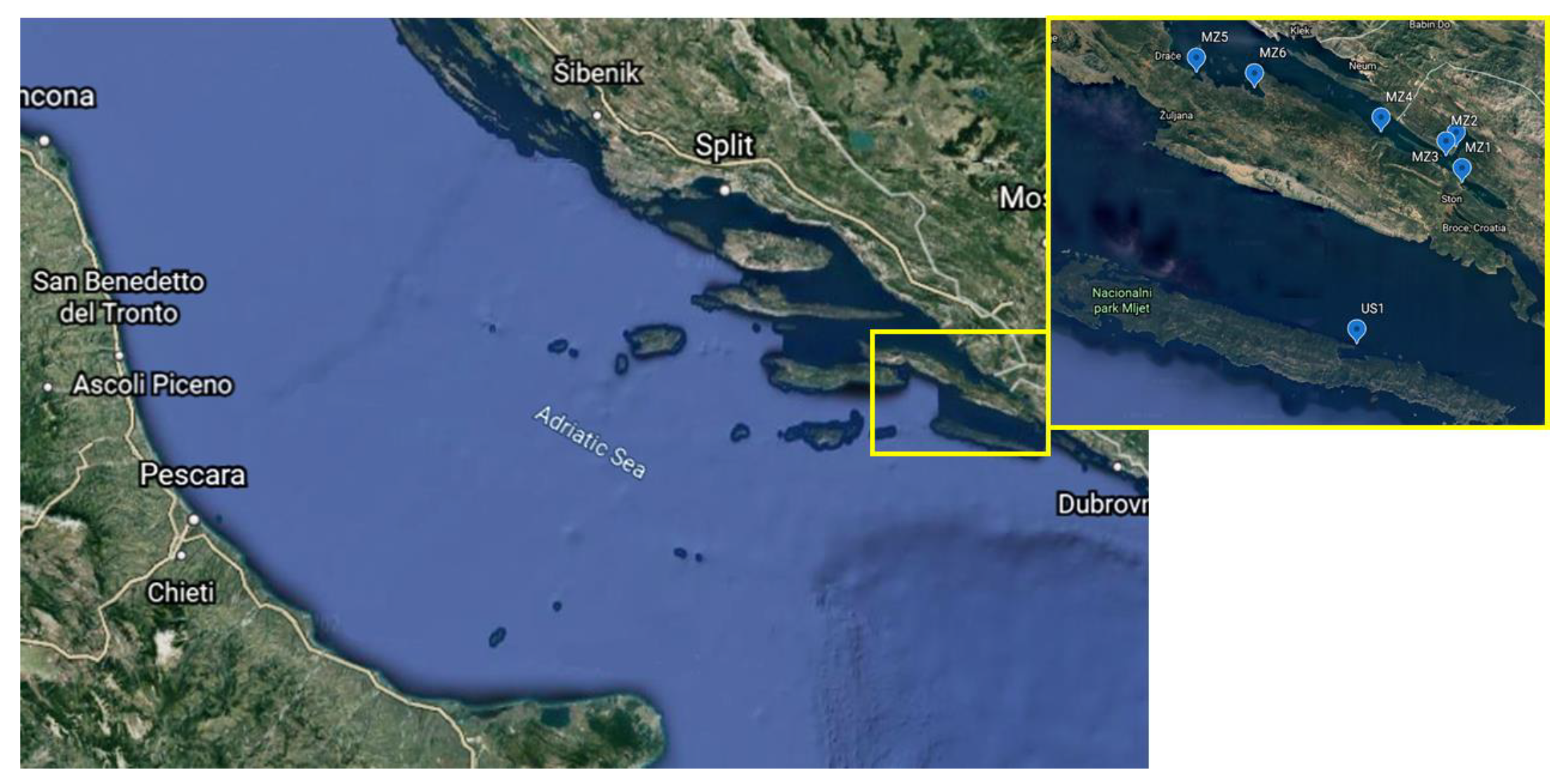

| Station Name | Station Mark on the Map | Longitude (N) and Latitude (E) of the Sampling Stations |
|---|---|---|
| Mali Ston Cove | MZ1 | 42°50.69′:17°42.69′ |
| Banja Cove | MZ2 | 42°52.16′:17°40.98′ |
| Bistrina Cove | MZ3 | 42°52.38′:17°42.17′ |
| Kanal Usko Cove | MZ4 | 42°52.94′:17°38.27′ |
| Sutvid Cove | MZ5 | 42°54.99′:17°28.57′ |
| Brijesta Cove | MZ6 | 42°54.35′:17°31.01′ |
| Sobra Cove | US1 | 42°44.73′:17°36.80′ |
| Station Name | Station Mark on the Map | The Concentration Range of Mn (mg kg−1) | Mean Value of Mn Concentration (mg kg−1) | Standard Error (SE) |
|---|---|---|---|---|
| Mali Ston Cove | MZ1 | 0.15–2.29 | 1.57 ± 0.60 | 0.17 |
| Banja Cove | MZ2 | 0.86–2.86 | 2.04 ± 0.55 | 0.16 |
| Bistrina Cove | MZ3 | 0.39–3.85 | 2.20 ± 1.00 | 0.25 |
| Kanal Usko Cove | MZ4 | 0.46–3.11 | 1.69 ± 0.87 | 0.27 |
| Sutvid Cove | MZ5 | 0.16–3.41 | 1.59 ± 0,94 | 0.22 |
| Brijesta Cove | MZ6 | 0.54–5.38 | 1.78 ± 0.98 | 0.23 |
| Sobra Cove | US1 | 0.22–2.35 | 1.22 ± 0.73 | 0.30 |
| Region | Concentration of Mn | Reference |
|---|---|---|
| Adriatic Sea, Bay of Mali Ston | 0.5–18.7 | Present study |
| Adriatic Sea, Montenegro | 7.3–85 | [40] |
| Adriatic Sea, Montenegro | 6.4–22.2 | [41] |
| Adriatic Sea, Albania | 12.3–139.3 | [20] |
| Safi coastal waters, Morocco | 7.2–27.5 | [38] |
| El Jadida coast, Morocco | 8.7–34.8 | [39] |
| Portonovo, Italy | 4.4–16.4 | [42] |
| Black Sea, Romania | 12.7–13.6 | [43] |
| Black Sea, Turkey | 5.7–22.8 | [44] |
| Aegean Sea, Greece | 7.2–25.3 | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuletić, N.; Lušić, J.; Anđelić, I. Analysis of Manganese Bioaccumulated in Mediterranean Blue Mussel (Mytilus galloprovincialis) from the Bay of Mali Ston (Adriatic Sea, Croatia) during Diarrhetic Shellfish Poisoning Toxicity. J. Mar. Sci. Eng. 2021, 9, 451. https://doi.org/10.3390/jmse9050451
Vuletić N, Lušić J, Anđelić I. Analysis of Manganese Bioaccumulated in Mediterranean Blue Mussel (Mytilus galloprovincialis) from the Bay of Mali Ston (Adriatic Sea, Croatia) during Diarrhetic Shellfish Poisoning Toxicity. Journal of Marine Science and Engineering. 2021; 9(5):451. https://doi.org/10.3390/jmse9050451
Chicago/Turabian StyleVuletić, Nenad, Jelena Lušić, and Ivana Anđelić. 2021. "Analysis of Manganese Bioaccumulated in Mediterranean Blue Mussel (Mytilus galloprovincialis) from the Bay of Mali Ston (Adriatic Sea, Croatia) during Diarrhetic Shellfish Poisoning Toxicity" Journal of Marine Science and Engineering 9, no. 5: 451. https://doi.org/10.3390/jmse9050451
APA StyleVuletić, N., Lušić, J., & Anđelić, I. (2021). Analysis of Manganese Bioaccumulated in Mediterranean Blue Mussel (Mytilus galloprovincialis) from the Bay of Mali Ston (Adriatic Sea, Croatia) during Diarrhetic Shellfish Poisoning Toxicity. Journal of Marine Science and Engineering, 9(5), 451. https://doi.org/10.3390/jmse9050451






