Differential Physiological Responses of Small Thalassiosira pseudonana and Large Thalassiosira punctigera to the Shifted-High Light and Nitrogen
Abstract
1. Introduction
2. Material and Methods
2.1. Culture Protocol
2.2. Experiment Design
2.3. Growth Rate
2.4. Pigment Content
2.5. Chrolophyll Fluorescence
2.6. Dark Respiration
2.7. Carbon and Nitrogen Contents
2.8. Protein Content and Superoxide Dismutase Activity
2.9. Data Analysis
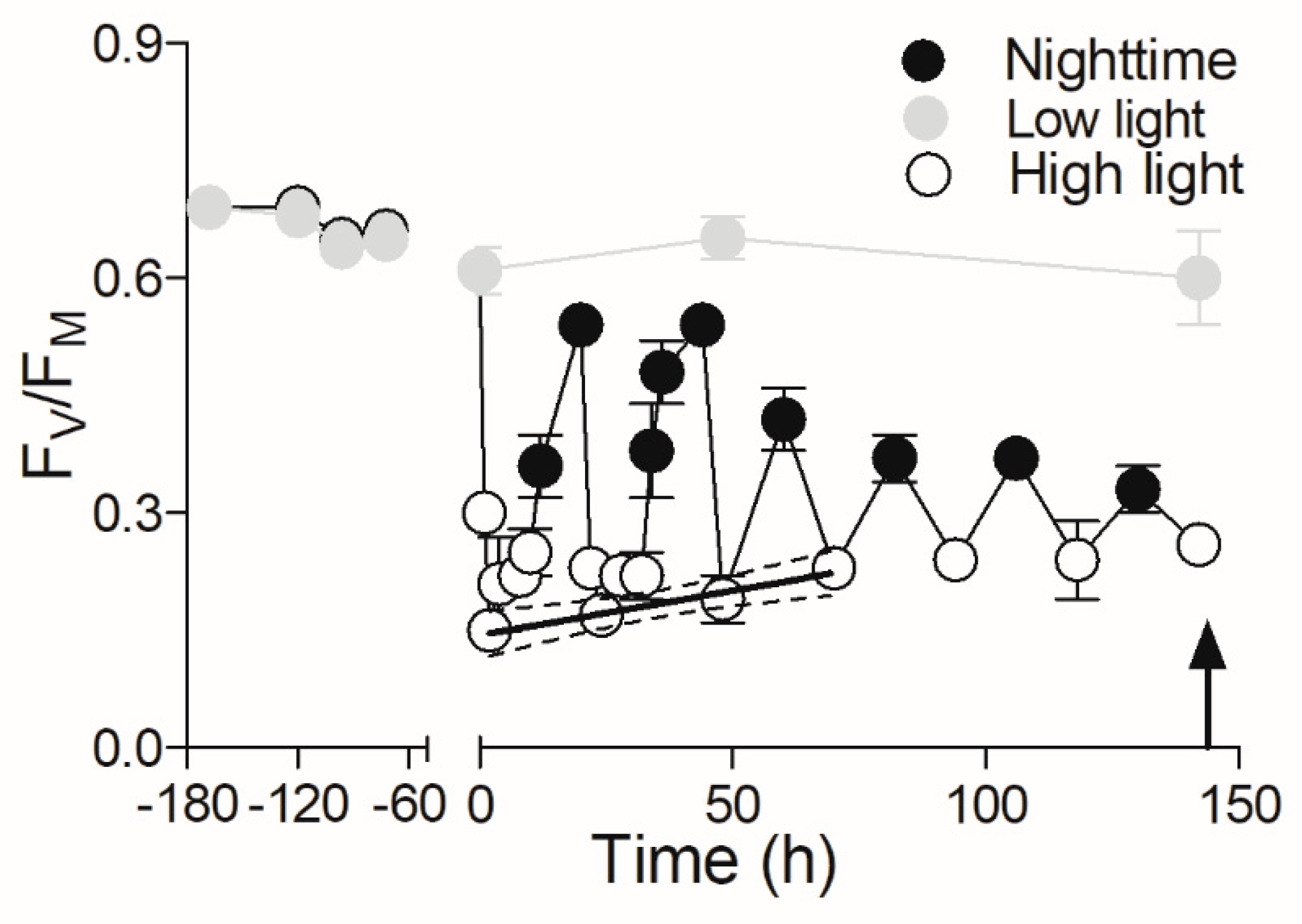
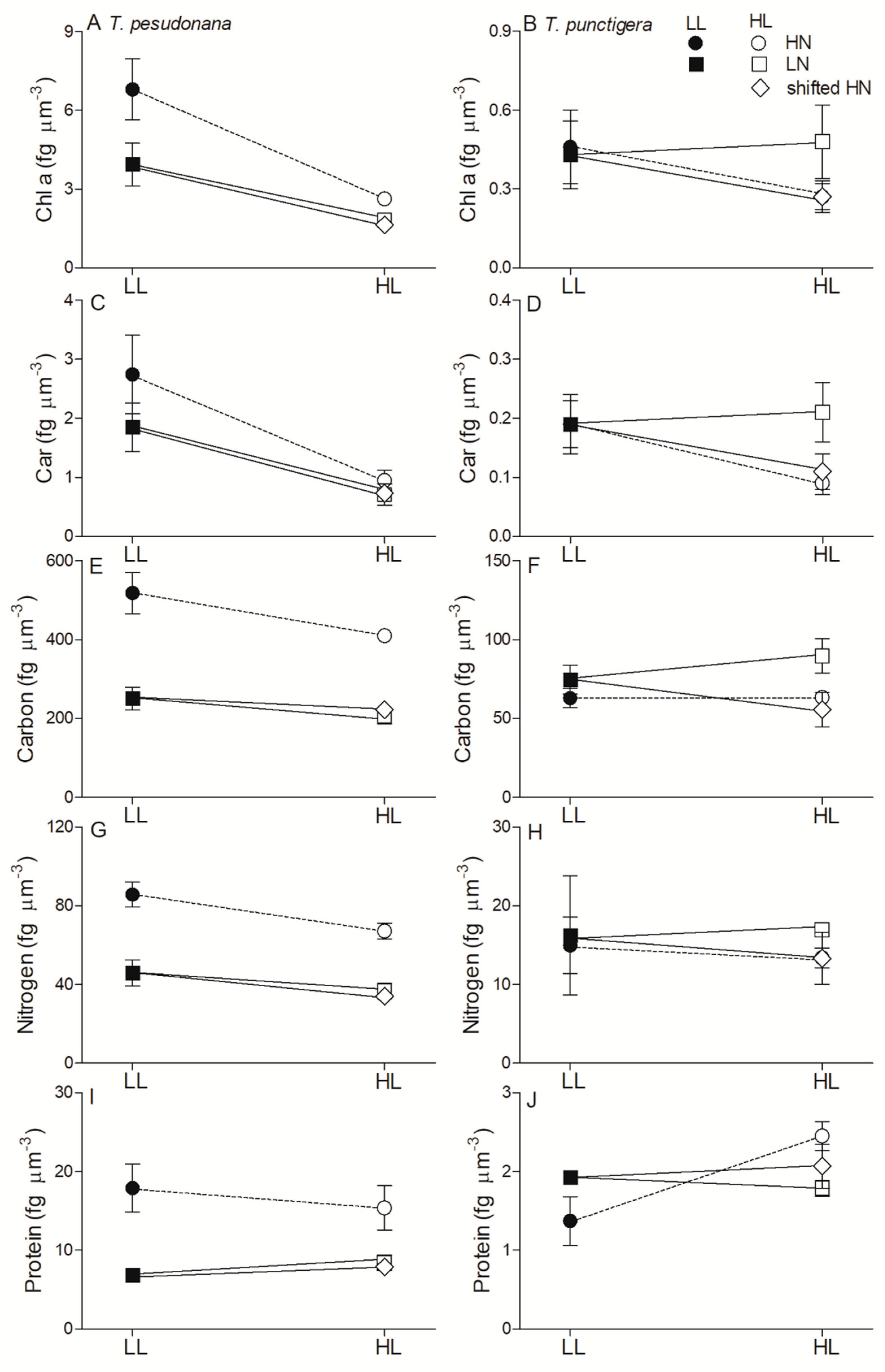
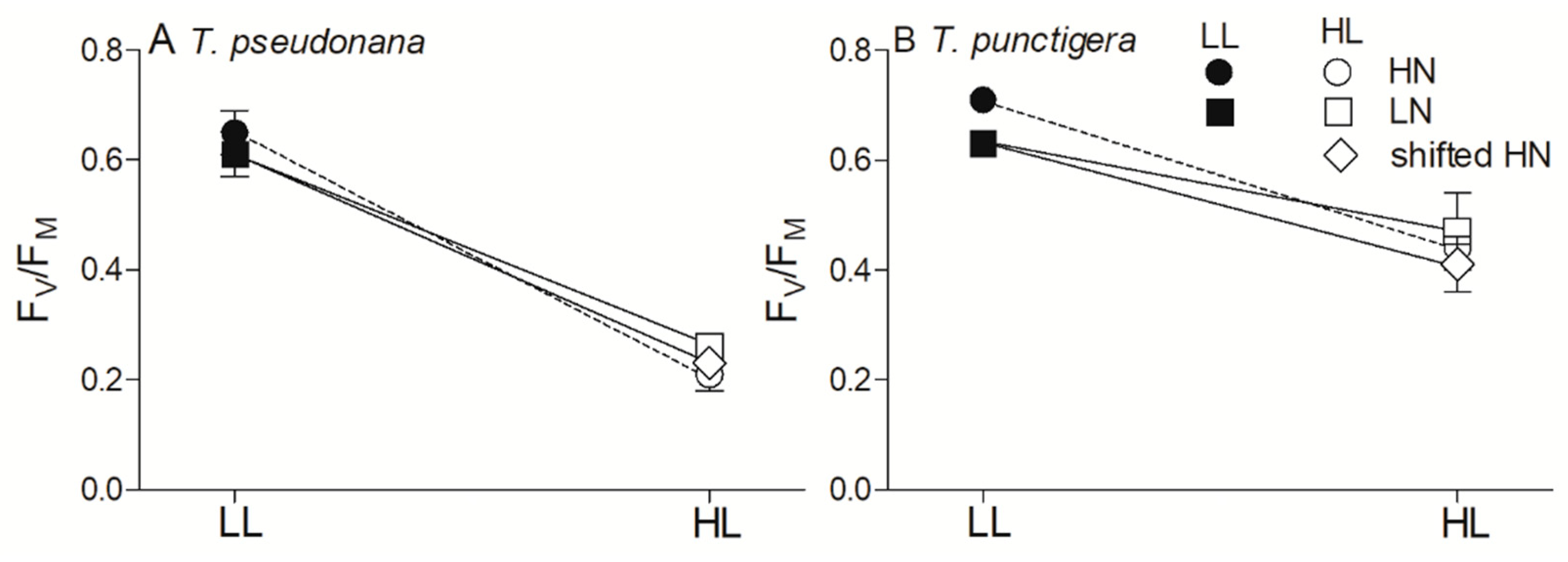
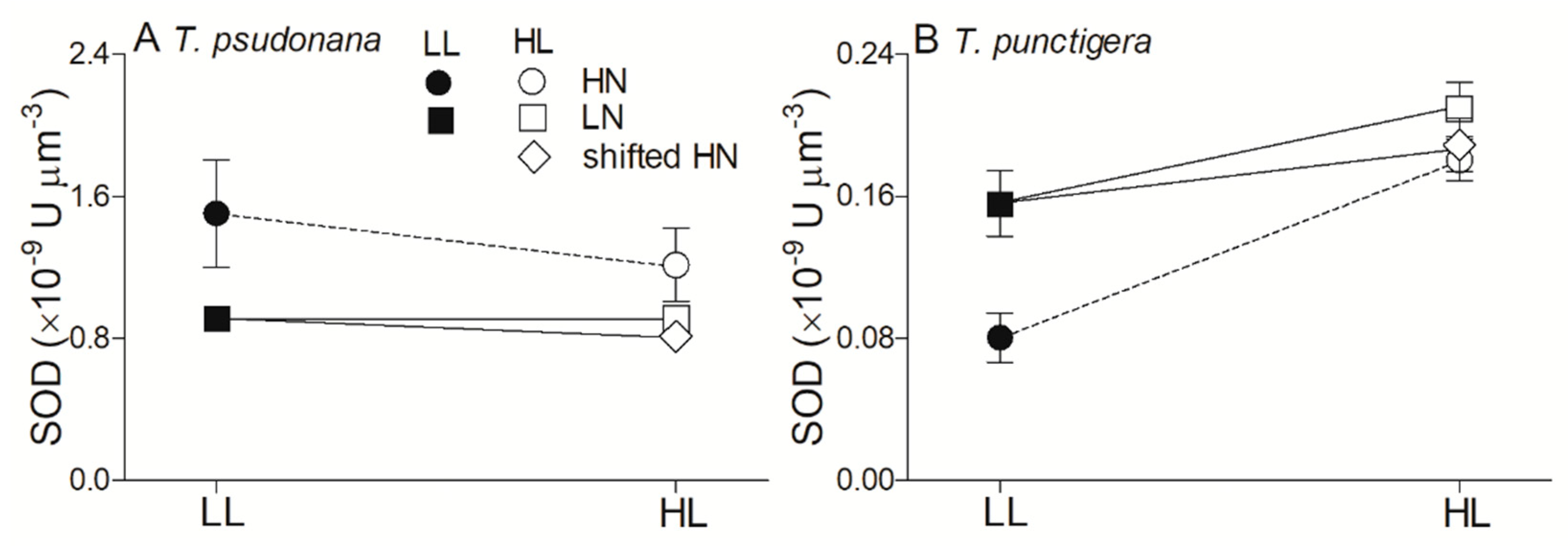
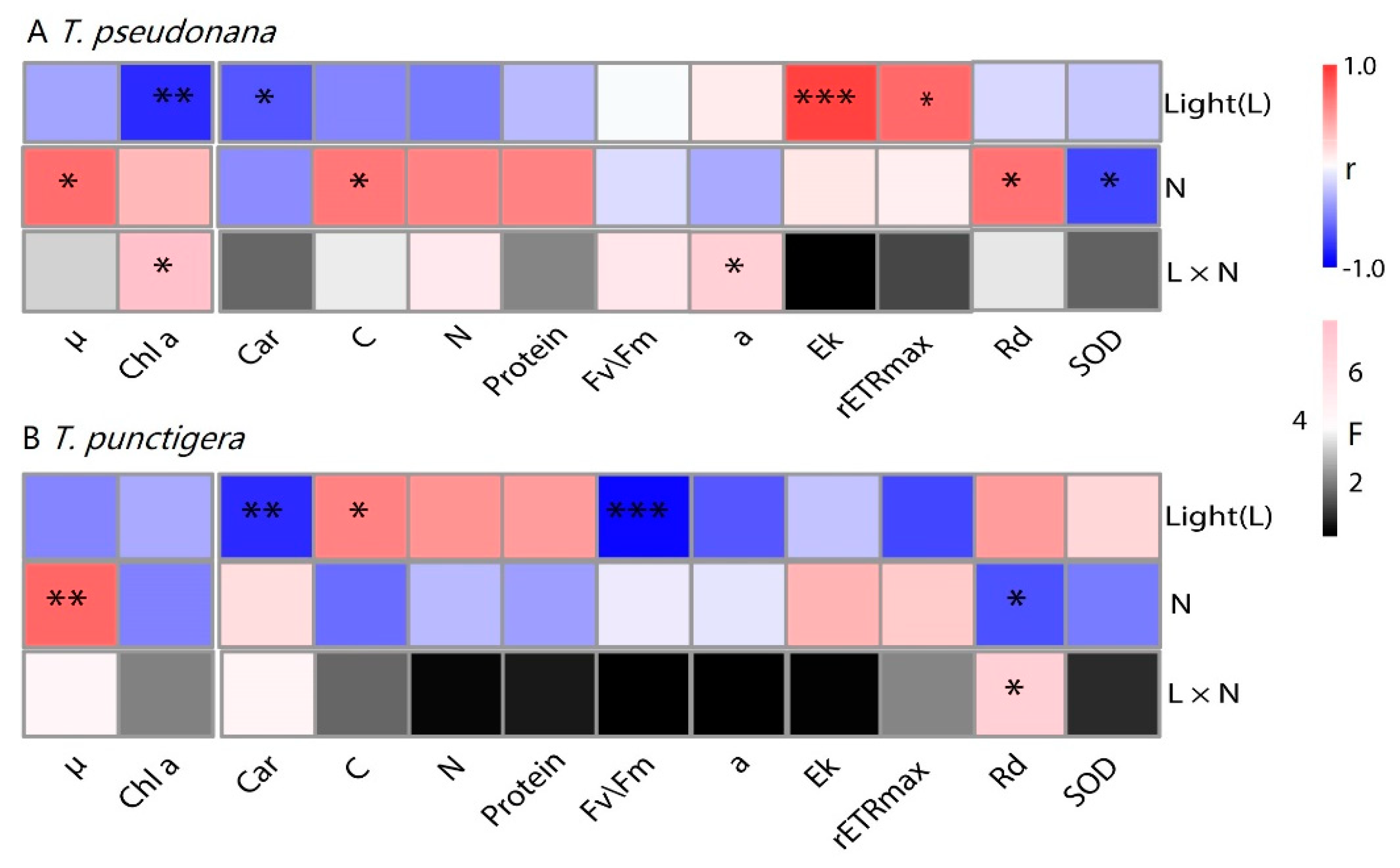
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ho, C.-H.; Baik, J.-J.; Kim, J.-H.; Gong, D.-Y.; Sui, C.-H. Interdecadal changes in summertime typhoon tracks. J. Clim. Chang. 2004, 17, 1767–1776. [Google Scholar] [CrossRef]
- Pan, J.; Huang, L.; Devlin, A.T.; Lin, H. Quantification of typhoon-induced phytoplankton blooms using satellite multi-sensor data. Remote Sens. 2018, 10, 318. [Google Scholar] [CrossRef]
- Mas, E.; Bricker, J.; Kure, K.; Adriano, B.; Yi, C.; Suppasri, A.; Koshimura, S. Field survey report and satellite image interpretation of the 2013 Super Typhoon Haiyan in the Philippines. Nat. Hazards Earth Syst. Sci. 2015, 15, 805–816. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Gao, K. Effects of typhoon Kaemi on coastal phytoplankton assemblages in the South China Sea, with special reference to the effects of solar UV radiation. J. Geophys. Res. 2009, 114, G04029. [Google Scholar] [CrossRef]
- Qiu, D.; Zhong, Y.; Chen, Y.; Tan, Y.; Song, X.; Huang, L. Short-term phytoplankton dynamics during typhoon season in and near the Pearl River estuary, South China Sea. J. Geophys. Res. 2019, 124, 274–292. [Google Scholar] [CrossRef]
- Lin, I.I.; Liu, W.T.; Wu, C.C.; Wong, G.T.F.; Hu, C.; Chen, Z.; Liang, W.D.; Yang, Y.; Liu, K.K. New evidence for enhanced ocean primary production triggered by tropical cyclone. Geophys. Res. Lett. 2003, 30, 1718. [Google Scholar] [CrossRef]
- Serra, T.; Vidal, J.; Casamitjana, X.; Soler, M.; Colomer, J. The role of surface vertical mixing in phytoplankton distribution in a stratified reservoir. Limnol. Oceanogr. 2007, 52, 620–634. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Kim, T. Typhoon-triggered phytoplankton bloom and associated upper-ocean conditions in the northwestern Pacific: Evidence from satellite remote sensing, Argo profile, and an ocean circulation model. J. Mar. Sci. Eng. 2020, 8, 788. [Google Scholar] [CrossRef]
- Wu, H.; Cockshutt, A.M.; McCarthy, A.; Campbell, D.A. Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms. Plant Physiol. 2011, 156, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Roy, S.; Alami, M.; Green, B.R.; Campbell, D.A. Photosystem II photoinactivation, repair, and protection in marine centric diatoms. Plant Physiol. 2012, 160, 464–476. [Google Scholar] [CrossRef]
- Anning, T.; Harris, G.; Geider, R.J. Thermal acclimation in the marine diatom Chaetoceros calcitrans (Bacillariophyceae). Eur. J. Phycol. 2001, 36, 233–241. [Google Scholar] [CrossRef]
- Li, G.; Campbell, D.A. Rising CO2 interacts with growth light and growth rate to alter Photosystem II photoinactivation of the coastal diatom Thalassiosira pseudonana. PLoS ONE 2013, 8, e55562. [Google Scholar] [CrossRef]
- Li, G.; Campbell, D.A. Interactive effects of nitrogen and light on growth rates and RUBISCO content of small and large centric diatoms. Photosynth. Res. 2017, 131, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tang, D.; Wang, Y. Comparison of phytoplankton blooms triggered by two typhoons with different intensities and translation speeds in the South China Sea. Mar. Ecol. Prog. Ser. 2008, 365, 57–65. [Google Scholar] [CrossRef]
- Zhao, H.; Shao, J.; Han, G.; Yang, D.; Lv, J. Influence of typhoon matsa on phytoplankton chlorophyll-a off East China. PLoS ONE 2015, 10, e0137863. [Google Scholar] [CrossRef]
- Tsai, A.Y.; Gong, G.C.; Chiang, K.P. Increase in phytoplankton production and consumption in response to a typhoon passing through the southern East China Sea. Aquat. Microb. Ecol. 2020, 84, 121–126. [Google Scholar] [CrossRef]
- Li, G.; Brown, C.M.; Jeans, J.A.; Donaher, N.A.; McCarthy, A.; Campbell, D.A. The nitrogen costs of photosynthesis in a diatom under current and future pCO2. New Phytol. 2015, 205, 533–543. [Google Scholar] [CrossRef]
- Song, X.; Tan, M.; Xu, G.; Su, X.; Liu, J.; Ni, G.; Tan, Y.; Huang, L.; Shen, P.; Li, G. Is phosphorus a limiting factor to regulate growth of phytoplankton in Daya Bay, northern South China Sea: A mesocosm experiment. Ecotoxicology 2019, 28, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Iudicone, D.; et al. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The diatoms: Biology and morphology of the genera. Aquat. Bot. 1991, 40, 301–302. [Google Scholar]
- Bowler, C.; Vardi, A.; Allen, A.E. Oceanographic and biogeochemical insights from diatom genomes. Annu. Rev. Mar. Sci. 2010, 2, 333–365. [Google Scholar] [CrossRef]
- von Dassow, P.; Petersen, T.W.; Chepurnov, V.A.; Armbrust, E.V. Inter- and intra-specific relationships between nuclear DNA content and cell size in selected members of the centric diatom genus Thalassiosira (Bacillariophyceae). J. Phycol. 2008, 44, 335–349. [Google Scholar] [CrossRef]
- Beardall, J.; Allen, D.; Bragg, J.; Finkel, Z.V.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Richardson, A.; Raven, J.A. Allometry and stoichiometry of unicellular, colonial and multicellular phytoplankton. New Phytol. 2009, 181, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Litchman, E.; Klausmeier, C.A.; Yoshiyama, K. Contrasting size evolution in marine and freshwater diatoms. Proc. Natl. Acad. Sci. USA 2009, 106, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Small is beautiful: The picophytoplankton. Funct. Ecol. 1998, 12, 503–513. [Google Scholar] [CrossRef]
- Finkel, Z.V.; Beardall, J.; Flynn, K.J.; Quigg, A.; Rees, T.A.V.; Raven, J.A. Phytoplankton in a changing world: Cell size and elemental stoichiometry. J. Plankton Res. 2010, 32, 119–137. [Google Scholar] [CrossRef]
- Key, T.; McCarthy, A.; Campbell, D.A.; Six, C.; Roy, S.; Finkel, Z.V. Cell size trade-offs govern light exploitation strategies in marine phytoplankton. Environ. Microbiol. 2010, 12, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Gao, K. Cell-size dependent effects of solar UV radiation on primary production in coastal waters of the South China Sea. Estuar. Coast. 2013, 36, 728–736. [Google Scholar] [CrossRef]
- Marañón, E.; Cermeño, P.; López-Sandoval, D.C.; Rodríguez-Ramos, T.; Sobrino, C.; Huete-Ortega, M.; Blanco, J.M.; Rodríguez, J. Unimodal size scaling of phytoplankton growth and the size dependence of nutrient uptake and use. Ecol. Lett. 2013, 16, 371–379. [Google Scholar] [CrossRef]
- Wu, Y.; Campbell, D.A.; Irwin, A.J.; Suggett, D.J.; Finkel, Z.V. Ocean acidification enhances the growth rate of larger diatoms. Limnol. Oceanogr. 2014, 59, 1027–1034. [Google Scholar] [CrossRef]
- Wu, Y.; Jeans, J.; Suggett, D.J.; Finkel, Z.V.; Campbell, D.A. Large centric diatoms allocate more cellular nitrogen to photosynthesis to counter slower RUBISCO turnover rates. Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef][Green Version]
- Yan, D.; Beardall, J.; Gao, K. Variation in cell size of the diatom Coscinodiscus granii influences photosynthetic performance and growth. Photosynth. Res. 2018, 137, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Tan, Y.; Wang, S.; Shen, P.; Ke, Z.; Huang, L.; Song, X.; Li, G. Cell size dependent responses of phytoplankton assemblages to nitrate and phosphate additions in surface waters of the northern South China Sea. J. Mar. Sci. 2014, 4, 61–67. [Google Scholar] [CrossRef][Green Version]
- Li, G.; Talmy, D.; Campbell, D.A. Diatom growth responses to photoperiod and light are predictable from diel reductant generation. J. Phycol. 2017, 53, 95–107. [Google Scholar] [CrossRef]
- Li, W.K.W. Macroecological patterns of phytoplankton in the northwestern North Atlantic Ocean. Nature 2002, 419, 154–157. [Google Scholar] [CrossRef]
- Raven, J.A.; Doblin, M.A. Active water transport in unicellular algae: Where, why, and how. J. Exp. Bot. 2014, 65, 6279–6292. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Lenton, T.M.; Williams, H.T.P.; Daines, S.J. Environmental selection and resource al-location determine spatial patterns in picophytoplankton cell size. Limnol. Oceanogr. 2013, 58, 1008–1022. [Google Scholar] [CrossRef]
- Grover, J.P. Resource storage and competition with spatial and temporal variation in resource availability. Am. Nat. 2011, 178, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.; Ryther, J.H. Studies of marine planktonic diatoms.1. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. BBA Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Eilers, P.H.C.; Peeters, J.C.H. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Modell. 1998, 42, 199–215. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, B.; Song, X.; Jian, X.; Tang, C.; Campbell, D.A.; Li, G. High antioxidant capability interacts with respiration to mediate two Alexandrium species growth exploitation of photoperiods and light intensities. Harmful Algae 2019, 82, 26–34. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Xu, G.; Liu, J.; Chen, B.; Li, G. Photoperiod mediates the differential physiological responses of smaller Thalassiosira pseudonana and larger Thalassiosira punctigera to temperature changes. J. Appl. Phycol. 2020, 32, 2863–2874. [Google Scholar] [CrossRef]
- Sun, Y.; Oberley, L.W.; Li, Y. A simple method for clinical assay of superoxide-dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Marcoval, M.A.; Villafañe, V.E.; Helbling, E.W. Interactive effects of ultraviolet radiation and nutrient addition on growth and photosynthesis performance of four species of marine phytoplankton. J. Photochem. Photobiol. B Biol. 2007, 89, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L. Spatial and seasonal variations of nitrate-based new production and primary production in the South China Sea. Deep-Sea Res. I 2005, 52, 319–340. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant. Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Waring, J.; Klenell, M.; Bechtold, U.; Underwood, G.J.C.; Baker, N.R. Light-induced responses of oxygen photoreduction, reactive oxygen species production and scavenging in two diatom species. J. Phycol. 2010, 46, 1206–1217. [Google Scholar] [CrossRef]
- Dong, H.; Dong, Y.; Cui, L.; Balamurugan, S.; Gao, J.; Lu, S.; Jiang, T. High light stress triggers distinct proteomic responses in the marine diatom Thalassiosira pseudonana. BMC Genom. 2016, 17, 994. [Google Scholar] [CrossRef] [PubMed]
- Hazzard, C.; Lesser, M.P.; Kinzie, R.A. Effects of ultraviolet radiation on photosynthesis in the subtropical marine diatom, Chaetoceros gracilis (Bacillariophyceae). J. Phycol. 1997, 33, 960–968. [Google Scholar] [CrossRef]


| N | Light | T. pseudonana | T. punctigera | ||||
|---|---|---|---|---|---|---|---|
| α | EK | rETRmax | α | EK | rETRmax | ||
| 882 | 35 | 0.20 ± 0.009 a | 348 ± 47.3 a | 70.5 ± 10.42 a | 0.24 ± 0.023 a | 524 ± 57.2 a | 125 ± 12.4 a |
| 250 | 0.09 ± 0.026 b | 264 ± 68.4 a | 8.15 ± 4.39 b | 0.13 ± 0.034 b | 263 ± 46.2 b | 30.8 ± 5.85 b | |
| 88.2 | 35 | 0.23 ± 0.023 a | 136 ± 17.9 b | 31.0 ± 4.58 c | 0.23 ± 0.022 a | 352 ± 34.3 c | 81.2 ± 14.04 c |
| 250 | 0.14 ± 0.011 c | 440 ± 62.4 c | 57.4 ± 7.64 a,d | 0.16 ± 0.020 b | 341 ± 73.2 c | 52.6 ± 11.40 b | |
| 882 | 250 | 0.11 ± 0.014 b,c | 342 ± 36.7 a,c | 35.8 ± 2.42 c | 0.16 ± 0.014 b | 218 ± 24.3 b | 34.7 ± 3.33 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Z.; Xia, X.; Mai, G.; Tan, Y.; Li, G. Differential Physiological Responses of Small Thalassiosira pseudonana and Large Thalassiosira punctigera to the Shifted-High Light and Nitrogen. J. Mar. Sci. Eng. 2021, 9, 450. https://doi.org/10.3390/jmse9050450
Qin Z, Xia X, Mai G, Tan Y, Li G. Differential Physiological Responses of Small Thalassiosira pseudonana and Large Thalassiosira punctigera to the Shifted-High Light and Nitrogen. Journal of Marine Science and Engineering. 2021; 9(5):450. https://doi.org/10.3390/jmse9050450
Chicago/Turabian StyleQin, Zhen, Xiaomin Xia, Guangming Mai, Yehui Tan, and Gang Li. 2021. "Differential Physiological Responses of Small Thalassiosira pseudonana and Large Thalassiosira punctigera to the Shifted-High Light and Nitrogen" Journal of Marine Science and Engineering 9, no. 5: 450. https://doi.org/10.3390/jmse9050450
APA StyleQin, Z., Xia, X., Mai, G., Tan, Y., & Li, G. (2021). Differential Physiological Responses of Small Thalassiosira pseudonana and Large Thalassiosira punctigera to the Shifted-High Light and Nitrogen. Journal of Marine Science and Engineering, 9(5), 450. https://doi.org/10.3390/jmse9050450








